Abstract
It is estimated that one third of the population in Western industrial countries suffers from constipation at least from time to time. Constipation may have somatopathic or functional causes. Furthermore, a great number of substances are known to cause medication-induced constipation, i.e. opioid-induced constipation is caused by linkage of the opioid to opioid receptors in the bowel and the central nerve system. Whenever possible, causal therapy should be undertaken. Patients in palliative care mostly suffer from chronic functional constipation. The treatment consists of basic measures and the application of laxatives. According to their mode of action, they are divided into bulk-forming laxatives, osmotic laxatives, stimulant laxatives, lubricating agents and others. Bulk-forming laxatives are not recommended for use in palliative care patients, for such patients are normally not able to take in the required amount of fluids. Osmotic laxatives are divided into (magnesium) salts, saccharine, alcohols and macrogols. Lactulose is the most popular saccharine laxative. Because of its side effects (flatulence, bloating and abdominal cramping), lactulose is not a laxative of our choice; instead, we prefer to give macrogol. Orally administered, macrogol is not metabolised and pH value and bowel flora remain unchanged. Macrogol hydrates hardened stools, increases stool volume, decreases the duration of colon passage and dilates the bowel wall that then triggers the defecation reflex. Even when given for some time, the effectiveness of macrogol will not decrease. Because of its high effectiveness and commonly good tolerance, macrogol has become the laxative of first choice in palliative care patients with all kinds of chronic constipation, if these patients are able to take in the necessary amount of fluids. From the general medical point of view, lubricating agents have become obsolete. In palliative care patients, however, they are still important laxatives for prophylactic treatment or therapy of constipation. Due to clinical experience, in palliative care a laxative ladder has proven successful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To date there has been no international consensus on a general definition and classification of constipation. Any definition that is, for example, based purely on the frequency of bowel movements does not do justice to the majority of patients who claim to suffer from constipation. The term constipation indicates a subjective impression that:
-
The frequency of bowel movements is dissatisfying
-
There is a sensation of incomplete evacuation
-
The consistency of the stool is too hard and/or
-
The stool is passed with discomfort [12].
Indications for constipation are given when there are less than three bowel movements per week, less than 35 g of stool per day, stool water weight is less than 70% and gastrointestinal transit time is longer than five days [3].
Causes of constipation
The possible causes of constipation are manifold, but they may be subsumed under two main categories, which are:
-
1.
Somatopathic causes
-
2.
Functional causes.
Somatopathic constipation may be a result of diseases such as diverticulitis, tumours/cancer, inflammatory processes in the anal area, neurological diseases, endocrinal diseases, recto-anal diseases or metabolic changes. Functional constipation may be due to a prolonged colon passage, defecation disorder, poor intake of fluids or dietary fibres, medication or situational factors such as way of life, lack of exercise/immobility.
A large number of substances are known to cause medication-induced constipation, i.e. antacids, antibiotics, anticholinergics, antihypertensives, anticonvulsants, anti-Parkinsonian medications, diuretics, excessive use of laxatives, muscle relaxants, neuroleptics, non-steroidal antiphlogistics, opioids and many others.
Opioid-induced constipation
Constipation is the most frequent and most persistent side effect of opioid treatment [7, 16, 18]. Unlike other side effects of opioid medication, such as nausea and emesis, there is no, or extremely slow, tolerance build-up to the constipatory effects of opioids. Therefore, patients tend to require regular laxative treatment for the duration of the opioid therapy.
Causes and pathophysiology of opioid-induced constipation
Peripheral as well as intrathecal and intraventricular application of opioids will lead to a prolonged colon passage of the bowel content due to the fact that opioid-induced constipation is caused by linkage of the opioid to opioid receptors in the bowel and the central nervous system [6, 7].
The inhibition of release of acetylcholine from the myenteric plexus leads to a relaxation of the longitudinal musculature of the colon and small intestine. Subsequently, the propulsive motor function decreases. Furthermore, opioids cause an increase of segmental intestinal contractions. Therefore, the faeces stay in the intestine for longer, leading to a withdrawal of water and a thickening and lengthening of the stool.
Furthermore, intestinal, gastric, biliary and pancreatic secretions are decreased. Hyperactivity of the tonus of the intestinal sphincters and an impaired defecation reflex add to the constipatory effect.
Diagnostics
Basic diagnostic measures include patient history, medical examination including inspection of the anal area, digital examination of the sphincter muscles and the rectum, stool examination and laboratory tests. Questions regarding the patient's history should include issues such as:
-
Beginning and duration of constipation
-
Pain in the abdominal area or during defecation
-
Sensation of fullness
-
Diet and eating habits
-
Medication
-
Sensation of incomplete evacuation
-
Stool consistency
-
Stool colour
-
Use of laxatives.
Details regarding these basic diagnostic measures, as well as details regarding measures such as sonography, X-ray and coloscopy, are beyond the scope of this paper.
Therapy
General measures
Whenever possible, causal therapy should be undertaken. The flow diagram (see Fig. 1) shows a systematic regimen for the assessment of an adequate strategy. If constipation is suspected, the first step must be to examine whether the patient suffers from gastrointestinal obstruction. If this is the case, the next step is to assess whether this gastrointestinal obstruction is complete or incomplete. In the case of complete gastrointestinal obstruction, no laxatives must be given, whereas they are indicated in the case of incomplete obstruction. Furthermore, the pros and cons of a potential surgical intervention should be weighed up in either case.
If a gastrointestinal obstruction has been eliminated, a rectal medical examination may reveal information for further diagnostic measures. If the rectum is full and the faeces are of a soft consistency, a stimulating suppository should be given in tandem with oral administration of a laxative, i.e. macrogol. If the faeces are hard, a stool-softening suppository in tandem with orally taken fluid and a laxative are indicated.
If the rectum is empty, the possibility of an obstruction must be considered, i.e. eliminated. An empty rectum but a full colon is an indication for the application of a clysma or enema in combination with oral administration of an osmotic laxative and stimulants. If both the rectum and colon are empty, other diagnostic measures must be considered.
Palliative care patients most frequently suffer from chronic functional constipation, the treatment of which includes basic therapeutic measures and the administration of laxatives.
Basic measures
Basic therapeutic measures include:
-
Information about the symptom complex, pathophysiology and potential causes of constipation
-
Information about physiotherapeutic measures
-
Increasing exercise
-
Increasing dietary fibre intake (to approximately 20–35 g/d)
-
Increasing intake of fluids (to about 1.5–2l/d).
However, palliative care patients are rarely able to undertake these important and effective basic measures, as most of them suffer from increasing fatigue, immobility and loss of appetite. Therefore, there is a clear medical indication for the application of laxatives.
Laxatives
Laxatives are substances that accelerate defecation. They have an impact on the transfer of water and electrolytes of the small and large intestinal mucosa, and they soften hardened faeces and stimulate defecation. According to their mode of action, they are divided into:
-
Bulk-forming laxatives
-
Osmotic laxatives
-
Stimulant laxatives
-
Lubricating agents
-
Others.
Bulk-forming laxatives
Chemically, bulk-forming laxatives are divided into different substances, which have in common that they cannot be broken down and are therefore only marginally absorbed, if at all. Mostly, they consist of natural or synthetic polysaccharides. By absorbing water in the intestine, bulk-forming laxatives increase the volume and softness of faeces, thus dilating the intestinal wall, which subsequently increases the propulsive motor function.
The use of bulk-forming laxatives in palliative care patients is not recommended, for these patients are usually confined to bed and cannot take in the required amount of fluids.
Osmotic laxatives
The substance group of osmotic laxatives is heterogeneous. The common denominator of osmotic laxatives is that they are not—or only marginally—reabsorbed during the bowel transit. Water taken in as a component of food remains bound and is transferred to the extracellular space in the bowel. Osmotic laxatives are divided into (magnesium) salts, saccharine, alcohols and macrogols. The following focuses on saccharine laxatives and macrogols only.
Saccharine laxatives
Lactulose is a disaccharide, consisting of glucose and fructose that cannot be broken down in the small intestine (see Fig. 2). Lactulose passes the gastrointestinal tract unabsorbed as far as the colon, where bacteria break down the sugar into short-chained fatty acids. Subsequently, the intraluminal pH value decreases, which causes an increase of peristaltic movements. Furthermore, the osmotic pressure in the colon lumen increases and water is retained in the intestine. The stool softens and increases in volume. Therefore, the bowel wall dilates, which then triggers the reflex action of the bowel peristalsis. Common side effects of lactulose are flatulence, abdominal cramps and bloating.
Polyethylene glycol (macrogol)
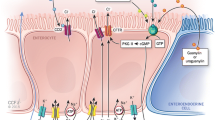
Originally, polyethylene glycol was given in relatively high doses in order to induce purgation, i.e. as a preparatory measure for gastroenterologic interventions such as coloscopy [4]. Orally administered, macrogol is not metabolised—i.e. there is no bacterial or enzymatic metabolisation—pH value and bowel flora remain unchanged and there is no fermentative production of intestinal gas (see Fig. 3).
Mode of action of lactulose and macrogol (mod. Müller-Lissner [13])
Not only is the water-binding capacity of macrogol exactly defined, it also does not lead to a loss of fluids and electrolytes because it only binds orally taken fluid [1].
Macrogol hydrates hardened stools, increases stool volume, decreases the duration of the colon passage and dilates the bowel wall, which then triggers the defecation reflex. The relation between dosage and effect is linear. Macrogol draws no water from the body and therefore shows a neutral balance. The effectiveness of macrogol does not decrease over time [2, 4, 8, 9]. Furthermore, its effectiveness is independent of both time of application (i.e. time of day) and time of food intake. After the first application, the initial effect is seen in 2 to 3 days. If taken regularly, the frequency of bowel movements tends to be one movement per day.
Because of its high effectiveness and good tolerance, macrogol has become the laxative of choice for palliative care patients with all kinds of chronic constipation and opioid-induced constipation, provided that these patients are able to take in the necessary amount of fluids (125 ml fluids per sachet).
Compared with the administration of lactulose, side effects occur much less frequently, in particular, the above-mentioned and very common flatulence, bloating and abdominal cramps.
Stimulants (antireabsorptive and secretagogue acting agents)
Antireabsorptive and secretagogue acting agents inhibit the reabsorption of liquid and sodium from the bowel lumen. Furthermore, they induce an inflow of sodium, chloride, calcium and liquid into the bowel lumen, and the sennosides increase the propulsive motor function due to their direct effect on the myenteric plexus (see Fig. 4). Stimulants include:
-
Anthracenes
-
Diphenols (phenolphthalein, bisacodyl, sodium picosulphate)
-
Fatty acids
The group of anthracenes includes senna preparations, which are reliable and effective laxatives. However, it has to be mentioned that they can cause cramp-like intestinal pain. Diphenols include bisacodyl and sodium picosulphate; the latter in particular is often used in palliative care patients. Castor oil (ricini oleum, Ph. Eur) belongs to the group of fatty acids. The special effect of castor oil is that it—unlike most other laxatives—enhances the peristaltic movement, even in the small intestine. Its laxative effect may be described as drastic, often accompanied with cramp-like abdominal pain. Therefore, castor oil can only be recommended for certain indications.
Lubricants
Lubricating agents are stool softeners and ease defecation due to their surfactant effect. This substance group includes non-reabsorbable oils, oils that are very difficult to reabsorb such as paraffin. Because of some side effects, lubricating agents are regarded as obsolete. In palliative care patients, however, they remain important agents for the prevention and treatment of constipation.
Other laxatives
Other laxative agents include prokinetics, metoproclamide, erythromycin, misoprostol and opioid antagonists. There is evidence that opioid antagonists (i.e. naloxone, orally administered, 8–10 mg/d) resolve opioid-induced constipation without having a negative impact on the analgetic effect of the opioid due to a displacement of the opioid from the intestinal opioid receptors [10, 16]. The analgesic effect of the opioid remains unchanged because of the high first-pass effect of naloxone.
Rectal laxatives
Rectal laxatives include suppositories, clysmas and enema preparations. Suppositories and clysmas contain mono-substances or combinations of secretagogue-acting agents or stool softeners for rectal application, i.e. bisacodyl, glycerol, sorbitol and docusate sodium. An enema is the application of larger amounts of fluids into the rectum, a high enema is used to reach as many segments of the colon as possible. The effect of the enema depends on:
-
The amount of applied fluid
-
The intraluminal pressure
-
The temperature of the enema
-
Additional substances such as glycerol, olive oil, sodium chloride and others.
Assessment of clinical use
Before any therapeutic measures can be implemented, a diagnosis must be undertaken based on a full holistic assessment as to whether the existing constipation is an independent syndrome, a symptom of a disease or possibly due to exogenic factors such as intake of medication with constipatory effects. Physicians must exclude organic or other constipation for which a causal treatment would be appropriate. Basic treatment of constipation includes:
-
Information for the patient about the nature, cause and options for the treatment of constipation
-
Promotion of exercise
-
Intake of an adequate amount of dietary fibres in order to enhance the water-binding capacity of the faeces
-
Intake of a diet that serves to promote bowel motility, i.e. fruit, dates, salad
-
Intake of the required amount of fluid (at least 1.5l/d)
-
Avoidance of constipation-promoting food (such as refined flour products, chocolate, bananas, tea other than herbal or fruit, etc).
These generally effective basic measures, however, are rarely applicable to palliative care patients, as most of them suffer increasingly from:
-
Increasing fatigue
-
Immobility and confinement to bed
-
Loss of appetite and aversion to a fibre rich diet
-
Decreasing intake of fluid.
Moreover, in order to achieve satisfying symptom control, the treatment of most of these patients requires relatively large amounts of drugs with constipatory side effects. Nevertheless, physicians must reconsider on a regular basis whether the application of constipation-causing substances may be terminated, although in most cases this is not possible. Physicians would not normally terminate opioid treatment for the management of severe pain, but would start an adjuvant therapy with laxatives.
Any decision to start a pharmacological therapy of constipation must be based on the following pre-conditions:
-
A causal therapy of the patient's disease is no longer possible
-
A causal therapy of the symptoms caused by this disease is also no longer possible
-
The goal of treatment is symptom control
-
The treatment of the patient's symptoms requires a great variety and number of drugs
-
The physician must be aware of the profile and the adequate treatment of side effects of these drugs.
Conclusion
Achievement of the best possible palliation of symptoms in palliative care patients frequently requires the use of drugs with a high profile of side effects. Symptom control, therefore, must be conducted in tandem with prophylactic or therapeutic treatment with adjuvant substances. Since most drugs used for palliative medical treatment have constipatory side effects, laxatives play a mayor role in the treatment of palliative care patients. Because of the way the diseases of these patients progress, laxative therapy differs greatly from the procedure used in other patient groups. In patients with an advanced tumour disease, for example, an intake of dietary fibre is not only not recommended, it may even be dangerous. The intake of dietary fibre may cause a promotion of constipation and result in faecal impaction, or worse, in bowel obstruction.
Stimulants are of great value in the treatment of palliative care patients—above all, senna preparations, sodium picosulphate and bisacodyl suppositories. Because of its drastic purgatory effect, the application of castor oil is only indicated in very special cases, i.e. if the therapy aims at a promotion of the peristaltic movement in the small intestine.
Among the group of osmotic laxatives, salinic laxatives are not important; saccharine alcohols (sorbitol, mannitol, glycerol) are almost exclusively used in the form of suppositories or enemas.
Our personal opinion is that the use of lactulose for the treatment of opioid-induced constipation is significantly overrated. Meteorism and flatulence are very frequent side effects that are very stressful for patients, and many patients also dislike the sweet taste of lactulose syrups.
In contrast to lactulose, macrogol, another osmotic laxative, is not broken down in the colon and remains unchanged during the intestinal passage. It binds a certain, defined amount of water and causes only negligible electrolyte imbalances. Compared with lactulose treatment, macrogol treatment very rarely causes meteorism and flatulence.
Macrogol is the laxative of choice for prophylactic treatment and therapy of opioid-induced constipation, provided that the patient is able to take in the required amount of fluids.
In contrast to general professional opinion, the application of lubricants (liquid paraffins) is a very useful and necessary additional measure in the treatment of palliative care patients.
The application of rectal laxatives is indicated if the therapeutic goal is to trigger the defecation reflex in order to evacuate soft or hardened bowel contents from a filled rectum. If the aim is also to shorten the duration of the colon passage, the combination with an orally applied laxative is recommended.
The information and clinical experiences discussed above have led to the development of a laxative ladder. This regimen for the treatment of constipation is easy to apply and has proven successful in palliative care (see Fig. 5), and it adds much to the patient's quality of life.
References
Cleveland M, Flavin DP, Ruben R A, Epstein RM, Clark GE (2001) New polyethylene glycol laxative for treatment of constipation in adults: a randomized, double-blind, placebo-controlled study. South Med J 94(5):478–481
Corazziari E, Badiali D, Bazzochi G, Basotti G, Roselli P, Mastropaolo G, Luca MG, Galeazzi R, Peruzzi E (2000) Long-term efficacy, safety and tolerability of low daily doses of isoosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut 46:522–526
Goerg K J, Wanitschke R, Loew D (1997) Obstipation und Laxanzien—eine Standortbestimmung, Der Allgemeinarzt. Fortbildung und Praxis für den Hausarzt. Kirchheim Verlag, Mainz. 19. Jg., 1+2, reprint
Hammer HF, Hammer J, Gasche C (2000) Polyäthylenglykol (Macrogol)—Übersicht über seine Verwendung in der Diagnostik und Therapie gastroenterologischer Erkrankungen. Wien. Klein. Wochenschr. 112:53–60
Heaton KW, O'Donnell LJ, Braddon FE, Mountford RA, Hughes RO, Cripps PJ (1992) Symptoms of irritable bowel syndrome in a British urban community: consulters and nonconsulters. Gastroenterology 102:1962–1967
Jurna I, Baldauf J (1993) Retardiert freigesetztes Naloxon oral: Aufhebung der Obstipation durch orales Morphin ohne Beseitigung der Analgesie. Der Schmerz 7:314–321
Kaufman PN, Krevsky B, Malmud LS, Maurer AH, Somers MB, Siegel JA, Fisher RS (1998) Role of opiate receptors in the regulation of colonic transit. Gastroenterology 94:1351–1356
Klauser AG, Mühldorfer BE, Vorderholzer WA, Wenzel G, Müller-Lissner SA (1995) Polyethylene glycol 4000 for slow transit constipation. Gastroenterology 33:5–8
Lasch HM, Bozymski EM (2000) A new weapon for the arsenal in the war against constipation? Am J Gastroenterol 95:341–342
Latasch L, Zimmermann M, Eberhardt B, Jurna I (1997) Aufhebung einer Morphin-induzierten Obstipation durch orales Naloxon. Anaesthesist 46:191–194
Locke GR III (1996) The epidemiology of functional gastrointestinal disorders in north America. Gastroenterol Clin North Am 25:1–19
Müller-Lissner S (1992) Nebenwirkungen von Laxantien. Z Gastroenterology 30:418–427
Müller-Lissner S, Beil W (2001) Moderne Therapie mit Laxantien, unter Mitarbeit von Erckenbrecht JF, Wagner S. UniMed Verlag. Bremen
Sonnenberg A, Koch TR (1989) Epidemiology of constipation in the United States. Dis Colon Rectum 32:1–8
Stewart WF, Liberman JN, Sandler RS, Woods MS, Sternhagen A, Chee E, Lipton RB, Farup CE (1999) Epidemiology of constipation (EPOC) Study in the United States: Relation of clinical subtypes to socioeconomic features. Am J Gastroenterol 94:3530–3539
Sykes N (1998) The treatment of morphine-induced constipation. European Journal of Palliative Care 5(1):12–15
Talley NJ, Weaver AL, Zinsmeister AR (1993) Functional constipation and outlet delay. A population-based study. Gastroenterology 105 781–790
Willweber-Strumpf A, Zenz M, Tryba M (1995) Leitlinien zur Therapie chronischer Schmerzen mit Opioiden. Anaesthesist 44:719–723
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented as an invited lecture at the 15th International Symposium Supportive Care in Cancer, Berlin, Germany, June 18–21, 2003.
Rights and permissions
About this article
Cite this article
Klaschik, E., Nauck, F. & Ostgathe, C. Constipation—modern laxative therapy. Support Care Cancer 11, 679–685 (2003). https://doi.org/10.1007/s00520-003-0525-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-003-0525-x