Summary
In addition to increasing the health risk to the individual patient, late diagnosis of HIV infection affects the costs of the medical care. Comprehensive data on the precise financial burden posed by late presentation are lacking.
This retrospective analysis in Austria aimed to compare the marginal costs of initial care after diagnosis in patients presenting with advanced HIV disease vs. non-late presenters.
Treatment-naïve late and non-late presenters were matched by age and risk group and were followed up for an average of 15 months. Using a marginal cost approach, the costs of medications, outpatient consultations, diagnostic interventions, and inpatient stays were compared.
Cases had significantly higher viral load and lower CD4 cell counts. At first diagnosis, 45.8 % of cases had CDC stage A vs 85.2 % of controls. Late presenters had 70 % more outpatient consultations (p < 0.01) and three-fold higher total marginal costs (€ 722,761 vs. 244,976). Cost per patient and month ranged from € 600 to 17,108 for cases and from € 102 to 26,958 for controls. Largest cost difference was noted for antiretroviral (ART) medication (monthly average € 1,089 per case vs. 77 per control), accounting for 42 % of overall costs for cases compared to 10 % of total costs for the controls. Higher costs were also seen for hospitalizations, diagnostics, and non-ART-medication in cases.
Late presentation places a significant economic burden on the Austrian healthcare system. Patients and society would benefit from effective screening programs to enable earlier diagnosis with more efficient linkage to care at least in the period immediately following diagnosis.
Zusammenfassung
Zusätzlich zu dem erhöhten individuellen Gesundheitsrisiko verändert eine späte Diagnose einer HIV-Infektion auch den Bedarf an medizinischer Versorgung. Genaue Daten über die finanziellen Folgen einer späten HIV-Diagnose fehlen jedoch.
Diese retrospektive Studie in Österreich untersuchte die Kosten, die aus der initialen medizinischen Versorgung eines Patienten mit fortgeschrittener HIV-Infektion („late presenter“) zum Zeitpunkt der Diagnosestellung erwachsen im Vergleich zu Patienten mit einer frühen Diagnosestellung.
Therapie-naïve late presenter wurden mit in Alter und Risikogruppe übereinstimmenden Patienten („Kontrollgruppe“) mit einer frühen HIV-Diagnose über im Mittel 15 Monate untersucht. Mit einem Grenzkosten-Ansatz wurden die Kosten für medikamentöse Therapie, Ambulanzbesuche, Labor- und bildgebende diagnostische Verfahren sowie stationäre Krankenhausaufenthalte verglichen.
Patienten mit eine späten HIV-Diagnose hatten signifikant niedrigere CD4 Zellwerte sowie eine höhere HI-Viruslast. Zum Zeitpunkt der Erstpräsentation befanden sich 45,8 % der late presenter und 85,2 % der Kontrollgruppe im CDC Stadium A.
Late presenters benötigten 70 % mehr ambulante Kontrollen (p < 0.01) mit 3-fach höheren Grenzkosten (€ 722.761 vs. 244.976). Die Kosten pro Patient pro Monat reichten von € 600 bis 17.108 in der Gruppe mit später HIV-Diagnose und von € 102 bis 26.958 in der Kontrollgruppe. Der größte Kostenunterschied zeigte sich in den Kosten für die ART (monatlich € 1089 vs. 77). Die Kosten für eine antiretrovirale Therapie verursachten 42 % der Gesamtkosten in der Gruppe der late presenter und 10 % der Gesamtkosten in der Kontrollgruppe. In der Gruppe der late presenter zeigten sich auch höhere Kosten für stationäre Aufnahmen, diagnostische Maßnahmen und Komedikationen.
Eine späte HIV-Diagnose stellt damit eine große finanzielle Belastung für das österreichische Gesundheitssystem dar. Patienten und die Gesellschaft würden daher von effizienten Screeningprogrammen profitieren, die eine frühzeitige Diagnosestellung und Einbindung in die medizinische Versorgung ermöglichen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the ever growing knowledge and awareness of the HIV epidemic, about 25 % of HIV-infected patients in Austria—as well as in other countries with reasonably good access to healthcare—are diagnosed in a late stage of the disease, i.e., either characterized by advanced immunosuppression or by clinical symptoms of AIDS [1–3]. Today, late presentation is defined as being diagnosed or presenting for care with < 350 CD4 cells/mm3, while presentation with advanced HIV disease is defined as presenting with < 200 CD4 cells/mm3 or an AIDS-defining event [4]. Several risk factors for late presentation have been identified and vary from denial of being at risk to cultural, social, and economic factors [5]. Contrary to current guidelines, which recommend initiation of highly active antiretroviral therapy (HAART) prior to severe immune depletion, these patients only begin antiretroviral therapy at low CD4 cell counts [6]. Patients, who begin treatment under such conditions have a poorer prognosis, benefit less from HAART, and tend to experience more adverse effects [7–10]. Furthermore, not only may serious medical problems arise from late presentation, but also earlier studies suggest that, because of their greater need for medical care, late presenters may also possibly impose a higher cost burden on the public healthcare system [11–13]. However, since late presenters differ in several aspects from non-late presenters (risk group, age, social background), these characteristics may also account for their different medical care needs and may overweigh the influence of CD4 cell levels. In addition, recent progress in HAART, testing and an increase in healthcare costs may have changed this picture, underscoring the need for further data on the economic burden of late presentation.
This study compares the marginal costs to the public healthcare system incurred during the initial period of care after diagnosis in two otherwise well-matched groups of patients, who are either presenting with advanced HIV disease (CD4 < 200 cells/µl and or AIDS) or are non-late (CD4 ≥ 350 cells/µl) in their disease, as respectively defined in the late-HAART era (2006–2009).
Patients and methods
Ethics statement
The study protocol was approved by the local ethics committee (Ethics committee of the Medical University of Vienna). Data were extracted from the institutions’ electronic patient management system. All patients provide their written consent before being included in this electronic database. Since the work presented here includes only retrospective analysis of patients’ charts the local ethics committee approved the study without the necessity of an additional informed consent.
Study population
All patients included in this study were cared for at the HIV unit of the Medical University of Vienna, which is part of the Austrian HIV cohort and cares for approximately 1,200 patients. The Austrian HIV cohort comprises approximately 4,000 HIV infected patients, i.e., 80 % of the HIV infected patients in Austria in regular follow-up. The HIV cohort cared for at Vienna is representative of the whole Austrian HIV cohort regarding patients’ characteristics (age, sex, and risk groups). Enrolled in the study were treatment-naïve patients, who were diagnosed HIV-positive after 1st July 2006 and presenting not later than 6 months after first diagnosis, with a continuous longitudinal follow-up of at least 4 months and at least two consecutive outpatient consultations or one inpatient stay. Patients were followed until 28th February 2009 (= censor date). Patients not seen within 6 months from the censor date were censored as alive at the date of the last visit. Excluded from the analysis were patients who were transferred from other HIV centers and who had relevant psychiatric comorbidities impacting HIV care. The study population was divided according to CD4 cell count and CDC stage. Patients presenting with a CD4 cell count < 200 CD4 cells/µl and/or AIDS were considered late presenters. Controls were defined as having ≥ 350 CD4 cells/µl at initial presentation. A cut-off of 200 CD4 cells was selected, because at the beginning of the study, this level was the cut-off point for late presentation (now classified as advanced AIDS disease). Another reason for selecting this cut-off point is the finding that patients presenting below this level have poorer prognosis, regardless of their clinical status at initial diagnosis. Presenting with ≥ 350 CD4 cells was considered to be a “timely” presentation, since current guidelines state that HAART should be started before the patient reaches this level. Since several predisposing factors for timely or late presentation (e.g., risk group) that could influence the outcome of this study have been identified, the control group and late presenters were matched with regard to age and risk group one by one. For this purpose, controls within the age range of cases were randomly selected 1:2 (intravenous drug use (IVDU) and heterosexual) or 1:3 (men who have sex with men (MSM)) from all non-late presenters during the study period. Due to this selection process, the selected controls are slightly older than the overall group of non-late presenters (38 vs. 35 years) and enriched in heterosexual infected patients (48 vs. 38 %) and women (41 vs. 33 %). The process for selecting the controls is shown in Fig. 1.
Data collection and cost calculation
Patients’ data were retrospectively collected from paper and electronic patient files from the HIV unit. To calculate the costs, the data were divided into five categories: medicines prescribed, in patient hospital care, outpatient consultations, laboratory, and imaging diagnostics. Costs for medicines were further subdivided into “costs for antiretroviral (ART)” and costs for “non-antiretroviral therapy (non-ART).” The cost calculation model uses a marginal cost approach, i.e., extra costs imposed on the Austrian healthcare system were taken into consideration. A marginal cost concept was chosen as the Austrian reimbursement system for hospitals is a lump-sum system and therefore could not been taken as an estimator of real expenses. Costs for medicines are based on published wholesale price lists, while costs of out-patient consultations and diagnostic interventions are derived from a list of costs of medical interventions published by the county of Vienna [16, 17]. Commercial laboratories provided the prices for laboratory diagnostics, and the costs for in patient treatments (costs of medical treatment and overnight stay in hospitals) are based on the Austrian public reimbursement system for hospitals [18, 19].
Statistics
Continuous variables were expressed as the mean ± 1 SD or as the median with the interquartile range (IQR), as appropriate, and were compared using unpaired t-tests for normally distributed data or Mann–Whitney rank sum tests, if the distribution was non-normal. Discrete variables were described as number of patients (percentage), and the χ2 or Fisher exact test was employed, as appropriate. Statistical significance was set at p < 0.05.
Results
Population profile and clinical outcome
A total of 24 cases and 27 controls were followed over an average of 15 months (15.5 ± 7.2 and 14.3 ± 9.0 months, respectively, p = 0.63). Cases and controls were well matched regarding age, gender, risk group, and migrational background (Table 1). Furthermore, regarding the distribution of age, gender, and risk group our cohort was comparable to the distribution of these data in the entire Austrian HIV cohort (patients currently in care: median age 44.5 years; 28 % female; 37 % MSM, 41 % heterosexual, 16 % IVDU) [1]. As expected, the cases had significantly higher viral load (5.11 ± 0.7 and 4.43 ± 1.04 log copies/ml, respectively; p < 0.01) and lower CD4 cell counts than controls (93 ± 66 versus 558 ± 187 cells/µl; p < 0.0001). There was a trend toward more symptomatic HIV disease in the cases. At first diagnosis, 11 (45.8 %) cases and 23 (85.2 %) control patients were in CDC stage A (p < 0.1). During the observation period, two patients from the group of cases progressed from stage A to B and one from stage A to C, while only one control patient showed disease progression from stage A to B. At end of the follow-up, the difference in CDC stages between the cases and controls reached borderline significance (p = 0.05). During the first 6 months after diagnosis, one stage C event (HIV encephalopathy) occurred in the group of cases and none in the control group. More than 6 months after first diagnosis, 2 CDC stage B events were observed in each group. There was no difference in the number of deaths in both groups. However, the death in the group of cases was related to a CDC stage C event, while the control patient died from (preexisting) severe heart disease.
Usage of the healthcare system
Usage of the healthcare system was evaluated on the basis of the mean number of visits or percentage of patients requiring hospitalization. Late presenters had 70 % more consultations than patients diagnosed with higher CD4 cell counts (p < 0.01). A similar trend was seen in the frequency of hospitalization: 42 % of late presenters required in-patient service, compared to only 15 % of the control patients. Furthermore, the majority of admissions of late presenters was due to AIDS/HIV-related diseases, while only half of the admissions in the control group occurred because of HIV-associated pathologies (Table 2).
Costs
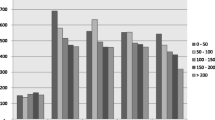
Total marginal costs during the observation period were three-fold higher for the cases than for the controls (€ 722,761 for cases vs. 244,976 for controls, median costs see Fig. 2). Cost per patient and month for cases ranged from € 600 to 17,108 with an average of € 1,948, cost per patient per month in the control group ranged from € 102 to 26,958 with an average of € 1,426. This difference was driven by higher costs in all the categories evaluated (Table 3). The greatest difference was seen in the costs for ART medication due to the immediate need for antiretroviral therapy in the group of cases. Cost for ART medication for the cases were on average € 1,089 per month, while the respective costs for the controls were € 77. In contrast only 41 % of the controls started antiretroviral therapy during the study period. For the cases, ART accounted for 42 % of the overall costs, whereas only 10 % of the total costs resulted from antiretroviral therapy in the control group. In addition, a higher percentage of patients with late HIV diagnosis had to be hospitalized, thereby resulting in eight-fold higher total costs for in-patient care (€ 135,523 vs. 17,644). In line with the higher number of out-patient consultations and hospital admissions, also the per patient costs for laboratory and imaging diagnostics were higher for the cases. Although the costs of non-ART medication accounted for more than half of the total costs incurred by the control patients, these costs were still lower than those incurred by the cases. Higher costs for non-ART medication in the cases were not only due to the need for prophylaxis or treatment of opportunistic infections, but were also because of treatment of comorbidities not directly related to HIV.
Discussion
In line with previous reports, we also show in this study that late presentation (< 200 CD4 cells/mm3 and/or AIDS at presentation) is associated with significantly higher costs for medical care in the initial period after HIV diagnosis. It has been established, however, that late presenters differ from non-late presenters in age, sex, risk activity, and ethnicity [6]. In contrast to these earlier studies, which simply stratified the patients by CD4 cell count at presentation, we have tried to control for these confounding factors by choosing a control group that is well-matched for the known risk factors, age, sex, risk group, as well as migrational background, except for the CD4 cell count at presentation. Using this case/control approach, we have now confirmed the hypothesis that late presentation is an independent and strong predictor of costs incurred by an HIV-infected patient, at least in the first months after diagnosis.
As there was an immediate need to start antiretroviral therapy, costs for ART were the main source driving this difference. However, we did indeed find statistically significant differences also in all the other categories analyzed. Thus, the excess costs of late presenters are also due to an increased need for consultations, hospitalization, and diagnostic procedures. In the case of non-late presenters, healthcare costs were more often associated with non-HIV-related causes, as could be observed when assessing the reasons for hospitalization. Furthermore, in non-late presenters, non-HAART medicines accounted for more than half of the overall costs. Nevertheless, the total non-ART costs were still significantly higher for late presenters—a finding which may reflect the greater overall disease burden of these patients.
According to the recently revised European definition, our patients would now be referred to as “individuals with advanced HIV/AIDS disease” [4]. It is unclear whether by using the new definition for late presentation, i.e., < 350 CD4 cells/mm3, we would also have found a significant cost difference in favor of non-late presentation or if an advanced immunosuppression is essential to produce excess costs. Previous studies, however, have shown that a gradual cost decrease with rising CD4 cell counts can be observed over several years [14].
It should be pointed out that our study has several limitations: the economic computation is limited by the fact that multiple sources had to be used to compute the costs, since the Austrian healthcare system does not provide costs for single treatments; the drug prices employed here are wholesale prices instead of retail prices; the reason of taking wholesale prices is that in the hospital sector usually wholesale prices are paid whereas in the primary sector higher prices paid by social insurances retail prices are refunded; as the study was conducted in the Austrian hospital sectors whole sale prices are appropriate. Information regarding the cost of laboratory diagnostics is derived from commercial sources; figures regarding out-patient consultations and diagnostic interventions are sourced from a municipal list for self-insured patients. Furthermore, we were unable to capture the social costs as well as any impact of late presentation on patients’ quality of life. It is also assumed that about half of HIV infections are transmitted by patients unaware of their status [20]. Thus, late diagnosis also allows for a longer transmission time and increases the disease burden on society. It is fair to say that the analysis represents an underestimation of the costs. Compounding this is our small sample size and the limited follow-up. Nevertheless, our results do confirm earlier data, and it has also been shown recently that higher costs incurred through late presentation persist over 15 years of follow-up [15]. Still, in order to confirm the long-term impact of late presentation on the public healthcare system, reevaluation at later time points would be absolutely essential.
The necessity to use multiple cost sources highlights the weakness of healthcare systems with multiple funding sources. The fragmented budgeting landscape of healthcare makes it very difficult to model the economic impact of therapeutic and diagnostic procedures. That said, as our model was based on real clinical data, we feel confident that the observed impact of late presentation represents a real finding.
Conclusion
In conclusion, late presentation in HIV disease is associated with a significant economic burden for the Austrian healthcare system and, thus, earlier diagnosis and linkage to care would not only improve survival, but would also reduce costs, at least during the initial period of care. Reducing late diagnosis of HIV infection through effective screening programs represents a significant benefit to society and should be a major objective of public healthcare systems.
Acknowledgements
The authors wish to thank Dr. Rita A. Klim, Munich, Germany for her medical writing and editorial support.
References
Zangerle, et al. 22nd report of the Austrian HIV cohort study. 2012.
Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the Unites States and Canada. Clin Infect Dis. 2010;50(11):1512–20.
Centers for Disease Control and Prevention. Late HIV testing—34 states, 1996–2005. Morb Mortal Wkly Rep. 2009;58:661–5.
Antinori A, Coenen T, Costagiola D, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12(1):61–4.
EACS Guidelines Version 6.0. October 2011.
Ndiaye B, Salleron J, Vincent A, et al. Factors associated with presentation to care with advanced HIV disease in Brussels and Northern France :1997–2007. BMC Infect Dis. 2011;11:11.
Fisher M. Late diagnosis of HIV infection: major consequences and missed opportunities. Curr Opin Infect Dis. 2008;21:1–3.
Egger M, May M, Chene G, et al. Prognosis of HIV infected patients starting antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29.
Smit C, Hallett TB, Lange J, et al. Late entry to HIV care limits the impact of antiretroviral therapy in the Netherlands. PLoS One. 2008;3(4):e1949.
Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trial Group protocol 384. Clin Infect Dis. 2009;48(3):350–61.
Fleishman JA, Yehia BR, Moore RD, et al. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48:1071–9.
Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Med. 2004;5:93–8.
Schackmann BR, Goldie SJ, Weinstein MC, et al. Cost-effectiveness of earlier initiation of antiretroviral therapy of uninsured HIV infected adults. Am J Public Health. 2001;91:1456–63.
Krentz HB, Gill MJ. Cost of medical care for HIV infected patients within regional population from 1997 to 2006. HIV Med. 2008;9(9):721–30.
Krentz HB, Gill MJ. The direct medical costs of late presentation (<350/mm) of HIV infection over a 15 year period. AIDS Res Treat. 2012;2012:757135.
Warenverzeichnis I Arzneispezialitäten und Mittel gemäß Gesamtvertrag Anlagen 2 und 3; Herausgeber Österr. Apotheker-verlagsgesellschaft m.b.H., Jän 2010.
Landesgesetzblatt für W, Jahrgang 2009, 15. Stück, 15. Verordnung: Festsetzung der Ambulatoriumsbeiträge für die Wiener städtischen Krankenanstalten.
Labor Dr. Haas, Luegerplatz 2, 1010 Wien, Stand 2009.
Programmpakete KDok für landesgesundheitsfondsfinanzierte Krankenanstalten, Federal Ministry of Health, Austria.
Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–50.
Conflicts of interest
This work was supported by a grant provided from Gilead Sciences and MS was a paid employee of Gilead. MS was involved in study design, data analysis and writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grabmeier-Pfistershammer, K., Rieger, A., Schröck, T. et al. Economic burden of late presentation in HIV disease in Austria: a comparison of the initial costs imposed by advanced HIV disease vs. non-late presentation. Wien Klin Wochenschr 125, 402–407 (2013). https://doi.org/10.1007/s00508-013-0392-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-013-0392-5