Abstract
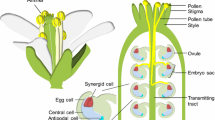
In higher plants, the double-fertilization process begins with the successful delivery of two sperm cells to the female gametophyte. The sperms cells are carried by a pollen tube that upon arrival at the micropylar end of the female gametophyte, bursts, and discharges its content into one of two specialized cells called the synergid cells. At their micropylar ends, both synergid cells form a thickened cell wall with a unique structure called the filiform apparatus. The filiform apparatus is believed to play a major role in pollen tube guidance and reception. It has also been assumed that the pollen tube enters the receptive synergid cell through the filiform apparatus. Here, we show that in Arabidopsis ovules, the arriving pollen tube appears to grow beyond the filiform apparatus to enter the synergid cell at a more distant site, where the tube bursts to release its contents. Thus, fertilization in Arabidopsis might involve two spatially and temporally separable stages, recognition and entry, with the latter apparently not requiring the filiform apparatus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the flowering plants, the female gametophyte consists of four cell types: two gametes—the egg cell and the central cell, three antipodal cells at its chalazal end and two synergid cells at the micropylar end. The growing pollen tube is guided to the female gametophyte where it interacts with one of the two synergid cells, eventually leading to pollen tube burst and discharge of its content into the receiving SC. The two released sperm cells then further migrate to fertilize the egg cell, and the central cell giving rise, respectively, to the embryo and its nursing tissue the endosperm (Berger et al. 2008).
The synergid cells have been shown to play a direct role in pollen tube attraction and guidance in Arabidopsis, Torenia, and Maize ovules (reviewed in Higashiyama 2002; Punwani and Drews 2008). At their micropylar ends, the two synergid cells together form a thickened cell wall with unique structure called the filiform apparatus. It is believed that the synergid cells are involved in pollen tube guidance, and its role in pollen tube reception has been highlighted by recent research showing that proteins required for sperm cell release are localized to the filiform apparatus (reviewed in Kessler and Grossniklaus 2011). Since the filiform apparatus is the thickest point on the synergid cell surface, the arriving pollen tube might be expected to penetrate into the receptive synergid cell through a point where the physical barrier of the cell wall is much thinner and not through the filiform apparatus. Yet, it has long been assumed that the filiform apparatus is the site of entry, for example, “the pollen tube enters the female gametophyte by growing through the filiform apparatus of the synergid cells” (Drews and Koltunow 2011; see also Kessler and Grossniklaus 2011) and that “After arrival at the micropyle, the pollen tube directly enters one of the two synergid cells via the filiform apparatus in plant species such as Arabidopsis or T. fournieri” (Dresselhaus and Ma′rton 2009).
Nevertheless, the direct evidence for this assumption is not definitive. One study cited in support of this conclusion is by Kasahara et al. (2005), who mention in their discussion that “the pollen tube enters the female gametophyte by growing through the filiform apparatus and into one of the synergid cells,” but they themselves cite the study by Weterings and Russell (2004). However, Weterings and Russell concluded only that “The tube enters near the filiform apparatus, elongates into the cytoplasm of the synergid, and discharges, usually forming an aperture at the tip,” citing an earlier review by (Russell 1992). The Russell 1992 review is in fact cautious on this point, stating that “Once in contact with the receptive synergid, the exact site of pollen tube penetration is usually described as being the filiform apparatus, which covers much of the outer wall of the synergid attached to the wall of the embryo sac, but the exact location of pollen tube penetration is difficult to observe. In many cases, the pollen tube appears to penetrate through the filiform apparatus; however, aligned fibrillar inclusions are frequently observed in the filiform apparatus, suggesting that rather than growing directly into the center of this structure, the filiform apparatus may instead redirect or deflect pollen tubes to the site of discharge.”
Thus, the literature briefly reviewed here only suggested that the filiform apparatus might be the entry site of the pollen tube into the synergid cell; however, to the best of our knowledge, there is no direct evidence for this conclusion, at least for Arabidopsis ovules. In this report, we show that in Arabidopsis ovules, the arriving pollen tube appears to avoid entering the receptive synergid cell through the filiform apparatus by growing beyond it to a certain zone of the SC, where the tube bursts to release its contents.
Methods
Plant materials
The C-terminal GFP translational fusion modified Gateway vector described recently by Leshem el al (2012) was used to clone a cDNA of At1g65450.3 (called here 450.3::GFP) and was found to be specifically expressed in the synergid cells of WT (Col) transgenic plants (n = 14/14, Fig. 1a). The central cell-specific GFP marker line DD65 was a gift from Gary Drews (University of Utah; Steffen et al. 2007). The LAT52::GUS pollen-specific marker was a gift from Sheila McCormick (UC- Berkeley; Twell et al. 1990). The LAT52::DsRed pollen-specific marker was a gift from Ravi Palanivelu (University of Arizona; Sandaklie-Nikolova et al. 2007). Microscopy procedures used for GFP/RFP visualization, and GUS staining was performed as described by Leshem et al. (2012). Emasculated WT (Ler) and 450.3::GFP flowers were pollinated with LAT52::GUS and LAT52::DsRed pollen, respectively. At indicated hours, after pollination (HAP) pistils were sampled; ovules were dissected and in the LAT52::GUS pollinated pistils—cleared in Hoyer’s solution, in a way that the filiform apparatus was visible by DIC microscopy.
The three sub-zones of the synergid cell. a Overlay of bright field image and green fluorescent image of unfertilized ovule expressing At1g65450.3 GFP C-terminal translational fusion in the SC. Scale bar = 30 μm. Inset is enlargement of the presented SC. The three sub-zones of the SC are indicated by white braces. b Green fluorescent image of unfertilized ovule from the CC-specific GFP marker line DD65. Scale bar = 30 μm. The three sub-zones of the present but unseen SC are indicated by white brackets
Results and discussion
Three sub-zones of the synergid cell
Based on our knowledge of the synergid cell macro-structure in Arabidopsis (Russell 1993; Punwani and Drews 2008), it can be further divided into three sub-zones: the synergid cell chalazal end, which is engulfed by the central cell cytoplasmic protrusions also called the synergids hooks or central cell apical pockets. This part of the synergid cell makes up most of its volume and is less available for physical contact with the arriving pollen tube, and here, we refer to it as zone I. The second part of the synergid cell corresponds to the narrow section that forms a “neck”-like shape. This region parallels the synergids hooks pockets that form a partial or complete cytoplasmic ring around the two synergid cells, and here is called zone II. The third part of the synergid cell is its micropylar end that forms a “head”-like shape, and is here referred to as zone III. This part of the synergid cell is external to the central cell pockets and is most accessible to the arriving pollen tube. The filiform apparatus is localized to the micropylar end of this zone. (Fig. 1a, b).
The route taken by the pollen tube after reaching the filiform apparatus.
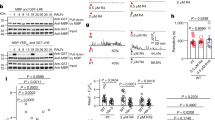
Tracking the fine details of the LAT52::GUS pollen tube route after its arrival to the synergid cell micropylar end revealed that the pollen tube did not invade the region of the filiform apparatus overlapping the junction between both synergid cells, but continued to grow along the external side of zone III of one of the synergid cell, past the filiform apparatus (Fig. 2a, b, c, and d). After covering a short distance from the filiform apparatus, the pollen tube has reached to a certain point where its growth ceased, rupture occurred, and its cytoplasmic contents were discharged into the receiving synergid cell (Fig. 2a, b, c and d). These details were observed in 13.2 % (n = 46/348) of the ovules reached by pollen tubes at 8–14 HAP. In the other 86.8 % cases (n = 302/348), the fine details at the point of reception were not discernible due to strong GUS staining (see representative image in Leshem et al. 2012, Supplemental Figure 1B) and thus should be considered as failed observations. Among the ovules where the details at the point of reception were clear, not a single case of pollen tube invasion into the filiform apparatus was observed. Thus, in case a certain percentage of the pollen tubes still enter into the synergid cell through the filiform apparatus as is the current prevailing view, we would have expected to see at least a fraction of these instances in the remaining 13.2 % of the ovules where the GUS did not obscure the fine details of the synergid cell micropylar end. From a statistical point of view, the probability of missing these instances in case the filiform apparatus is the major entry site, is very low (P < 0.008 for the hypothesis that pollen tube entry through the filiform apparatus is at least as likely entry at another site, calculated for this sample size). Following the latter statistical hypothesis leads us to conclude that an alternative site is more likely to be the site where the pollen tube enters into the synergid cell than the filiform apparatus. Nevertheless, due to a potential bias in the GUS staining procedure in the remaining 86.8 % of the examined ovules, we cannot rule out the possibility that details at the reception site were obscured in that group, including entry of the pollen tube at the filiform apparatus.
The route taken by the pollen tube after reaching the filiform apparatus. Emasculated WT flowers (Ler) were pollinated with LAT52::GUS pollen. At the indicated times, pistils were sampled and ovules were dissected out. Images a–c were captured during 5–6 HAP for which pollen tube arrival had occurred for only 52 % of the ovules (n = 57/110). Image d was captured during 8–14 HAP where pollen tubes had reached over 90 % of the ovules (n = 348/382). Black arrows point to the filiform apparatus (FA), red arrow in d indicates point of pollen tube rupture (R).n = 15 for (a–c) and 46 for (d). Scale bar = 10 μm
In most of the fixed ovules where the GUS stain did not obscure the fine details of the synergid cell micropylar end, the cell’s shape was still altered in a way that made it difficult to determine the exact entry site of the pollen tube into this cell. However, in 20 % of these ovules (n = 9/46), the “head” and “neck” shapes of zone III and II, respectively, could be clearly identified and so was the probable region of zone I (Fig. 3a, b). In these ovules, the GUS signal, which represents the pollen tube cytoplasm, was unevenly distributed throughout the receptive synergid cell. A strong GUS signal was observed approximately at the transition between zone III and zone II of the receptive synergid cell (Fig. 3a). The strong GUS signal is associated with the point of the pollen tube’s tip where rupture occurs (Fig. 3b and Supplemental Figure 1) that was observed in 65.2 % of the ovules (n = 30/46). Since the strong GUS signal indicates a high density of cytoplasm at the entry site that would be later diluted with the cytoplasm of the receptive synergid cell, we suspect that this region might be harboring the alternative entry site of the pollen tube into the receptive synergid cell.
Distribution of the LAT52::GUS signal in the synergid cell after pollen tube cytoplasm has been discharged (a) and near the point of pollen tube rupture (b) Ovules were processed as in Fig. 2. In each column, the bottom image is a duplication of the top image, but indicates in dotted lines the probable borders of the synergid cell, and in black square braces, the three sub-zones demonstrated also in Fig. 1. Red arrows point to the strong GUS signal thought to be the general region where pollen tube rupture has occurred (R). Note that in column A zone III and II are in focus. Zone I which was not in the same plain can still be recognized. In column B, black arrow in top image points to the synergid hooks pockets (SH) and black arrow in bottom image point to the filiform apparatus (FA). Scale bar in top images = 10 μm. The synergid cell’s shape was preserved as in the presented images in 9/46 of the fixed ovules
Live imaging of pollen tube entry at the micropylar end of the ovule.
Since the GUS observations were carried out in fixed ovules, we wanted to find out whether these pollen tube growth patterns could be also seen in live ovules. Therefore, we emasculated flowers of 450.3::GFP plants, in which the GFP is specifically expressed in the synergid cells and highly concentrated in the filiform apparatus pole (see Fig. 1a). The flowers were then pollinated with pollen of the LAT52::DsRed pollen tube-specific marker. The ovules were dissected out of the pistils 8 h later and observed under an epifluorescence microscope. Out of the total 214 examined ovules only 69 (32 %) exhibited relatively clear red and green signals, in a way that both the pollen tube and synergid cell structures could be determined simultaneously. The high frequency of unsuccessful observations was caused by substantial auto-fluorescence background noise in the red fluorescence channel, and the cell modifications that occur naturally in the shape of the synergid cell during fertilization, which affected the signal coming from the green fluorescence channel. In 78 % of the successful observations (n = 55/69), the pollen tube had already ruptured. That was indicated by the strong intensity of the red fluorescence signal high above the auto-fluorescence background in the receptive synergid cell (Fig. 4c). In the remaining 22 % cases (n = 14/69), based on the low RFP signal intensity, there was no positive evidence that pollen tube rupture has occurred (Fig. 4a, b). In both cases, the ruptured and the arriving pollen tubes had bypassed the filiform apparatus and had not grown through it. Moreover, in all of the cases, the pollen tube had reached to the approximate region of zone II (Fig. 4b, c, and Supplemental Figure 3), which was the last point where the pollen tube was visible prior to tube rupture (Fig. 4c and Supplemental Figure 3). These observations suggest that pollen tube rupture has occurred at least at this point or beyond it. In all of the cases, the pollen tube was not seen to grow directly through the filiform apparatus, which remained always free from RFP signal, indicating that pollen tube rupture has not occurred at that point. These suggestions derived from the live imaging study are consistent with the GUS observations performed on fixed ovules (compare Fig. 2d and supplemental Figure 3).
Dynamics of pollen tube arrival at the micropylar end of the receptive synergid cell. Emasculated 450.3::GFP flowers were pollinated with LAT52::DsRed pollen and, ovules were dissected out of the pistils 8 h later and observed under an epifluorescence microscope. Shown are representative fluorescent images of LAT52::DsRed pollen tubes; arriving at the filiform apparatus pole—row (a), avoiding the FA—row (b) and after the occurrence of pollen tube rupture—row (c). In each row provided are the separated red and green fluorescent images, the merged image of both and their bright field image. The three sub-zones of the synergid cell are indicated with white braces in the green filter image of row C. White arrows throughout the figure indicate the last point where the pollen tube is clearly visible. Note that in C, the pollen tube has reached zone II, and the subsequent rupture has occurred at least at this point or beyond. Scale bar = 10 μm, n = 14 for a, and b and 55 for c
Previously, Sandaklie-Nikolova et al. (2007) studied the dynamics of pollen tube arrival and synergid cell degeneration using an in vitro assay. In their study, they noted that after arrival at the synergid cell, the pollen tube continues to grow around or along the synergid cell. Although the specific site of entry into the synergid cell was not identified in that study, the images provided therein are consistent with the above conclusions.
Interestingly, we observed a few cases where it appeared that the pollen tube had grown further toward the chalazal end of the synergid cell (Fig. 3b, supplemental Figure 2 and Figure 4B). Since this pattern was observed in only a few ovules (n = 3 for GUS s and 2 for RFP), we are careful not to make any conclusions. However, these observations indicate that some of the pollen tubes may be able to reach to zone I. This observation is in line with the images presented by Sandaklie-Nikolova et al. (2007, Fig. 4) which show that the LAT52::DsRed pollen tube can grow deeper into the approximate region of zone I (though the SC sub-zones could not be determined in that study).
Possible sources for pollen tube guidance from the filiform apparatus to the site of entry into the synergid cell
The signal(s) that guide(s) the growth of the pollen tube during the short distance from the filiform apparatus to the alternative site of entry is (are) unknown. We can speculate that there might be chemical attractants secreted by the “synergids hooks” pockets, where the central cell cytoplasm is enclosing a region of the synergid cell, as it is known that the central cell plays a role in pollen tube guidance (Chen et al. 2007; Takeuchi and Higashiyama 2012). The synergids hooks pockets might also play a passive role by forming an incomplete/complete ring around the synergid cell that could physically block further growth of the tube and in concert with other factors force it to burst at that point. As mentioned above, at zone II, the synergid cell sides are collapsed/bent inside, a feature which may indicate a region of weakened cell wall that allows the synergids hooks pockets to press the synergid cell inwards. In that case, the thickened cell wall barrier formed by the filiform apparatus is significantly reduced here, allowing better access of the pollen tube to the synergid cell plasma membrane.
In summary, the interaction of the pollen tube with the synergid cell in Arabidopsis ovules appears to involve two spatially and temporally separable stages. The first is the interaction with the micropylar end where the FA is located; this stage likely primes the pollen tube for gamete release through the action of the FER, LRE, and NTA gene products (reviewed in Kessler and Grossniklaus 2011). The second stage is the additional growth of the pollen tube toward the synergids hooks, reaching the site where it will enter the synergid cell and release the sperm cells. Detailed electron microscopy studies should yield a better understanding and full characterization of the exact location and features of this entry site. Further studies are also needed to understand why successful fertilization requires these two stages to be spatially distinct, as well as elucidating the mechanisms governing the second stage.
References
Berger F, Hamamura Y, Ingouff M, Higashiyama T (2008) Double fertilization—caught in the act. Trends in Plant Sci 13:437–443
Chen YH, Li HJ, Shi DQ, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang WC (2007) The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19:3563–3577
Dresselhaus T, Ma′rton ML (2009) Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr Opin Plant Bio 12:773–780
Drews GN, Koltunow AMG (2011) The female gametophyte. The Arabidopsis Book e0155. doi:10.1199/tab.0155
Higashiyama T (2002) The synergid cell: attractor and acceptor of the pollen tube for double fertilization. J Plant Res 115:149–160
Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17:2981–2992
Kessler SA, Grossniklaus U (2011) She’s the boss: signaling in pollen tube reception. Curr Opin Plant Biol 14:622–627
Leshem Y, Johnson C, Wuest SE, Song W, Ngo QA, Grossniklaus U, Sundaresan V (2012) Molecular characterization of the glauce mutant: a central cell-specific function is required for double fertilization in Arabidopsis. Plant Cell 24:3264–3277
Punwani JA, Drews G (2008) Development and function of the synergid cell. Sex Plant Reprod 21:7–15
Russell SD (1992) Double fertilization. Int Rev Cytol 140:357–388
Russell S (1993) The egg cell: development and role in fertilization and early embryogenesis. Plant Cell 5:1349–1359
Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN (2007) Synergid cell death in arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol 144:1753–1762
Steffen JG, Kang IH, Macfarlane J, Drews GN (2007) Identification of genes expressed in the Arabidopsis female gametophyte. Plant J 51:281–292
Takeuchi H, Higashiyama T (2012) A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol 10(12):e1001449. doi:10.1371/journal.pbio.1001449
Twell D, Yamaguchi J, McCormick S (1990) Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109:705–713
Weterings K, Russell SD (2004) Experimental analysis of the fertilization process. Plant Cell 16(Suppl):S107–S118
Acknowledgments
The authors are grateful to Aneesh Panoli for his useful comments and helpful discussions and to Ravi Palanivelu for the LAT52::DsRed line. This research was supported by a National Science Foundation grant IOS-1051951 to V.S. Y.L. was partially supported by a Vaadia-BARD Postdoctoral Fellowship Award FI-392-07 from BARD, the U.S.–Israel Binational Agricultural Research and Development Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dolf Weijers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leshem, Y., Johnson, C. & Sundaresan, V. Pollen tube entry into the synergid cell of Arabidopsis is observed at a site distinct from the filiform apparatus. Plant Reprod 26, 93–99 (2013). https://doi.org/10.1007/s00497-013-0211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-013-0211-1