Abstract
Cynara cardunculus is a native plant with flowers that are used traditionally in the manufacture of ewe’s cheese in the Iberian Peninsula. Milk clotting ability of the plant is attributed to the high concentrations of aspartic proteinases (APs), named cardosins, found in the flowers. Although these enzymes are well characterised on a molecular and biochemical basis, the biological role of the majority of plant APs is yet unassigned. We suspected APs play an important role in ovule function, and we characterised the maturation of the ovules of C. cardunculus and its Polygonum-type embryo sacs. The internal layer of the integument differentiates into an endothelium as described for other Asteraceae, with differentiation of two nucellar layers, a podium and a hypostase coinciding with the onset of pollen receptivity. In flowering plants, programmed cell death (PCD) events are essential for the success of nucellar maturation and consequent differentiation of a fully functional embryo sac. In C. cardunculus, nucellar PCD is integral to the maturation of the embryo sac, which in turn is closely correlated with the accumulation of the AP cardosin B specifically in the hypostase. The onset of cardosin B expression temporally coincides with the degeneration of nucellar cells. In fully mature embryo sacs, cardosin B is localised in both the hypostase and epistase, two regions that differentiate through PCD. Thus, cardosin B localisations closely correlate with events of PCD in the nucellus of C. cardunculus suggesting involvement in ovule and embryo sac development and further suggest the biological significance of APs like cardosin B, in this particular process. This work contributes new data to the plant AP research field and indicates an involvement of cardosin B in the PCD-dependent degeneration of the nucellus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The embryo sac differentiates from the megasporocyte cell in the nucellus. In Oenothera biennis and Spinacia olearacea, several zones in the nucellus can be distinguished during differentiation, when cells undergo different patterns of cell degeneration and exert distinct functions (Bouman 1984). In some species, the nucellar and chalazal tissue facing the antipodal end of the embryo sac differentiate into a hypostase. Additionally, the remnants of the nucellar base may form a podium which resembles a pedestal for the embryo sac. At the micropylar end of the female gametophyte, nucellar remains may also persist, forming an epistase (Bouman 1984). Also, the inner layer of the inner integument surrounding the embryo sac may differentiate to an endothelium, which in Asteraceae becomes bi- or multilayered with radially stretched cells (Bouman 1984).
The general cellular anatomy of the Polygonum-type mature embryo sac comprises of an egg cell, two synergids, a central cell and the antipodals (Huang and Russell 1992). The egg cell is located at the proximal end of the embryo sac, adjacent to the two synergids. One of the latter two cells receives the contents of the pollen tube and plays an important role in the transport of the sperm nuclei into the egg and central cells. The central cell contains the polar nuclei, which may fuse before being fertilised (Huang and Russell 1992; Coimbra and Salema 1999). The antipodals are localised at the distal end of the embryo sac, and cell wall projections have been observed extending from these cells into the chalazal nucellus, suggesting the flow of metabolites between the nucellar region and the embryo sac (Raiser and Fischer 1993).
Differentiation of a fertile embryo sac largely depends on programmed cell death (PCD), which is critical in providing nucellar space needed for embryo sac expansion (Bouman 1984; Hiratsuka et al. 2002; Greenwood et al. 2005), a common phenomenon that is essential to sexual plant reproduction (Wu and Cheung 2000). In barley ovules, some degeneration occurs prior to pollination, although nucellar cell degeneration is generally stimulated by pollination/fertilisation and begins with the inner nucellar cells, progressively moving towards the outermost cells (Chen and Foolad 1997). Nucellar PCD is characterised by cytoplasmic degeneration with accumulation of vesicles, emergence of multivesicular bodies and disintegrated nuclear fragments in the cell content (An and You 2004; Greenwood et al. 2005). In barley, nucellar cells undergoing cytoplasmic disorganisation and degeneration are spatially restricted such that the integrity of the surrounding nucellus is retained, displaying persistent cell walls and membranes (Chen and Foolad 1997). In wheat, nucellar cells that undergo PCD show a dense cytoplasm, increased vacuolisation and membrane fragmentation due to organelle lysis (Domínguez et al. 2001). These observations point towards nucellar functions both as a source and a passage way of nutrients to the developing endosperm (Bouman 1984; Chen and Foolad 1997; Linnestad et al. 1998; Greenwood et al. 2005).
Simultaneously with the onset of PCD processes, several proteolytic enzymes are expressed in the nucellus (Chen and Foolad 1997; Domínguez and Cejudo 1998; Linnestad et al. 1998; Xu and Chye 1999; Greenwood et al. 2005). In animals, apoptotic proteases have been suggested as common effectors for various incoming cell death signals (Wylley 1995). Plant aspartic proteolytic enzymes are known to be associated with developmentally regulated PCD phenomena such as programmed organ senescence, floral wilting (Panavas et al. 1999; Beers et al. 2000), tracheary element differentiation (Runeberg-Roos and Saarma 1998) and sexual reproduction (Ge et al. 2005; Wu and Cheung 2000). Aspartic proteinases (APs) are ubiquitous in nature and have been described in several plant species and organs (Mutlu and Gal 1999; Simões and Faro 2004). Although their functions are still not clearly defined, APs have been implicated in storage protein degradation and proprotein processing (Mutlu and Gal 1999). APs in the pistil may be involved in the restructuring of the extracellular matrix to provide a physically more permissive environment for pollen tubes to grow or to the mobilisation of resources necessary to nurture pollen tube elongation (Wu and Cheung 2000). Alternatively, pistil APs may activate other proteins that are more directly involved in pistil cell death processes. In the style, PCD could be interpreted as a mean to provide a biochemically more enriched and physically more accommodating medium for pollen tube penetration. It could also prevent the invasion of the ovary by pathogens (Wu and Cheung 2000).
Cardosins are APs found in Cynara cardunculus flowers at unusually high levels. These proteins are biochemically well characterised and their tissue-specific localisation in C. cardunculus pistils has been previously described (Veríssimo et al. 1996; Ramalho-Santos et al. 1997; Faro et al. 1999; Vieira et al. 2001). Among cardosins, cardosin B is an extracellular enzyme which is localised in the cell wall and extracellular matrix of the stigma and style transmitting tissue during floral development (Vieira et al. 2001). However, so far no definite biological role has been assigned to any of the cardosins and this is also true for most plant APs (Mutlu and Gal 1999; Simões and Faro 2004).
In this paper, we describe the structure of the embryo sac of C. cardunculus at selected developmental stages, from closed inflorescences to open capitula. A characterisation of nucellar development of C. cardunculus was completed and two distinct nucellar layers were identified: the hypostase and the podium. Differentiation of the internal layer of the integument into an endothelium was also observed, a feature previously described for other Asteraceae. Assays for PCD detection carried out with C. cardunculus ovules revealed that, in ovules with mature embryo sacs, the hypostase and the podium layers of the nucellus appear in distinct stages of cell degeneration. Furthermore, the distribution of cardosin B in the ovule of C. cardunculus was characterised throughout maturation of the embryo sac. It was observed that cardosin B occurs both in the hypostase and epistase of C. cardunculus nucellus after the embryo sac has fully matured. Interestingly, prior to the development of these nucellar layers, neither PCD events nor cardosin B presence are observed in C. cardunculus ovules.
Materials and methods
Plant material
Cynara cardunculus L. plants were harvested in the field at three different stages: at two successive stages prior to stigma emergence in closed inflorescences, after stigma emergence and concomitant anthesis and in open inflorescences. All experiments were repeated at least three times for each microscopic technique performed.
Electron microscopy
For transmission electron microscopy (TEM), ovaries were fixed for 1 h at room temperature in 2.5% (v/v) glutaraldehyde in 1.25% (w/v) piperazine-N,N′-bis [2-ethanesulfonic acid] (PIPES) buffer pH 7.2, and washed in three 10 min changes of 2.5% (w/v) PIPES buffer pH 7.2. Specimens were then post-fixed for 1 h at room temperature in 2% (w/v) osmium tetroxide in 1.25% (w/v) PIPES buffer pH 7.2 and dehydrated in a graded ethanol series, infiltrated using propylene oxide–resin mixtures and embedded in Epon 812 (TAAB, Berks, UK). Ultrathin sections were cut using an LKB Nova Ultratome (LKB Nova, Bromma, Sweden) with a diamond knife and mounted on coated 400 mesh copper grids. Sections were subsequently labelled with uranyl acetate and lead citrate for 5 min in each solution. Observations were carried out using a Zeiss EM 10C electron microscope (Carl Zeiss, Inc., Oberkochen, Germany) operating at 60 kV.
Light microscopy and immunolocalisation studies
For light microscopy and immunolocalisation studies ovaries were pre-fixed for 2 h at room temperature in 16% (v/v) paraformaldehyde and 2.5% (v/v) glutaraldehyde in PIPES buffer pH 7.2. The pieces were then dehydrated in an ethanol series, infiltrated in ethanol–resin mixtures and embedded in LR White (TAAB, Berks, UK). Semi-thin sections (≈1 μm thick) were cut using glass knives and stained with 1% (w/v) Azure II in distilled water: 1% (w/v) methylene blue in 1% (w/v) sodium borate (1:1; v/v). The sections were observed and photographed using a Leica DMLB microscope.
For immunolocalisation studies, semi-thin sections of ovules were collected on 0.1% poly-l-lysine-coated glass slides using droplets delimited with a hydrophobic pen. After a 10 min treatment with saturated sodium metaperiodate (Sigma) solution, the sections where washed briefly in double-distilled and deionised water followed by a 5 min incubation with filtered Tris–buffered saline (0.01 M Tris–HCl, pH 7.5) containing 500 mM NaCl (TBS 500). A 1 h incubation with blocking solution [10% (v/v) non-immune goat serum (Sigma) in TBS 500 plus 0.2% Tween-20 (TBS-T)] followed. The sections where then exposed to the primary antibody, anti-cardosin B (1:40) in blocking solution, for approximately 16 h, at 4°C. Cardosin B immunodetection was performed using a monospecific antibody against the synthetic peptide sequence CVIHPRYDSGD (Vieira et al. 2001). Following a thorough wash with TBS-T (3–5 times for 2 min, immunodetection was performed with Histostain-Plus (Zymed) according the manufacturer’s instructions with the following modifications: washing steps were carried out with TBS-T 500 and the substrate chromogen solution was replaced with SIGMA FAST BCIP/NBT alkaline phosphatase substrate diluted in 10% (w/v) polyvinyl-alcohol (PVA 40–70 kDa, Sigma). Sections were mounted using double-distilled deionised water. For immunofluorescence, sections were labelled 1 h at room temperature with 1:400 goat anti-rabbit IgG secondary antibody conjugated with AlexaFluor 488 probe (Molecular Probes, Oregon USA) in TBS-T. Sections were then rinsed in abundant TBS-T (3–5 times for 2 min), mounted in anti-fading mounting medium (Vectashield mounting medium, Vector Laboratories, Peterborough, UK), and observed using a confocal microscope (model PCS SP2AOBS, Leica).
In situ cell death detection
After treatment with sodium metaperiodate solution as described above, the sections were washed briefly in distilled and deionised sterilised water, followed by a 5 min incubation with filtered phosphate-buffered saline (PBS) buffer. Tissue permeabilisation was achieved by incubating the sections 8 min. in 0.1% Triton X-100 in freshly prepared 0.1% sodium citrate. After two PBS washes, the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) reaction (Roche Applied Science) was performed as indicated by the manufacturer. In the negative control, the terminal deoxynucleotidyl transferase was omitted. The positive control was incubation in DNAse I (Promega) solution for 10 min at room temperature before TUNEL labelling reaction. Slides were observed as described above.
Results
Ultrastructural characterisation of the mature C. cardunculus embryo sac
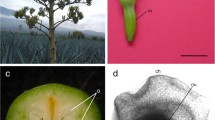
Cynara cardunculus florets possess a single ovulate ovary. The ovule is anatropous, with thick integuments that surround the embryo sac and the nucellus (Fig. 1a, b).
The ovary (Ov) of Cynara cardunculus, observed in a light microscope. Semi-thin sections (≈1 μm) stained with 1% (w/v) Azure II in distilled water: 1% (w/v) methylene blue in 1% (w/v) sodium borate (1:1; v/v). a Embryo sac (es) region of the ovule (Ovl). b Cross-section of the nucellus (nu), endothelium (e) and embryo sac. cal chalaza, h hypostase nucellar cells, my micropyle, vr ventral region of ovary wall. Bars 100 μm
Our observations of ovules obtained from closed inflorescences with mature female gametophytes revealed that C. cardunculus possesses an embryo sac of the Polygonum type, comprised of an egg cell and two synergids at the proximal end (Fig. 2a, c), a central cell (Fig. 2a, b) in the medial region, and at least three antipodal cells at the opposite distal end (Fig. 2b).
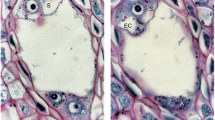
a, b Transmission electron microscopy of the mature embryo sac of Cynara cardunculus before pollination. a Narrow cell walls (triangles facing each other) border synergids (S) and egg cell (E). At this stage, central cell (C) has numerous vacuoles (v) and a cytoplasm rich of vesicles. Some are attached to the large vacuole (open arrow). Nucellar remnants (nr) lining the central cell distal end are observed. b Partial view of the embryo sac showing central cell and antipodals (A). Cell wall ingrowths (in) near the polar nuclei (pn) are frequent. c Synergids show the filiform apparatus (fa) at the proximal end. e n Nucleus. Bars 1 μm
The egg cell nucleus is polarised at the distal end with a single nucleolus (Fig. 2a). The egg cell is surrounded by two synergids, similar in size to the first but polarised towards the proximal end. In C. cardunculus, the filiform apparatus is evident at the proximal end of the female gametophyte (Fig. 2c), as observed in embryo sacs of other Asteraceae (Huang and Russell 1992). The synergids show a central, well-developed nucleus where nucleoli are often evident (Fig. 2c). These cells reveal a strongly polarised cytoplasm with most vacuoles localised at the distal end, while in the proximal end, abundant mitochondria, rough endoplasmic reticulum and polyribosomes are present (results not shown).
The central cell, which is the largest cell in the embryo sac, shows a large central vacuole and several smaller ones at the distal end (Fig. 2a, b). Both polar nuclei are localised at the centre of the cell and near these nuclei, cell wall ingrowths can also be observed (Fig. 2b). At its distal end, near the border of the central cell, numerous electron dense structures corresponding to nucellar remnants are present (Fig. 2a, b). In the cytoplasm, a large number of secretory vesicles, as well as lipid droplets can be observed (Fig. 2a). It is interesting to notice that in C. cardunculus, as for other dicots (Bouman 1984), the antipodal cells persist after fusion of the polar nuclei (Fig. 2b).
Prior to pollination, two specialised nucellar layers can be distinguished in C. cardunculus ovules (Figs. 1b, 3a): a hypostase and a podium. The podium cells are located adjacent to the distal end of the embryo sac and show a rich cytoplasm and a large nucleus with condensed chromatin at the periphery (Fig. 3b). This nucellar cell type presents narrow cell walls. Surrounding the podium, one can find the hypostase, where thick cell walls, electron-dense cytoplasm with amorphous condensed material, remains of the nucleus and strong vesiculation can be observed (Fig. 3c), as has been described for other plant species (Bouman 1984). All these morphological features are typical of cells undergoing PCD, as previously referenced. At the micropylar end, the nucellar remnants stain strongly with methylene blue and correspond to the epistase, as described for other plant species (Fig. 4).
a The ovule of Cynara cardunculus before pollination, observed in a light microscope. Semi-thin sections (≈1 μm) stained with 1% (w/v) Azure II in distilled water: 1% (w/v) methylene blue in 1% (w/v) sodium borate (1:1; v/v). Bar 200 μm. b, c Nucellar cells that involve C. cardunculus embryo sac, observed in a transmission electron microscope. b Detail of the podium nucellar cells. c The hypostase cells show electron dense cytoplasm with amorphous condensed chromatin (asterisk), remains of the nucleus and fusing vacuoles as typical PCD signals. e Endothelium cells, h hypostase, n nucleus, p podium nucellar cells, v vacuole, arrow condensed cromatin, asterisk vesicles. Bars 0.5 μm
The ovule of Cynara cardunculus after pollination, observed in a light microscope. Semi-thin sections (≈1 μm) were stained with 1% (w/v) Azure II in distilled water: 1% (w/v) methylene blue in 1% (w/v) sodium borate (1:1; v/v). e Endothelium cells, E egg cell, ep epistase, h hypostase, p podium. Bar 100 μm
The embryo sac of C. cardunculus is encircled by a differentiated internal layer of the inner integument, the endothelium. This region of radially oriented cells becomes bilayered as ovule maturation proceeds, a phenomenon observed for other Asteraceae. The endothelium cells are characterised by a rich cytoplasm comprising abundant dictyosomes, vesicles, inclusion bodies and vacuoles, and shows several ingrowths consistent with potentially high secretory activity (results not shown). At the chalazal end, this inner integument layer of endothelium is limited by the hypostase. Nucellar remnants are observed surrounding the embryo sac in the endothelium region (Fig. 2a, b).
Programmed cell death in C. cardunculus ovules
Analysis of results obtained with TUNEL reactions indicates an absence of nuclear DNA fragmentation in very young ovules, prior to nucellar differentiation and embryo sac maturation (Fig. 5a). However, after embryo sac differentiation, nucellus cells both at the hypostase and podium levels reveal genomic DNA cleavage, reflecting that during this developmental stage nucellar layers of the hypostase and podium are both undergoing PCD (Fig. 5d). In the positive control, all nuclei are revealed as TUNEL-positive after DNAse pretreatment (Fig. 5b, e). In ovules from closed inflorescences in which mature embryo sacs are present, part of the cells in the hypostase layer have already lost their nuclei, as observed by unlabelled regions in the positive control (Fig. 5e). The negative controls did not display any observable labelling (Fig. 5c, f).
Localisation of PCD in sections of ovules of Cynara cardunculus, before and after embryo sac maturation. Sections were treated with TUNEL reaction, fluorescein, (Roche) and observed in a confocal microscope. a Ovule with an immature embryo sac (es) where nucellus cells (nu) show no PCD. b Positive control treated with DNAse. c Negative control without terminal deoxynucleotidyl transferase. d Ovule with a mature embryo sac where hypostase cells (h) and podium cells (p) show different stages of PCD. e Control treated with DNAse. f Control without terminal deoxyncleotidyl transferase. e Endothelium cells, my micropyle. Bars 100 μm
Immunolocalisation of cardosin B in C. cardunculus ovule
Previous results obtained using immunodetection assays revealed that in C. cardunculus florets, cardosin B is constantly present along the transmitting tissue of the lower stigma and style (Vieira et al. 2001). Here we show labelling in the ventral side of the ovary wall, through the micropyle and also at the nucellar region of the ovule (Fig. 6a). This localisation largely coincides with the pollen tube path to the egg cell. The presence of cardosin B is particularly evident in the hypostase (Figs. 6b, 7a), where it localises in the cell wall and extracellular matrix. Interestingly, cardosin B is only detected in this TUNEL-positive nucellar layer (Figs. 7a, 5d) when these cells show strong vesiculation, condensed cytoplasmic material and thick cell walls—all morphological cell events characteristic of late PCD degeneration (Fig. 3c). In fact, cardosin B expression is highly restricted to the hypostase, since no immunolabelling is detected in other tissues such as inner and outer integuments, or in the podium, a region that is in direct contact with the embryo sac. Control sections reflect an absence of cardosin B labelling (Figs. 6c, 7b).
Immunocytochemistry localisation of cardosin B sections of ovules (Ovl) of Cynara cardunculus, prior pollination. Sections labelled with anti-cardosin B visualised by staining for alkaline phosphatase activity. a Longitudinal section of an ovary (Ov). Bar 500 μm. b Transverse section of the hypostase, endothelium and embryo sac region of the ovule. Bar 25 μm. c Control without primary antibody. Bar 500 μm. cal Chalaza, e endothelium cells, h hypostase cells, my micropyle, nu nucellus, Sy style, tt transmitting tissue, vr ventral region of ovary wall
Immunofluorescence localisation of cardosin B in sections of ovules of Cynara cardunculus, prior and after pollination. Sections labelled with anti-cardosin B with Alexa Fluor® 488 (Molecular Probes) secondary antibody and observed in a confocal microscope. a Ovule with a developed embryo sac. b Control without the primary antibody. Bars 500 μm. c Ovule after pollination. d Same region; control without primary antibody. e Micropyle (my). f Control without primary antibody. Bars 100 μm. e Endothelium cells, es embryo sac, h hypostase cells, my micropyle, S synergids
In the ovule of C. cardunculus pollinated florets, the embryo sac (Fig. 4) is surrounded by a high number of small cells that have differentiated into an endothelium, that contain a rich cytoplasm and prominent nuclei, an indication that these cells are metabolically active. The podium region is composed of cells that stain strongly with methylene blue (Fig. 4). Outside this highly stained region, one can observe cells of the hypostase already degenerated, with condensed cytosolic material and cell walls with irregular profiles (Fig. 4). During the late stages of embryo sac development, cardosin B is restricted to this nucellar layer of degenerated cells, being confined to the cell walls and extracellular matrix of the hypostase (Fig. 7c). Cells of the micropylar canal also label for cardosin B in their extracellular space, as observed before fertilisation (Fig. 7e). In the remaining ovule tissues, such as inner and outer integuments and in the control sections (Fig. 7d, f), no immunofluorescence for cardosin B presence was detected.
Discussion
The embryo sac of C. cardunculus presents characteristics typical of the Polygonum type. The structure of the synergids reflects the active physiological role attributed to these cells, particularly the production of secretions putatively responsible for pollen tube attraction (Huang and Russell 1992; Higashiyama et al. 2003). In C. cardunculus, as in other plant species, the filiform apparatus of the synergid displays the transfer cell wall characteristics that are associated with cells involved in intense exchanges, absorption or/and secretion of substances (Willemse and van Went 1984). The structural characteristics of the central cell reflect that this cell is metabolically active, suggesting its capacity for nutrient uptake from the endothelium, presumably a prerequisite for initiation of endosperm development and embryo support (Huang and Russell 1992; Coimbra and Salema 1999).
The podium is a remnant of the nucellar base that resembles a pedestal for the embryo sac. It consists of nucellar tissue resistant to encroachment by the female gametophyte. The cup-shaped hypostase is common to other plant species and many functions have been attributed to this structure (Bouman 1984). It may connect the vascular supply with the embryo sac, thus facilitating transport or being responsible for the production of specific molecules (Bouman 1984). In C. cardunculus, maturation of the nucellar cell layers coincides with two morphologically distinct PCD degeneration events. Before the occurrence of pollination, cytoplasm degenerates, with concomitant accumulation of secretory vesicles, multivesicular bodies, increased electron density in the tonoplast and occurrence of disintegrated nuclear fragments in the hypostase. These morphological characteristics, along with nuclear DNA fragmentation (Wang et al. 1996; Wan et al. 2002), are considered as indicative of PCD events and have been observed in the nucellus of other plant species (Hiratsuka et al. 2002; Greenwood et al. 2005). In mature ovules prior pollination, the nucellar podium also shows chromatin condensation at the nuclear periphery, but the degree of cellular disorganisation is limited. After pollination, the cells of the hypostase become highly disorganised, often devoid of cytoplasm and markedly deformed. In C. cardunculus, progression of this degradative process in nucellar layers is a highly specific and localised phenomenon.
The development of endothelial cells is believed to be crucial in the nutrition of the embryo sac in plants that possess this layer. Presumably, the endothelium may serve as an intermediary layer between the embryo sac and neighbouring tissues, receiving, metabolising and transferring dissolution products before they enter the embryo sac. In C. cardunculus, embryo sac nutrition is probably linked to endothelium secretion activity. The nutrient supply of the endothelium most possibly occurs through nutritional delivery from the hypostase, a region that labels positively for cardosin B.
In C. cardunculus, Vieira et al. (2001) have shown that cardosin B is expressed in the stylar transmitting tissue. It is interesting to note that the shape of nucellar intercellular spaces of the hypostase is structurally similar to that previously described for the transmitting tissue in the style of this plant. Here we show the localisation of the aspartic proteinase cardosin B in the ventral side of the ovary wall and along the micropylar canal to the embryo sac. Thus, cardosin B is present along the pollen tube pathway leading to the synergids and the egg cell. The presence of cardosin B in these specific regions may be in association with a role in softening and loosening of the cell walls, presumably facilitating pollen tube progression. In C. cardunculus mature ovules, the strongest labelling for cardosin B is observed in the hypostase, both prior to and after pollination. It is noteworthy that this protein is completely absent in the nucellus prior to the beginning of nucellar degeneration by PCD or during embryo sac maturation. Therefore, we hypothesise that the presence of the aspartic proteinase cardosin B may be related with the specific occurrence of PCD during degeneration of the hypostase. Cardosin B presence in the hypostase may be associated with proteolytic activity that results in the supply of nutrients to the growing embryo sac. It is also possible that cardosin B activity is simply related to the loosening of hypostase cell walls, which may facilitate the passage of nutritive substances through this region, nourishing the embryo sac.
In several plant species around the time of fertilisation, the cells of the nucellus begin to undergo PCD (Bi et al. 2005). In barley, Chen and Foolad (1997) have shown localisation of an AP-like protein named nucellin that is expressed exclusively in nucellus degenerating cells. Another proteinase, nucellain, is a nucellar cysteine proteinase that has been reported as having a similar expressional pattern. Cell wall reorganisation, particularly modification or processing of wall polypeptides must be catalysed by enzymes such as nucellain that are located in the cell wall itself (Linnestad et al. 1998). APs may be involved in restructuring the extracellular matrix to provide a physically more permissive medium for pollen tubes or for resource mobilisation to nurture the developing pollen grains, the elongating pollen tubes in the pistil, the developing embryo sac and the zygote in the seed (Wu and Cheung 2000). Alternatively, APs may activate other proteins that have a more direct role in pistil cell death processes (Wu and Cheung 2000; Chen and Foolad 1997). Ge et al. (2005) reported that hyperactivation of the AP constitutive disease resistance 1 in Arabidopsis causes spontaneous cell death, whereas another AP, promotion of cell survival, functions to promote cell survival during embryogenesis and gametogenesis. These findings suggest that embryonic tissues are equipped with PCD machinery, and both anti- and pro-cell death pathways coordinate to ensure development of a functional embryo (Ge et al. 2005). Another hypothesis raised for nucellin is that this enzyme, a major hydrolytic proteinase, converts cell death-related proteins into nutrients for embryo and endosperm development (Chen and Foolad 1997). Wu and Cheung (2000) also considered that the role of proteases in pistil cell wall polymer hydrolysis would be to release low molecular weight oligosaccharides that may act as signals for developmental events, including PCD. Cardosin B has broader specificity and higher proteolytic activity than cardosin A, the other AP reported in C. cardunculus pistils (Veríssimo et al. 1995).
References
An L, You R (2004) Studies on nuclear degeneration during programmed cell death of synergid and antipodal cells in Triticum aestivum. Sex Plant Reprod 17:195–201
Beers E, Woffenden B, Zhao C (2000) Plant proteolytic enzymes: possible roles during programmed cell death. Plant Mol Biol 44:399–415
Bi X, Khush G, Bennett J (2005) The rice nucellin gene ortholog OsAsp1 encodes an active aspartic protease without a plant-specific insert and is strongly expressed in early embryo. Plant Cell Physiol 46:87–98
Bouman F (1984) The ovule. In: Johri B (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 123–157
Chen F, Foolad M (1997) Molecular organization of a gene in barley which encodes a protein similar to aspartic protease and its specific expression in nucellar cells during degeneration. Plant Mol Biol 35:821–831
Coimbra S, Salema R (1999) Ultrastructure of the developing and fertilized embryo sac of Amaranthus hypochondriacus L. Ann Bot 84:781–789
Domínguez F, Cejudo F (1998) Germination-related genes encoding proteolytic enzymes are expressed in the nucellus of developing wheat grains. Plant J 15:569–574
Domínguez F, Moreno J, Cejudo F (2001) The nucellus degenerates by a process of programmed cell death during early stages of wheat grain development. Planta 213:352–360
Faro C, Ramalho-Santos M, Vieira M, Mendes A, Simões I, Andrade R, Veríssimo P, Lin X, Tang J, Pires E (1999) Cloning and characterisation of cDNA encoding cardosin A, an RGD-containing plant aspartic proteinase. J Biol Chem 274:28724–28729
Ge X, Dietrich C, Matsuno M, Li G, Berg H, Xia Y (2005) An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Rep 6:282–288
Greenwood J, Helm M, Gietl C (2005) Ricinosomes and endosperm transfer cell structure in programmed cell death of the nucellus during Ricinus seed development. Proc Natl Acad Sci USA 102:2238–2243
Higashiyama T, Kuroiwa H, Kuroiwa T (2003) Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol 6:36–41
Hiratsuka R, Yamada Y, Terasaka O (2002) Programmed cell death of Pinus nucellus in response to pollen tube penetration. J Plant Res 115:141–148
Huang BQ, Russell SS (1992) Female germ unit: organization, isolation and function. Int Rev Cytol 140:233–293
Linnestad C, Doan D, Brown R, Lemmon B, Meyer D, Jung R, Olsen O (1998) Nucellain, a barley homolog of the dicot vacuolar-processing protease, is localized in nucellar cell walls. Plant Physiol 118:1169–1180
Mutlu A, Gal S (1999) Plant aspartic proteinases: enzymes on the way to a function. Physiol Plant 105:569–576
Panavas T, Pikula A, Reid P, Rubinstein B, Walker E (1999) Identification of senescence-associated genes from daylily petals. Plant Mol Biol 40:237–248
Raiser L, Fischer R (1993) The ovule and the embryo sac. Plant Cell 5:1291–1301
Ramalho Santos M, Pissarra J, Veríssimo P, Pereira S, Salema R, Pires E, Faro C (1997) Cardosin A, an abundant aspartic proteinase, accumulates in protein storage vacuoles in the stigmatic papillae of Cynara cardunculus L. Planta 203:204–212
Runeberg-Roos P, Saarma M (1998) Phytepsin, a barley vacuolar aspartic proteinase, is highly expressed during autolysis of developing tracheary elements and sieve cells. Plant J 15:139–145
Simões I, Faro C (2004) Structure and function of plant aspartic proteinases. Eur J Biochem 271:2067–2075
Vieira M, Pissarra J, Veríssimo P, Castanheira P, Costa Y, Pires E, Faro C (2001) Molecular cloning and characterisation of cDNA encoding cardosin B, an aspartic proteinase accumulating extracellularly in the transmitting tissue of C. cardunculus L. Plant Mol Biol 45:529–539
Veríssimo P, Esteves C, Faro C, Pires E (1995) The vegetable rennet of Cynara cardunculus contains two proteinases with chymosin and pepsin-like specificities. Biotech Lett 17:621–626
Veríssimo P, Faro C, Moir A, Lin Y, Tang J, Pires E (1996) Purification, characterisation and partial amino acid sequencing of two new aspartic proteinases from fresh flowers of Cynara cardunculus L. Eur J Bioch/FEBS 235:762–768
Wan L, Xia Q, Qiu X, Selvaraj G (2002) Early stages of seed development in Brassica napus: a seed coat-specific cysteine proteinase associated with programmed cell death of the inner integument. Plant J 30:1–10
Wang H, Li J, Bostock R, Gilchrist D (1996) Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8:375–391
Willemse M, van Went J (1984). The female gametophyte. In: Johri B (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 159–196
Wu H, Cheung A (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44:267–281
Wyllie A (1995) The genetic regulation of apoptosis. Curr Opin Genet Dev 5:97–104
Xu F, Chye M (1999) Expression of cysteine proteinase during developmental events associated with programmed cell death in brinjal. Plant J 17:321–327
Acknowledgements
The authors thank Rui Fernandes and Dr Paula Sampaio for all the technical support related to image acquisition and processing. The authors also thank the corresponding editor for useful suggestions that have undoubtedly improved the quality and interest of this manuscript. Raquel Figueiredo was recipient of a fellowship by the “Fundação para a Ciência e Tecnologia” associated to the project POCTI/39765 BME/2001, financed by FEDER and the Portuguese Government. Patrícia Duarte was beneficiary of a PhD grant from “Fundação para a Ciência e para a Tecnologia”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Feijó
Rights and permissions
About this article
Cite this article
Figueiredo, R., Duarte, P., Pereira, S. et al. The embryo sac of Cynara cardunculus: ultrastructure of the development and localisation of the aspartic proteinase cardosin B. Sex Plant Reprod 19, 93–101 (2006). https://doi.org/10.1007/s00497-006-0026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-006-0026-4