Abstract
The effect of different external factors on pollen germination and pollen tube growth is well documented for several species. On the other hand the consequences of these factors on the division of the generative nucleus and the formation of callose plugs are less known. In this study we report the effect of medium pH, 2-[N-morpholino]ethanesulfonic acid (MES) buffer, sucrose concentration, partial substitution of sucrose by polyethyleneglycol (PEG) 6000, arginine (Arg), and pollen density on the following parameters: pollen germination, pollen tube length, division of the generative nucleus, and the formation of callose plugs. We also studied the different developmental processes in relation to time. The optimal pH for all parameters tested was 6.7. In particular, the division of the generative nucleus and callose plug deposition were inhibited at lower pH values. MES buffer had a toxic effect; both pollen germination and pollen tube length were lowered. MES buffer also influenced migration of the male germ unit (MGU), the second mitotic division, and the formation of callose plugs. A sucrose concentration of 10% was optimal for pollen germination, pollen tube growth rate and final pollen tube length, as well as for division of the generative nucleus and the production of callose plugs. Partial substitution of sucrose by PEG 6000 had no influence on pollen germination and pollen tube length. However, in these pollen tubes the MGU often did not migrate and no callose plugs were observed. Pollen tube growth was independent of the migration of the MGU and the deposition of callose plugs. In previous experiments Arg proved to be positive for the division of the generative nucleus in pollen tubes cultured in vitro. Here, we found that more pollen tubes had callose plugs and more callose plugs per pollen tube were produced on medium with Arg. After the MGU migrated into the pollen tube (1 h after cultivation), callose plugs were deposited (3 h). After 8 h the first sperm cells were produced. The MGU moved away from the active pollen tube tip until the second pollen mitosis occurred, thereafter the distance from the MGU to the pollen tube tip diminished. Callose plug deposition never started prior to MGU migration into the pollen tube. Pollen tubes without a MGU also lack callose plugs (±30% of the total number of pollen tubes). Furthermore, we found a correlation between the occurrence of sperm cells in pollen tubes and the synthesis of callose plugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen germination and tube growth are essential processes that ensure the reproduction of flowering plants. Pollen tubes are vectors carrying the male sperm cells to the egg cell in the ovule, and are strictly tip-growing. They are characterised by an axially oriented cytoskeleton, a typically zonated cytoplasm, and a so-called reverse fountain-like cytoplasm streaming pattern (Cai et al. 1997; Taylor and Hepler 1997; Derksen et al. 2002). In vitro germination assays are helpful in the study of the growth requirements of pollen tubes. Important external factors influencing pollen germination in vitro include the carbon source, boron and calcium, temperature, water potential, pH, and pollen density, and optimum conditions vary according to pollen species (Túpy and Rihova 1984; Read et al. 1993a; Holm 1994; Shivanna and Sawhney 1995; Rihova et al. 1996; Pasonen and Kapyla 1998; Holdaway-Clarke et al. 2003; Schreiber and Dresselhaus 2003).
Although in vitro germination provides a controlled experimental environment, tube growth in vitro does not completely mimic growth in vivo (Derksen et al. 2002). For species with binucleate pollen, such as Aechmea fasciata, at some point after pollen germination, pollen switches from predominantly autotrophic to predominantly heterotrophic growth, the generative cell (GC) divides (‘second pollen mitosis’) and the first callose plugs are produced. The growth of bicellular pollen is biphasic, the growth rate is slow during the first few hours after germination (autotrophic), followed by a 2- to 5-fold increase in growth rate (heterotrophic) (Mulcahy and Mulcahy 1988; de Graaf et al. 2001; Stephenson et al. 2003). The term pollen growth transition (PGT) is used to denote the time, and associated cellular processes, when pollen tubes switch from slow to rapid growth. It is generally accepted that PGT fails to occur in vitro (Lubliner et al. 2003).
In their attempts to optimise in vitro media for certain species, most researchers focus only on the effects of the in vitro medium on pollen germination and pollen tube growth (Rihova et al. 1996; Fan et al. 2001; Holdaway-Clarke et al. 2003; Hudson and Stewart 2004; Tang et al. 2004; Wolukau et al. 2004). However, other processes, such as the division of the generative nucleus into two sperm nuclei (SN) in binucleate pollen and the formation of callose plugs, may be induced by several components of the medium (Read et al. 1993a; Li et al. 1997; Laitiainen et al. 2002; Vervaeke et al. 2004a). The vegetative nucleus (VN) and the GC or the two sperm cells are proposed to form a functional assemblage, the male germ unit (MGU). The sperm and vegetative nuclei move as the MGU and this association can be important for successful transmission of the male gametes (Palevitz 1993; Lalanne and Twell 2002). Callose, a 1,3-β-glucan with some 1,6 linked branches, is deposited as the major component in the wall of growing pollen tubes, but is confined to the inner wall layer behind the tube tip, and to transverse walls named plugs (Ferguson et al. 1998). The control of callose deposition may be related to the mechanisms controlling tip-growth of pollen tubes and their polar cytoplasmic organisation (Taylor and Hepler 1997).
An optimal medium that enables at least 90% pollen germination and normal pollen tube morphology is useful for both basic studies on pollen function and applied aspects such as in vitro pollination and fertilisation. The proper transport of the vegetative and the generative nucleus and the formation of sperm cells is a prerequisite for successful fertilisation (Laitiainen et al. 2002; Vervaeke et al. 2004a, 2004b). In previous studies, A. fasciata (Bromeliaceae) proved to be well-suited for studies on fertilisation (in vivo and in vitro), and in vitro and semi in vivo pollen tube growth (Vervaeke et al. 2002, 2004a, 2004b, 2005), and therefore we decided to use A. fasciata as a model plant for this study.
The aim of this study was to determine the most favourable medium for pollen germination and tube length, but also for the division of the generative nucleus and the formation of callose plugs. Therefore, different external factors were studied: medium pH, 2-[N-morpholino]ethanesulfonic acid (MES) buffer, sucrose concentration, partial substitution of sucrose by polyethyleneglycol (PEG) 6000, arginine (Arg), and pollen density. Furthermore, the different developmental processes were studied in relation to time.
Materials and methods
Aechmea fasciata plants were cultivated in greenhouses and maintained at average day/night temperatures of 21±0.5°C/19.5±0.5°C, respectively, and at 65±5% relative humidity. Normal nursery practice for pest control, watering, ventilation and fertilisation was conducted. Flower induction was started by ethylene.
Anthers were taken from flowers at anthesis between 8 a.m. and 10 a.m. and were transferred to a 1.5 ml Eppendorf tube. Pollen was isolated from these anthers by shaking them in 1 ml liquid modified Nitsch medium [80 mg/l NH4NO3, 125 mg/l KNO3, 500 mg/l Ca(NO3)2·4H2O, 125 mg/l KH2PO4, 3 mg/l ZnSO4·4H2O, 0.5 mg/l ZnSO4·7H2O, 10 mg/l H3BO3, 0.025 mg/l CuSO4·5H2O, 0.025 Na2MoO4·2H2O; Higashiyama et al. 1998]. The anther-medium mix was then placed on 4 ml solid medium (5 g/l Difco agar) in a Petri dish (diameter 55 mm). The medium was autoclaved (15 min at 121°C and 1.5 bar), thus obtaining a microorganism-free medium. For each determination, ten flowers (ten Petri dishes) were observed. Petri dishes were incubated at 20°C for 24 h in a growth chamber with continuous light at a light intensity of 40 μmol m−2 s−1 (lamp type: OSRAM L36/10).
Pollen germination was determined by light microscopy. Pollen grains were scored as germinated when the pollen tube length was at least twice the pollen grain length. For each determination, 300 pollen grains were evaluated. Pollen tube length of approximately 100 tubes was measured. For 4′,6-diamidino-2-phenylindole (DAPI) staining of germinated pollen grains, 0.6 ml Partec Cystain UV Ploidy solution was added to the pollen grains. DAPI stains the generative and vegetative nuclei and makes them visible under fluorescence microscopy. In each pollen sample, at least 40 pollen tubes were examined for division of the generative nucleus. After 1 h, ethanol (70%, 1 ml) was added to the Petri dishes. After evaporation of ethanol, pollen tubes were stained with aniline blue (AB; 0.05% in 0.06 M K2HPO4-K3PO4, pH 11) for at least 16 h in the dark. Callose stains with AB and consequently callose plugs in pollen tubes can be observed by means of UV fluorescence microscopy. The percentage of pollen tubes with callose plugs was determined; at least 40 pollen tubes per pollen sample were scored. For each determination, the number of callose plugs was counted in 100 pollen tubes. Microscopy was performed by means of an OLYMPUS BX 40 microscope with a BP-360–370 nm excitation filter (DM-400 dichroic mirror and BA420 barrier filter) connected to a JVC camera and a computer with MicroImage image analysis software.
Different external factors influencing pollen germination, pollen tube length, division of the generative nucleus and the formation of callose plugs were studied. These were: pH (pH was adjusted with 1 M KOH before autoclaving), MES buffer, sucrose concentration, partial substitution of sucrose by PEG 6000, Arg (1 mM), and pollen density (one-half, one, two or four anthers were added to 1 ml liquid medium; the average number of pollen grains per square millimetre was subsequently determined by image analysis). Medium pH before and after autoclaving was measured using a Consort P901 and a Hamilton Slimtrode (PIN238150/04, pH 0–14). For the optimal medium (100 g/l sucrose, 1 mM Arg, pH 8.3 before autoclaving, two anthers per Petri dish), pollen germination, pollen tube length, movement of the MGU, division of the generative nucleus, distance of the MGU from the pollen tube tip, the formation of callose plugs (% pollen tubes with one or more plugs and the average number of plugs per pollen tube) were determined in relation to time after start of pollen germination on the in vitro medium (0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24 and 48 h). After these time intervals DAPI (600 μl Partec Cystain UV Ploidy Solution), which inhibited further pollen tube growth, was added to the pollen grains. In order to fix the pollen tubes and improve AB staining, ethanol (70%, 1 ml) was added. AB staining was completed as described above.
Results
Influence of pH and MES buffer
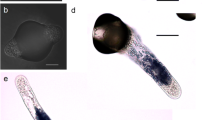
In unbuffered modified Nitsch medium, the pH was lowered after autoclaving (Table 1). In general, cultivation of pollen grains and pollen tubes did not influence the medium pH. Only when the medium pH was 7.5 (after autoclaving), did the pH drop to 6.9 due to pollen germination and tube growth. Pollen germination, pollen tube length, division of the generative nucleus, and the formation of callose plugs were optimal when the pH after autoclaving was 6.7 (Fig. 1a). When the pH was 7.8 before and 5.9 after autoclaving pollen, the germination rate was high (83±13%); however, pollen tubes were shorter and produced fewer sperm cells and callose plugs. The formation of sperm cells was optimal at pH 6.7–7.5 after autoclaving (Fig. 1b). Not all pollen tubes had a MGU, these pollen tubes also lacked callose plugs (±30% of the total number of pollen tubes produced when the pH after autoclaving was 6.7). When the pH after autoclaving was 5.9, pollen tubes showed normal migration of the MGU, but the second pollen mitosis was often inhibited (Fig. 1c). At this lower pH pollen tubes often showed characteristic curling (Fig. 1c). Pollen tubes cultured at high pH (8.1 after autoclaving) without normal callose plugs showed high fluorescence, were thicker and the pollen tube tips were swollen (Fig. 1d).
a Callose plugs, pH 8.3 before autoclaving (AB staining). b Pollen tube with sperm cells and vegetative nucleus, pH 8.8 before autoclaving [4′,6-Diamidino-2-phenylindole (DAPI) staining]. c Curling of pollen tube and irregular formation of callose, pH 7.3 before autoclaving (DAPI and AB staining).d Normal pollen tubes with callose plugs and thicker pollen tube with strong fluorescence (arrow), pH 8.8 before autoclaving [aniline blue (AB) staining]. Bars a, c 50 μm; b 200 μm; d 100 μm
MES buffer, with a pH range between 5.5 and 6.7, had a negative influence on the in vitro germination and tube growth of A. fasciata pollen (Table 2). Pollen grains scored as not germinated had a burst pollen tube (Fig. 2a). Bursting was characterised by an irregular mass of cytoplasm protruding from the pollen grain. Autoclaving and pollen germination had no influence on pH when 12.5 mM MES was added to the medium. When the pH was adjusted to 6.3 or lower with 12.5 mM MES, the generative and vegetative nucleus did not migrate into the pollen tube (Fig. 2b) and no callose plugs were produced (Fig. 2c). From pH 6.7 onwards, MES buffer was less effective and the medium pH was lowered after autoclaving. There was still an overall negative effect of the MES buffer, which resulted in lower pollen germination and a reduced pollen tube length. In media of pH 6.7 and higher, the generative and vegetative nucleus migrated into the pollen tube, but the GC did not divide into sperm cells (Fig. 2d) and no callose plugs were produced.
Influence of 2-[N-morpholino]ethanesulfonic acid (MES) buffer (12.5 mM). a Abnormal pollen tube formation, pH 5.9 before autoclaving (DAPI and AB staining). b Pollen tube without nuclei and without callose plugs, pH 5.5 before autoclaving (DAPI and AB staining). c Pollen tube tip without callose and nuclei, pH 5.9 before autoclaving (DAPI and AB staining). d Generative and vegetative nucleus [male germ unit (MGU)] in pollen tube, pH 7.1 before autoclaving (DAPI staining). Bars a, c 50 μm; b, d 200 μm)
Influence of sucrose and PEG 6000
In previous experiments a sucrose concentration of 20% was used (Vervaeke et al. 2004a). The results in Table 3 show that a sucrose concentration of 10% was optimal for both pollen germination and pollen tube length. In 94% of the pollen tubes with a MGU, the generative nucleus divided into two sperm cells. The percentage of pollen tubes with one or more callose plugs was not influenced by the sucrose concentration, but more callose plugs were formed on a medium with 10% sucrose (average of 11.5 on medium with 10% sucrose in contrast to 2.7 and 2.9 on 5 and 20% sucrose, respectively). Because pollen tubes were often swollen at the tip on medium containing sucrose (Fig. 3a), sucrose was partly replaced with PEG 6000 (Table 3). PEG 6000 had no influence on pollen germination or pollen tube length. However, these pollen tubes lacked a MGU and consequently no sperm cells were formed. Also, either no callose plugs were produced (Fig. 3b), or callose deposition was abnormal, with no clear plugs (Fig. 3c). When cultured on medium containing PEG 6000, the pollen tube tips showed characteristic bursting (Fig. 3d).
Influence of sucrose and PEG 6000. a 10% sucrose; pollen tube tip swollen (DAPI and AB staining). b 5% sucrose, 5% PEG 6000; pollen tubes without nuclei and callose plugs (DAPI and AB staining). c 5% sucrose, 5% PEG 6000; abnormal deposition of callose (AB staining). d 5% sucrose, 5% PEG 6000; burst pollen tube tip (DAPI staining). Bars a, b, d, 50 μm; c 100 μm
Influence Arg and number of pollen grains per square millimeter
To study the influence of the pollen density, the number of pollen grains per square millimeter was counted when one-half, one, two and four anthers were shaken in 1 ml liquid medium and the pollen were deposited on solid medium in a Petri dish. In previous experiments, two anthers were always used, resulting in an average density of 1.8±0.9 pollen grains per square millimeter (Table 4). When four anthers were used it was impractical to measure the pollen tube length because all pollen tubes were intertwined. Visually, they were not shorter than when pollen density was lower. On medium with Arg at lower densities, pollen tube length and the number of callose plugs were lower. On medium without Arg, pollen tube length, percentage of pollen tubes with sperm cells, percentage of pollen tubes with callose plugs, and the number of callose plugs were lower. Only pollen germination was not influenced by Arg. On medium without Arg the different parameters evaluated (see Table 4) were enhanced with increasing pollen density; however, values obtained on medium with Arg were never reached.
Timing of the second pollen mitosis and formation of callose plugs
After 30 min cultivation on modified Nitsch medium with 20% sucrose, pH 8.3 before autoclaving, and 1 mM Arg, pollen grains were hydrated and small pollen tubes were sometimes formed (Fig. 4a). Nevertheless, any tubes formed were still smaller than the pollen grain diameter (Table 5). After 1 h, pollen germination was almost at the maximal level and the MGU started to migrate into the pollen tube. Between 1.5 h and 2 h there was strong pollen tube growth (1.1 mm h−1), and at 3 h the first callose plugs were formed (Fig. 4b). Between 3 h and 10 h pollen tube growth almost ceased but the percentage of pollen tubes with callose plugs and the number of callose plugs per pollen tube increased. The active pollen tube tip did not show callose deposition (Fig. 4c), until the first tips started to swell at 10 h (Fig. 4d). The distance of the MGU to the pollen tube tip increased until 8 h and decreased again when the first SN were formed (Fig. 4e). Pollen tubes continued to elongate for between 12 h and 24 h; however, the number of callose plugs did not increase, due to the increasing distance between the callose plug last formed and the MGU. About 30% of the callose plugs were ‘empty’ (they had no MGU nor callose plugs, Fig. 4f); these pollen tubes were smaller and had an average length of 609±229 μm.
a Hydrated pollen grain with small pollen tube at 0.5 h. b Callose plugs 4 h after start (AB staining). c Pollen tube tip without callose wall at 2 h (AB staining). d Pollen tube with MGU and swollen tips 48 h after start. e MGU with vegetative and two sperm nuclei (SN) after 8 h (DAPI staining). f Pollen tube without MGU and callose plugs at 12 h (DAPI and AB staining). Bars a, b, d, e 50 μm; c 100 μm; f 200 μm
Discussion
Influence of pH and MES buffer
The optimal pH in unbuffered medium differed for pollen germination, tube elongation, generative nucleus division and the formation of callose plugs. At a relatively high pH (7.5) the GC divided into two sperm cells in 99±1% of pollen tubes with a MGU. After 24 h cultivation of pollen tubes, the medium pH decreased from 7.5 to 6.9. In Crotalaria retusa, pollen germinated and grew well in unbuffered media of pH ranging from 4.0 to 9.0, whereas buffered medium of pH 6.0 appeared to be the best medium for germination. This was probably due to the ability of pollen to shift the pH of unbuffered solutions to the optimum level by releasing a range of metabolites (Sharma and Shivanna 1983). A. fasciata pollen grains had less influence on medium pH in unbuffered media, even at higher pollen densities (results not shown). For A. fasciata pollen grains, a pH of 6.7 after autoclaving was optimal for all parameters tested. In general, the optimal pH differs between different species; e.g. pH 7.0 was optimal for Borago officinalis (trinucleate) (Montaner et al. 2003), pH 8.0 for Brassica napus (trinucleate) (Shivanna and Sawhney 1995), pH 5.8 for Arabidopsis thaliana (trinucleate) (Fan et al. 2001), pH 5.9 for Nicotiana tabacum (binucleate) (Read et al. 1993a), and pH 5.5 for Lilium longiflorum (Messerli and Robinson 2003). In L. longiflorum the cytosolic pH was very sensitive to values exceeding pH 7.0, where growth stopped immediately (Fricker et al. 1997). Lowered pH may weaken the pectin cell wall as the greater [H+]0 will compete with Ca2+ to reduce pectin cross-linking. Also, low pH should keep pectin methyl esterase (PME) in a less active state, leading to a higher degree of pectin esterification and a weaker cell wall. In less acidic media, Ca2+ will bind pectin more extensively and PME will be in a more active state, strengthening the cell wall, eliminating growth oscillations and slowing growth (Messerli and Robinson 2003).
Germination and pollen tube growth of N. tabacum results in progressive medium acidification and total growth inhibition. An increase of the buffering capacity of the culture medium by MES buffer enhanced pollen tube growth, e.g. as reported by Túpy and Rihova (1984). In A. fasciata, MES buffer had a negative effect on both pollen germination and tube length, even at the optimal pH. At pH 7.1, the MGU migrated into the pollen tube but callose plugs were never observed. MES buffer was not appropriate to study the difference between a buffered and an unbuffered medium. Since A. fasciata pollen germination and tube growth did not result in strong acidification, with the consequent tube growth inhibition, no further buffering molecules (with fewer or no toxic effects) were studied.
Influence of sucrose and PEG 6000
In previous experiments, medium with 20% sucrose was used (Vervaeke et al. 2004a, 2005). However, 10% sucrose was superior, especially for pollen tube length and number of callose plugs per pollen tube. The optimal sucrose concentration differs for different species and is in general higher for trinucleate species (>20%) than for binucleate species (10–15%) (Shivanna 2003). In Zea mays, 16% sucrose was optimal. When sucrose was partly substituted by PEG, 10% sucrose and 6% PEG 6000 was optimal (Schreiber and Dresselhaus 2003). PEG is a non-penetrating osmotic agent that decreases the water potential of the culture medium. In pollen grains, PEG is considered to regulate the permeability of the plasma membrane and to give stability to the pollen tube membrane (Read et al. 1993b). In A. fasciata PEG had no influence on pollen germination and pollen tube length. Most pollen tubes had no MGU and no callose plugs. This observation indicates that pollen tube growth was independent of MGU migration and the production of callose plugs. In general, the movement of pollen tube organelles relies on cytoskeletal elements. The microtubule (MT) cytoskeleton is required for the proper transport of the VN and the GC from the pollen grain to the tube tip (Cai et al. 1997; Laitiainen et al. 2002). The GC and VN migrate progressively through the pollen tube, moving from the central part into the apical zone only after prolonged growth (Pierson et al. 1990). The actin cytoskeleton is principally responsible for cytoplasmic streaming and tube growth (Cai et al. 1997). It appears that MTs are not an absolute requirement for pollen tube growth (Cai et al. 1997). Our results also confirm that movement of the MGU and pollen tube growth are autonomous processes.
Influence of Arg and number of pollen grains per square millimeter
The phenomenon of density-dependent pollen germination and tube growth in vitro has long been well documented in many plant species and is termed the ‘pollen population effect’ (Pasonen and Kapyla 1998; Chen et al. 2000). Brewbaker and Majumder (1961) suggested that it results from the presence of a diffusable, water-soluble pollen growth factor in pollen grains and in plant tissues. This factor was later proved to be the calcium ion (Brewbaker and Kwack 1963). We could not confirm the putative effect of pollen density on pollen germination, most probably because there was sufficient Ca present in the germination medium [500 mg/l Ca(NO3)2·4H2O]. Chen et al. (2000) showed that phytosulfokine-α (PSK- α) is a native bioactive factor contributing to the pollen population effect. Exogenous PSK-α stimulated pollen germination in a low density pollen culture of N. tabacum. On medium without Arg, a higher pollen density resulted in longer pollen tubes, more division of the generative nucleus and more callose plug synthesis. When Arg was added, the effect was seen only for the production of callose plugs.
Vervaeke et al. (2005) previously reported that Arg as single amino acid was the limiting factor for the occurrence of second pollen mitosis in pollen tubes of A. fasciata cultured in vitro. The involvement of Arg is probably related to protein synthesis. In this study we found that addition of Arg also increased the number of callose plugs.
Timing of the second pollen mitosis and formation of callose plugs
In previous experiments, pollen germination, pollen tube growth and timing of division of the generative nucleus were studied on unautoclaved medium (pH 7.3) and 20% sucrose (Vervaeke et al. 2005). On this latter medium, pollen germinated slower, growth rates were lower, and tubes were shorter. The growth pattern also differed: on medium with 20% sucrose, pollen tube growth was logarithmic (Parton et al. 2002); on medium with 10% sucrose, pollen tube growth rates were very high at the start (1.1 mm/h), almost as fast as when pollen tubes grow through a compatible style (2 mm/h, Vervaeke et al. 2003). However, already 2 h after cultivation, during which time callose plugs were deposited and the second pollen mitosis occurred, pollen tube growth ceased. Between 12 h and 24 h, pollen tubes extended again but no more callose plugs were produced. The first generative nuclei started to divide 8 h after cultivation, as on medium with 20% sucrose (Vervaeke et al. 2005). Therefore, we can conclude that the timing of the second pollen mitosis is independent of the sucrose concentration and of pollen tube growth rate. Up to 10 h after germination pollen tubes of N. tabacum contain a single generative nucleus (Read et al. 1993a). In N. tabacum pollen tubes, a cross wall consisting mainly of callose forms a plug in the subapical part of the tube after about 6 h of growth in vitro. The callose plug maintains living cytoplasm and the MGU in the apical region of the tube. New callose plugs are formed repeatedly during tube elongation in vivo and in vitro. After 12 h there were 3.4±0.1 callose plugs in N. tabacum pollen tubes (Laitiainen et al. 2002). In A. fasciata more callose plugs (11.5±3.4) were formed after 12 h. In Petunia inflata callose plugs were seen in 50% or more of the tubes cultured for at least 8 h in vitro. In general, the division of the generative nucleus coincides with callose plug deposition (Lubliner et al. 2003). In A. fasciata, callose plugs were deposited 5 h earlier than the division of the generative nucleus.
Conclusions
Pollen germination, pollen tube growth rate, maximal pollen tube length in vitro, division of the generative nucleus, and formation of callose plugs are basically different processes that occur in pollen tubes cultured on artificial medium and in vivo. These processes are influenced by different external factors in vitro. Pollen tubes grown in vitro lack the biochemical, physiological and physical environment of the pistil, and possible interactions with adjacently growing pollen tubes (de Graaf et al. 2001). Pistil transmitting tract cells secrete free sugars, glycolipids, glycoproteins, polysaccharides, lipids, and proteins into the extracellular matrix, providing the nutrients and resources that support rapid growth of pollen tubes after the materials stored within the pollen grain are exhausted (Swanson et al. 2004). In vitro, pH, sucrose concentration and the presence of 1 mM Arg influences pollen tube growth rate. Medium composition also influenced division of the generative nucleus and callose deposition, two events necessary to produce a pollen tube with the competence to achieve double fertilisation. In A. fasciata callose plugs were never observed in pollen tubes in vitro without a MGU (±30% of total number of pollen tubes). These pollen tubes are neither functional nor are they able to fertilise ovules.
The sequence of events that we found in pollen tubes cultured in vitro most probably also occurs during in vivo pollen tube growth. Lubliner et al. (2003) stated that callose plugs are deposited by default and that division of the GC in vitro is triggered if basic nutritional requirements are met. pH, sucrose and Arg affected both the second pollen mitosis and the formation of callose plugs. Therefore, A. fasciata pollen tubes cultured on artificial medium can undergo pollen growth transition if favourable external factors are present. However, there are still differences as regards in vivo grown pollen tubes, as plasmatubules and secretory vesicle clusters are present in the tip of pollen tubes growing in vivo (Derksen et al. 2002).
Laitiainen et al. (2002) declared that the movement of the GC and the VN is independent of the synthesis of callose plugs in vitro. The role of the cytoskeleton in callose plug synthesis is currently unknown. Although VN and GC transport precedes synthesis of callose plugs during normal pollen development, it seems that callose plug synthesis requires an intact MT cytoskeleton. The absence of an MT cytoskeleton also reduced the number of callose plugs synthesised in N. tabacum pollen tubes (Laitiainen et al. 2002). Our results indicate that movement of the generative nucleus and, subsequently, callose plug synthesis can be inhibited by a commonly used medium supplement like PEG without any influence on pollen germination or pollen tube length. Furthermore, we found a correlation between the occurrence of the second pollen mitosis (presence of two sperm cells) and percent of pollen tubes with callose plugs (R2: 0.74).
References
Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50:747–858
Brewbaker JL, Majumder SK (1961) Cultural studies of the pollen population effect and the self-incompatibility inhibition. Am J Bot 48:457–464
Cai G, Moscatelli A, Cresti M (1997) Cytoskeletal organization and pollen tube growth. Trends Plant Sci 2:86–91
Chen YF, Matsubayashi Y, Sakagami Y (2000) Peptide growth factor phytosulfokine-α contributes to the pollen population effect. Planta 211:752–755
Derksen J, Knuiman B, Hoedemaekers K, Guyon A, Bonhomme S, Pierson ES (2002) Growth and cellular organization of Arabidopsis pollen tubes in vitro. Sex Plant Reprod 15:133–139
Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52:1603–1614
Ferguson C, Teeri TT, Siika-aho M, Read SM, Bacic A (1998) Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206:452–460
Fricker MD, NS White, G Obermeyer (1997) pH gradients are not associated with tip growth in pollen tubes of Lilium longiflorum. J Cell Sci 110:1729–1740
Graaf BHJ de, Derksen JWM, Mariani C (2001) Pollen and pistil in the progamic phase. Sex Plant Reprod 14:41–55
Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1998) Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10:2019–2031
Holdaway-Clarke TL, Weddle NM, Kim S, Robi A, Paris C, Kunkel JG, Hepler PK (2003) Effect of extracellular calcium, pH and borate in Lilium formosanum pollen tubes. J Exp Bot 54:65–72
Holm SO (1994) Pollen density—effects on pollen germination and pollen tube growth in Betula pubescens Ehrh. in northern Sweden. New Phytol 126:541–547
Hudson LC, Stewart CN (2004) Effects of pollen-synthesized green fluorescent protein on pollen grain fitness. Sex Plant Reprod 17:49–53
Laitiainen E, Nieminen KM, Vihinen H, Raudakoski M (2002) Movement of the generative cell and vegetative nucleus in tobacco pollen tubes is dependent on microtubule cytoskeleton but independent of the synthesis of callose plugs. Sex Plant Reprod 15:195–204
Lalanne E, Twell D (2002) Genetic control of male germ unit organization in Arabidopsis. Plant Physiol 129:865–875
Li H, Bacic A, Read SM (1997) Activation of pollen tube callose synthase by detergents. Plant Physiol 114:1255–1265
Lubliner N, Singh-Cundy DT, Singh-Cundy A (2003) Characterization of the pollen growth transition in self-incompatible Petunia inflata. Sex Plant Reprod 15:243–253
Messerli MA, Robinson KR (2003) Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta 217:147–157
Montaner C, Floris E, Alvarez JM (2003) Study of pollen cytology and evaluation of pollen viability using in vivo and in vitro test, in borage (Borago officinalis L.). Grana 42:33–37
Mulcahy G, Mulcahy DL (1988) The effect of supplemented media on the growth in vitro of bi- and trinucleate pollen. Plant Sci 55:213–216
Palevitz BA (1993) Relationship between the generative cell and the vegetative nucleus in pollen tubes of Nicotiana tabacum. Sex Plant Reprod 6:1–10
Parton E, Vervaeke I, Delen R, Vandenbussche B, Deroose R, De Proft MP(2002) Viability and storage of bromeliad pollen. Euphytica 125:155–161
Pasonen HL, Kapyla M (1998) Pollen-pollen interactions in Betula pendula in vitro. New Phytol 138:481–487
Pierson ES, Lichtscheidl IK, Derksen J (1990) Structure and behaviour of organelles in living pollen tubes of Lilium longiflorum. J Exp Bot 41:1461–1468
Read SM, Clarke AE, Bacic A (1993a) Requirements for division of the generative nucleus in cultured pollen tubes of Nicotiana. Protoplasma 174:101–115
Read SM, Clarke AE, Bacic A (1993b) Stimulation of growth of cultured Nicotiana tabacum W38 pollen tube by poly(ethylene glycol) and Cu(II) salts. Protoplasma 177:1–14
Rihova L, Hrabetova E, Túpy J (1996) Optimization of conditions for in vitro pollen germination and tube growth in potatoes. Int J Plant Sci 157:561–566
Schreiber DN, Dresselhaus T (2003) In vitro pollen germination and transient transformation of Zea mays and other plant species. Plant Mol Biol Rep 21:31–41
Sharma N, Shivanna KR (1983) Pollen diffusates of Crotalaria retusa and their role in pH regulation. Ann Bot 52:165–170
Shivanna KR (2003) Pollen biology and biotechnology. Science, Enfield
Shivanna KR, Sawhney VK (1995) Polyethylene glycol improves the in vitro growth of Brassica pollen tubes without loss in germination. J Exp Bot 46:1771–1774
Stephenson AG, Travers SE, Mena-Ali JI, Winsor JA (2003) Pollen performance before and during the autotrophic–heterotrophic transition of pollen tube growth. Phil Trans R Soc London 358:1009–1017
Swanson R, Edlund AF, Preuss D (2004) Species specificity in pollen-pistil interactions. Annu Rev Genet 38:793–818
Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J 39:343–353
Taylor LP, Hepler PK (1997) Pollen germination and pollen tube growth. Annu Rev Plant Physiol Plant Mol Biol 48:461–491
Túpy J, Rihova L (1984) Changes and growth effect of pH in pollen tube culture. J Plant Physiol 115:1–10
Vervaeke I, Parton E, Maene L, Deroose R, De Proft MP (2002) Pollen tube growth and fertilization after different in vitro pollination techniques of Aechmea fasciata. Euphytica 124:75–83
Vervaeke I, Delen R, Wouters J, Deroose R, De Proft MP (2003) Flower biology of six cultivars of the Bromeliaceae. II. Pollination and fertilization. Selbyana 24:87–94
Vervaeke I, Delen R, Wouters J, Deroose R, De Proft MP (2004a) Division of the generative nucleus in cultured pollen tubes of the Bromeliaceae. Plant Cell Tissue Organ Cult 76:17–28
Vervaeke I, Delen R, Wouters J, Deroose R, De Proft MP (2004b) Semi in vivo pollen tube growth of Aechmea fasciata. Plant Cell Tissue Organ Cult 76:67–73
Vervaeke I, Stichelbout L, Londers E, Deroose R, De Proft MP (2005) Influence of arginine, DFMO and polyamines on the division of the generative nucleus in cultures pollen tubes of Aechmea fasciata (Bromeliaceae). Plant Cell Tissue Organ Cult (in press)
Wolukau JN, Zhang S, Xu G, Chen D (2004) The effect of temperature, polyamines and polyamines synthesis inhibitor on in vitro pollen germination and pollen tube growth. Sci Hortic 99:289–299
Acknowledgements
This research is supported by IWT (Flemish Institute to Promote Scientific and Technological Research in the Industry), grant IWT ozm 030507. The authors thank Deroose Plants NV for the plant material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vervaeke, I., Londers, E., Piot, G. et al. The division of the generative nucleus and the formation of callose plugs in pollen tubes of Aechmea fasciata (Bromeliaceae) cultured in vitro. Sex Plant Reprod 18, 9–19 (2005). https://doi.org/10.1007/s00497-005-0243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-005-0243-2