Abstract
The Gewurztraminer (GW) and the Pinot noir (PN) cultivars of grapevine differ in their sensitivity to environmental factors that can cause flower abscission, cv. GW being the most sensitive. In order to further define the mechanisms leading to abscission, and owing to the importance of sugars in the achievement of sexual organ ontogenesis, we attempted to correlate the chronology of flower ontogenesis with the variations of carbohydrates in the inflorescence. In the vineyard, under optimal climatic conditions, fruit set of cv. GW and cv. PN was 82% and 65%, respectively. The sugar distribution was different in their inflorescences during the entire duration of flower development. Between stages 15 and 17, flowers of GW and PN reached the crucial meiosis stage. At that time, the inflorescences of cv. GW exhibited higher concentrations of starch and sucrose, whereas those of PN presented higher levels of glucose and fructose. Despite higher starch concentrations in GW inflorescences, starch reserves were present in the ovules and anthers of PN but not in those of GW. These results suggest that the higher content of reserve and transport carbohydrates in the inflorescences of GW favour flower development and fruit set under optimal environmental conditions. Furthermore, since meiosis represents a key step of female development, the different sugar concentrations in the inflorescences of the two cultivars at stages 15 and 17 could be related to the sensitivity to flower abscission under climatic stress. In particular, the presence of starch granules in PN ovules and anthers might explain the higher resistance of this cultivar to flower abscission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grapevine (Vitis vinifera L.) is affected by flower abscission to various extents, meaning that some of the flowers are physiologically removed during development of the inflorescence. Under optimal growth conditions, the intensity of flower abscission remains compatible with economical purposes. However, under environmental stress, flower abscission may increase dramatically depending on the cultivar, reaching up to 80% in cv. Gewurztraminer (GW) (Huglin and Schneider 1998).
The first hallmarks of flower abscission consist of abnormalities in ovary development at the time of meiosis (Fougère-Rifot et al. 1993), which is a key step in the formation of the female organ. Several hypotheses have been proposed to explain flower abscission, such as hormonal balance perturbation (Jackson 1991) or bad climatic conditions during flowering (Jackson 1991). More recently, nutrient deficiency in the inflorescences has been suspected as a putative originator of flower abscission in grapevine. It has been shown that carbon metabolism may be involved in the process of flower necrosis in cv. Pinot noir (PN) (Gu et al. 1996).
In higher plants, carbohydrate supply is of fundamental importance for flowering and the achievement of sexual reproduction. Developing flowers need carbohydrates from the stage of floral initiation (Yu et al. 2000) up to maturation of the floral organs (Clément et al. 1996; Rodrigo et al. 2000) and fruit setting (Jean and Lapointe 2001; Ruiz et al. 2001; Iglesias et al. 2003). The two main carbohydrates involved in flower nutrition are (1) sucrose, as the main circulating form of carbohydrate from the source tissues (Winter and Huber 2000; Truernit 2001), and (2) starch, which is actively mobilised during the development of floral tissues (Clément et al. 1996; Rodrigo et al. 2000). Sugars supplied to the reproductive structures originate from root and stem reserves (Nishizawa and Shihido 1998) or from photosynthesis performed either in mature leaves (Gregory et al. 1996; Voznesenskaya et al. 1999; Miyazawa and Terashima 2001) or in the flowers themselves (Vemmos and Goldwin 1994; Clément et al. 1997).
In grapevine, the carbohydrates required for inflorescence development may originate either from perennial woody organs or from annual leaves. In spring, carbon reserves of roots are mobilised and sustain the expansion of new young roots, annual stems and leaves, as well as emerging inflorescences (Zapata et al. 2004). During flower development, the leaves acquire their own photosynthetic autonomy and begin to provide photoassimilates to sink organs. At this time there is a global change in the whole plant physiology regarding sugar supply to developing organs. The mobilisation of carbohydrate reserves from the roots is progressively replaced by increasing photosynthesis in the leaves (Zapata et al. 2004). This source transition occurs during the development of sexual organs, making them particularly sensitive to physiological perturbations.
Whatever its cause, any perturbation of sugar physiology during flower development usually leads to gametophyte abortion (Dorion et al. 1996; Jean and Lapointe 2001), reducing the success of fertilisation and the yield for cultivated species (Prymakowska-Bosak et al. 1999; Siddiqi et al. 2000). The reproductive organs are particularly sensitive to modifications of carbohydrate physiology when fertile tissues reach meiosis (Saini 1997; Jean and Lapointe 2001).
Since there is a strong transition in the source of sugars from the roots to the leaves during flower development (Zapata et al. 2004), we suspected that inflorescences are affected by these changes at crucial steps of flower development (Dorion et al. 1996; Jean and Lapointe 2001). Therefore, we attempted to correlate the rate of flower abscission with sugar fluctuations in the inflorescences, focusing on key steps of sexual organ formation. We followed the variations of carbohydrate content and the location of starch in flowers, in parallel with the progress of male and female organ development, in vineyard-grown plants. For this purpose we used cvs. GW and PN, which differ significantly in their fruit set under normal conditions and their sensitivity to flower abscission under climatic stress.
Materials and methods
Plant material and sampling
Thirty-year-old field-grown grapevines Vitis vinifera L. flower abscission sensitive cv. GW (clone 47) and the non-sensitive cv. PN (clone 162), grafted on SO4 rootstocks and planted in an INRA vineyard in Bergheim, France, were used in this study in 2002 and 2003. Plants under investigation were grown in the same location and were subject to rigorously similar cultural practices.
The sampling stages were determined according to the classification of Eichhorn and Lorenz (1977). Inflorescences were followed during their entire development, from the “visible cluster” stage (stage 12) up to fruit set (stage 27), representing six developmental stages: 12, 15, 17, 21, 25 and 27. Moreover, the period between stage 15 and 17 was split into two additional stages: “separated cluster” stage (stage 15), stage 15+2 days (15+2d), stage 15+8 days (15+8d), and “separated floral buds” stage (stage 17). Inflorescences were collected at each development stage and frozen in liquid N2 and stored at −80°C until the determination of sugars, or treated immediately after harvest for microscopy analysis.
Dry weight analyses
Dry weight (DW) of inflorescences was estimated after freeze-drying for 72 h with a CS5L device (Serail Lyophilisateur).
Determination of fruit set rate
Fruit set rate is the major parameter used for assessing the success of sexual reproduction. It was evaluated as the ratio of berries over flowers after counting the number of flower scars per inflorescence and the number of berries per bunch (stage 27).
Preparation for microscopy
Flowers were fixed in 2% glutaraldehyde (v/v in a 0.1 M phosphate buffer) at pH 7.2 in the presence of 2% (w/v) sucrose and 1‰ (v/v) Tween 20 for 24 h under continual agitation at room temperature. After three rinses (5 min each) in buffer with 2% (w/v) sucrose, flowers were postfixed with 1% (w/v) osmium tetroxide in the same buffer for 4 h. Flowers were then rinsed three times (5 min each) in buffer, dehydrated in an alcohol series, transferred to acetone, and embedded in araldite.
Semi-thin sections (1 μm) were collected on glass sides and the periodic acid Schiff polysaccharide specific reaction was carried out. Sections were first immersed in 1% (w/v) periodic acid for 4 h, then in Schiff’s reagent without rinsing for 16 h, and finally in 5% (w/v) sodium metabisulfite for 20 min. Sections were then rinsed in distilled water, air-dried, and mounted in Eukitt.
Carbohydrate analysis
Extraction
Lyophilized inflorescences were ground in a mortar with Fontainebleau sand and 10 volumes of ethanol (80°). Sugars were then extracted for 15 min at 84°C under continual agitation. After adjusting the volume to 5 ml with distilled water, the extract was centrifuged at 4°C for 10 min at 11,000 g. For soluble sugars, the supernatant was used for soluble sugar determination. For starch, the pellet previously obtained was suspended in a mixture containing dimethylsulfoxide:8 N hydrochloric acid (8:2) and starch was dissolved over 30 min at 60°C under continual agitation. After cooling, the extract was centrifuged at 20°C for 10 min at 13,000 g and the supernatant was kept at −80°C until use.
Sucrose, glucose and fructose assay
Sucrose, glucose and fructose were assayed because they are the major sugars in grapevine (Glad et al. 1992). d-Glucose was phosphorylated and oxidised in the presence of NADP to gluconate-6-phosphate and NADPH,H+. The amount of NADPH,H+ formed was determined by means of its absorbance at 340 nm. Fructose was phosphorylated to fructose-6-phosphate by a hexokinase in the presence of ATP. Fructose-6-phosphate was then converted to glucose-6-phosphate by a phosphoglucoisomerase. Glucose-6-phosphate formed was tested as described above and a blank was performed without phosphoglucose isomerase. Sucrose was hydrolyzed to d-glucose and d-fructose in the presence of a β-fructosidase. d-Glucose formed was then determined as described above and compared with a blank without β-fructosidase.
Starch assay
Aliquots of 100 μl extract were used to determine starch concentration. The aliquot was mixed with 100 μl Lugol iodine solution (0.03% I2 and 0.06% KI in diluted 0.05 N HCl). After 15 min, the absorbance was read spectrophotometrically at 600 nm. A blank was performed with the starch solvent (DMSO:HCl, 8:2) instead of the extract.
Statistical analysis
At least five assays were performed for each stage of flower development, and three independent readings were carried out for each extract. For carbohydrate determination, results are expressed in milligrams/gram DW ±SE. Statistical analyses were carried out using Student’s t test. A 2% probability was considered significant. For microscopy, four samples were analysed at each phenological stage to check the developmental stage of the anther and the ovule, and to localise starch in the flower organs.
Results
Fruit set
The number of flowers per inflorescence was statistically identical in the two cultivars but the number of berries was higher in GW (Table 1). As a consequence, the percentage of fruit set was 81.8±5.7% in GW against 65.1±2.7% in PN.
Inflorescence growth and development
Phenological development was synchronous in the two cultivars and could be divided into three periods (Fig. 1). The inflorescence developed from stage 12 to stage 15 in 4 days, and then to stage 17 within 9 days. Thereafter, development slowed since 18 days were required to reach stage 21 (flowering onset). Full bloom (stage 25) occurred within the following 24 h and an additional 7 days were required to reach fruit set (stage 27), representing about 29 days after stage 12.
Phenological development of inflorescences in grapevine (Vitis vinifera L.) cvs. Gewurztraminer (GW) and Pinot noir (PN). Numbers on graph correspond to number of days between two successive stages of development (according to the classification of Eichhorn and Lorenz 1977). Stages: 12 Visible clusters, 15 separated clusters, 17 separated floral buds, 21 10% opened flowers (early bloom), 25 90% opened flowers (late bloom), 27 fruit set
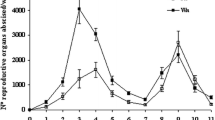
During the whole of flower development, PN inflorescences were significantly bigger than those of GW except at the fruit set stage (Fig. 2). From stage 12 to stage 25 (bloom), inflorescence DW regularly increased, from 29±4 mg to 200±17 mg in GW and from 27±8 mg to 396±24 mg in PN. Afterwards, inflorescence weight rose suddenly in both cultivars, reaching 1,040±350 mg in GW and 1,450±320 mg in PN at fruit set (stage 27).
Weight variations of cvs. GW and PN inflorescences during flower development. Open circles GW, closed circles PN. Stages as in Fig. 1, plus 15+2d separated clusters +2 days, 15+8d separated clusters +8 days. Values are means (n=5) ±SE. Asterisks indicate a 0.1% probability that was considered significant
Reproductive organ development
From stage 12 to stage 15, female reproductive cells were sporogenous tissue in the two cultivars (Fig. 3a–c) and reached the macrospore mother cell stage at 15+2d (Fig. 3d). The time-course of female development differed thereafter. In PN, ovules underwent meiosis between stages 15+2d and 15+8d, whereas meiosis occurred between stages 15+8d and 17 in GW. The residual macrospore generating the embryo sac was formed at stage 15+8d in PN (Fig. 3e) and at stage 17 in GW (Fig. 3f). In PN, the embryo sac still developed at stage 17 (Fig. 3g). However, at the onset of anthesis, the embryo sac was fully developed in both cultivars.
Female organ development and starch localisation; Ow ovary wall, O ovary, Oc ovarian cavity, f funicle, Nu nucellus, Tg tegument, et external tegument, it internal tegument. A stage 12: Sporogenous tissue (ST) stage in PN. Starch grains (arrowheads) detected in ovary wall; ×1,200. B Stage 15: archespore (star) stage in PN. Starch (arrowheads) located in ovary wall; ×500. C Stage 15: archespore (star) stage in GW. No starch detected in ovary wall, open arrowheads polyphenols; ×560. D Stage 15+2d: macrospore mother cell (star) stage in PN. No starch detected; ×400. E Stage 15+8d: embryo sac mother cell (star) stage in PN. Macrospore (star) and surrounding nucellus contained amylaceous reserves (arrowheads); ×450. F Stage 17: embryo sac mother cell (star) stage in GW. No starch detected; ×250. G Stage 17: developing embryo sac (star) stage in PN. Starch (arrowheads) still abundant in ovary wall; ×200
The time-course of pollen development was also different in the two cultivars. Sporogenous cells were detected in the anthers of both cultivars at stage 12 (Fig. 4a, b). Meiosis occurred thereafter at stage 15 in PN and between stages 15 and 15+2d in GW, giving rise to tetrads at stages 15 and 15+2d in PN and GW, respectively (Fig. 4c, d). In PN, tetrads released the microspores in the loculus at stage 15+2d (Fig. 4e) and at the stage 15+8d in GW (Fig. 4f). Microspores underwent vacuolisation until stage 15+8d (Fig. 4g) in PN. Sporal mitosis occurred at stage 17 (Fig. 4h) and pollen maturation extended to stage 21, leading simultaneously to mature pollen grains at anthesis in both PN and GW.
Anther development and starch localisation during flower development; SC sporogenous cell, e epidermis, E endothecium, L middle layer, t tapetum, T tetrads, S septum. A Stage 12: sporogenous cell in PN. Abundant amylaceous reserves (arrowheads) detected in epidermis and sporogenous cells. No starch detected in endothecium; ×500. B Stage 12: sporogenous cell stage in GW. Few starch grains (arrowheads) present in GW in epidermis and sporogenous cells. Endothecium devoid of starch; ×500. C Stage 15+2d: tetrads in GW. Starch grains (arrowheads) observed in endothecium, tapetum and tetrads but not in epidermis or middle layer; ×400. D Stage 15: late tetrads with primexine stage in PN. Amylaceous reserves (arrowheads) present only in endothecium and tetrads); ×400. E Stage 15+2d: young microspores (YM) stage in PN. Starch present only in endothecium; ×700. F Stage 15+8d: young microspore stage in GW. Amylaceous reserves (arrowheads) present only in endothecium; ×700. G Stage 15+8d: vacuolated microspore (VM) stage in PN. Amylaceous reserves (arrowheads) abundant in endothecium, middle layer, and septum as well in microspores; ×700. H Stage 17: bicellular pollen grain (BPG) stage in PN. Starch grains (arrowheads) present in endothecium (E). Starch (arrowheads) abundant in vegetative cell (vc) of pollen grain, but not in generative cell (gc); ×600
Starch location
The presence of starch was examined in each floral organ (Fig. 5a) during the entire developmental period in both cultivars and the location of starch revealed that differences were not cultivar-dependant but stage-dependant, with the exception of the ovule. In sterile organs, amylaceous reserves were abundant in the floral peduncle (Fig. 5b) and receptacle (Figs. 5c, d). Starch was located mainly in the parenchyma or in the epidermis. No starch variation was detected in these organs during the whole of floral development. Similarly, in the petals, starch was constant in the parenchyma during floral development (Fig. 5e). In the sepals (Fig. 5f, g) and the anther filament (Fig. 5 h, i) starch progressively disappeared during development. In these organs, starch was located in both the parenchyma and the epidermis.
Starch localisation in vegetative parts during flower development; e epidermis, P parenchyma. A Longitudinal section of PN flower at stage 17 showing starch accumulation in various sites. A anther, f funicle, F anther filament, FP floral peduncle, FR floral receptacle, M microspores, Nu nucellus, Ov ovule, Pt petal, S sepal ×80. B Stage 17: floral peduncle in PN. Starch (arrowheads) observed in epidermis and parenchyma; ×200. C,D Stage 17: floral receptacle in PN. Starch grains (arrowheads) abundant and regularly distributed in epidermis, parenchyma and vascular bundles; C ×180, D ×450. E Stage 17 petal in PN. Amylaceous reserves (arrowheads) located in epidermis and parenchyma; ×280. F Stage 12: anther filament in PN. Starch (arrowheads) present in epidermis and parenchyma; ×160. G Stage 17: anther filament in PN. Starch grains (arrowheads) poorly represented in epidermis and parenchyma; ×240. H Stage 12: sepal in PN. Starch (arrowheads) located in epidermis and abundant in parenchyma; ×350. I Stage 17: sepal in PN. Starch grains (arrowheads) less abundant in epidermis and parenchyma than in previous stage; ×320
Differential starch fluctuations were detected in the fertile organs of the two cultivars. Considering the female organ, starch grains were present in the macrospore and the surrounding nucellus in PN (Fig. 3e) at stage 15+8d, whereas no starch was present in the ovules of GW (Fig. 3f). At stage 17, starch was present in the ovary parenchyma as well as in the integuments throughout floral development in both cultivars (Fig. 3f, g).
In the anther, starch was present in both the sporophytic and gametophytic tissues during pollen development. In the sporophytic cell layers, starch was localised in the epidermis, endothecium, middle layers and tapetum (Fig. 4a, c, d, g). During flower development, this localisation of starch did not vary, and no major difference was observed between the two cultivars. Conversely, in the sporogenous tissues, differences between the two cultivars appeared at premeiosis. Indeed, at stage 12, the sporogenous tissue of PN exhibited abundant amylaceous reserves (Fig. 4a), while no trace was detected in GW (Fig. 4b). Later, at stage 15+8d, microspores of PN had accumulated starch grains (Fig. 4g), but those of GW had not (Fig. 4f).
Carbohydrate content
In the two cultivars, starch concentration in the inflorescences was maximal at stage 12, corresponding to 7.1±0.5 mg (g DW)−1 and 8.2±0.6 mg (g DW)−1 in GW and PN, respectively (Fig. 6a). Afterwards, starch variation in the inflorescences could be divided into two phases: (1) the first phase extended from stage 12 to stage 17 and consisting of a steady starch decrease, down to 2.7±0.3 mg (g DW)−1 and 2.8±0.4 mg (g DW)−1 in GW and PN, respectively; (2) the second phase extended from stage 17 to stage 27 and was characterised by a succession of amylogenesis/amylolysis sequences, with a maximum observed at stage 25. Although starch concentration in both GW and PN decreased from stage 12 to stage 17, significant differences were noticed between stages 15 and 17, and particularly at stage 15+2d. At this step (female structures at macrospore mother cell stage and male structures at tetrad stage), the starch concentration in the inflorescences of GW was 2-fold higher than in those of PN. In addition, the starch level in inflorescences at full bloom (stage 25) was 50% higher in GW than in PN.
Changes in some carbohydrate content of cvs. GW and PN inflorescences during flower development. Open circles GW, closed circles PN. Starch (A) and soluble carbohydrates sucrose (B), glucose (C) and fructose (D) were measured. Values are means (n=5) ±SE. Asterisks indicate a 2% probability that was considered significant
In GW inflorescences, the sucrose concentration increased from 2.9±2.4 mg (g DW)−1 to 16.6±1.1 mg (g DW)−1 between stages 12 and 15+2d, before dropping rapidly to 0.2±0.2 mg g DW−1 at stage 17 (Fig. 6b). From stage 17 up to fruit set (stage 27), sucrose content alternatively increased and decreased between 0.2±0.2 mg (g DW)−1 at stage 17 and 4.5±1.1 mg (g DW)−1 at stage 27. In PN inflorescences, the concentration of sucrose fluctuated irregularly between 0 and 7.1±4.1 mg (g DW)−1 during the whole flower development. From stage 15 to stage 17, the sucrose level was significantly higher in the inflorescences of GW than in those of PN. However at stage 17, the sucrose concentration was higher in the PN inflorescences.
Glucose was the most represented soluble sugar in the inflorescence during the entire flower development. Glucose concentration in cvs. GW and PN was higher in stage 12, at 32.7±7.8 and 43.5±12.5 mg (g DW)−1 inflorescences, respectively (Fig. 6c), decreasing to 15.8±0.8 and 22.7±5.2 mg (g DW)−1 in GW and PN, respectively, at stage 15, then remaining stable until stage 25. Finally, glucose concentration at anthesis (stage 25) fell slightly to 20.9±3.8 and 19.7±4.3 mg (g DW)−1 in GW and PN, respectively. Significant differences between the two cultivars were noticed at stages 15, 15+8d and at anthesis (stage 25). In all cases, glucose content was significantly higher in PN inflorescences than in those of GW.
Fructose concentration presented a similar profile during flower development in both GW and PN cultivars. The lowest fructose levels were observed at stage 12 [11.2±6.4 and 6.5±3.6 mg (g DW)−1, respectively] and at stage 27 [6.8±0.6 and 6.1±0.9 mg (g DW)−1, respectively; Fig. 6d]. Between these stages, fructose concentration was higher, ranging from 15 to 25 mg (g DW)−1, and significant differences between the two cultivars were observed at stage 15+8d. At this stage, fructose content was higher in PN inflorescences than in those of GW, at 24.5±1.6 and 20.4±2.4 mg (g DW)−1, respectively.
Discussion
Chronology of flower development
Following the whole of flower development, we showed that the ontogenesis of male and female organ is not synchronous in cvs. GW and PN. Both female and male meiosis occurred earlier in PN than in GW, the delay corresponding to 2–6 days in the ovule and 2 days in the anther. The criteria used to determine the stage of inflorescence development in grapevine are based on morphological traits (Eichhorn and Lorenz 1977) and do not necessary reflect the stages of development for the fertile organs, especially at key steps such as meiosis. For example, in cvs. Merlot, Cabernet-Sauvignon and Chardonnay, meiosis occurred 10, 15 and 16 days before anthesis, respectively (Fougère-Rifot et al. 1993). Therefore, the duration of reproductive development between meiosis and bloom is cultivar-dependent. These results are in agreement with previous data obtained in various cultivars of apple (Sato et al. 1988) or in apricot (Albuquerque et al. 2002).
Under unfavourable environmental conditions, asynchronous development of male and female gametophytes may have dramatic consequences on the success of fertilisation and the subsequent yield. In apricot, female sterility is partly due to delayed ovule development at anthesis and causes a low fruit set (Lillecrapp et al. 1999; Albuquerque et al. 2002). In the case of grapevine, male and female gametophytes were fully developed at anthesis under favourable conditions, enabling some pollination and fertilisation in GW (81.8±5.7%) and in PN (65.1±2.7%). However, when climatic stress occurs, the delay of ovule development in GW may generate development abnormalities and lead to abscission.
Carbohydrates in the inflorescences
The main differences in carbohydrate content in the inflorescences of GW and PN were detected between stages 12 and 17. This period coincides with (1) the occurrence of meiosis in male and female organs of both cultivars and (2) a strong transition in the whole plant physiology, since the carbohydrate source originating from root and trunk reserves is progressively replaced by photosynthesis in the leaves (Zapata et al. 2004). During this period starch and sucrose concentrations were higher in GW inflorescences, whereas glucose and fructose concentrations were higher in PN.
Under stress conditions, grapevine accumulates high amounts of starch, which participates in the resistance to unfavourable environmental conditions (Aït Barka and Audran 1996; Saladin et al. 2003). The amount of starch in the ovule at meiosis may be correlated to the sensitivity of grapevine cultivars to flower abscission. Indeed, between stages 15 and 17, starch was present in the ovule of PN but not in GW. In other species, it has been demonstrated that the initiation of ovule formation (Rodrigo et al. 2000), and achievement of ovule development, is conditioned by the presence of starch within the ovular tissues (Rodrigo and Herrero 1998). This strongly suggests that starch content in the female organ is crucial for ovule fate (Rodrigo et al. 2000; Jean and Lapointe 2001; Ruiz et al. 2001). In this respect, the greater sensitivity of GW to flower abscission when the plant is subject to drastic fluctuations in climatic conditions, may be correlated with the lack of amylaceous reserves in the ovule at key steps of development. However, the absence of starch in the ovule of GW does not seem to interfere with development under favourable conditions since the fruit set under such conditions is significantly higher.
Similarly, in the anther prior to meiosis (stage 12), starch is present in the male sporogenous tissue of PN but not in GW. It has been shown that the development of the male gametophyte may be affected under stress conditions, especially when the stress occurs close to meiosis (Dorion et al. 1996; Saini 1997). Again the presence of starch in the male gametophyte may enable PN to more easily counteract the damage caused by climatic changes and allow the proper achievement of pollen development.
At the onset of female and male meiosis, although the global amount of starch is higher in GW inflorescences, the anatomical analysis of starch repartition indicates that amylaceous reserves are more abundant in the ovules and anthers of PN. These differences are due to the level of starch stored in the stalks (data not shown), explaining the finding that higher amounts of starch could be stored in bunches of GW compared to those of PN.
A strong correlation has often been pointed out in woody plants between successful development of fertile sexual organs and the amount of carbohydrates available in the flower at various stages of development (Rodrigo et al. 2000; Jean and Lapointe 2001; Ruiz et al. 2001; Iglesias et al. 2003). Considering the differential sensitivity to flower abscission of cvs. GW and PN, the higher amount of soluble sugars in GW could explain the higher fruit set in GW under optimal conditions, whereas the presence of starch in PN ovules could contribute to the higher tolerance of this cultivar to flower abscission when abiotic stress cause changes in carbohydrate metabolism (Saini 1997).
References
Aït Barka E, Audran JC (1996) Response of grapevines to subzero temperatures: effect of controlled cooling on sugar reserves of the leaf bud complex preceding and during budding. Can J Bot 3:492–505
Alburquerque N, Burgos L, Egea J (2002) Variability in the developmental stage of apricot ovules at anthesis and its relationship with fruit set. Ann Appl Biol 141:147–152
Clément C, Burrus M, Audran JC (1996) Floral organ growth and carbohydrate content during pollen development in Lilium. Am J Bot 83:459–469
Clément C, Mischler P, Burrus M, Audran JC (1997) Characteristics of the photosynthetic apparatus and CO2-fixation in the flower bud of Lilium. II. Corolla. Int J Plant Sci 158:794–800
Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol 111:137–145
Eichhorn KW, Lorenz DH (1977) Phöenologische Entwicklungsstadie. Der Rebe. Nachrichtenbl Dtsch Pflanzenschutzdienstes (Braunschweig) 29:119–120
Fougère-Rifot M, Benharbit N, Brun O, Bouard J (1993) Relations entre le développement défectueux des ovaires et l’involution des ovules chez Vitis vinifera L. var. Chardonnay. J Int Sci Vigne Vin 27:99–112
Glad C, Regnard JL, Querou Y, Brun O, Morot-Gaudry JF (1992) Flux and chemical composition of xylem exudates from Chardonnay grapevines: temporal evolution and effect of recut. Am J Enol Viticult 43:275–282
Gregory PJ, Palta JA, Batts GR (1996) Root systems and root:mass ratio—carbon allocation under current and projected atmospheric conditions in arable crops. Plant Soil 187:221–228
Gu S, Lombard P, Price S (1996) Effect of shading and nitrogen source on growth, tissue ammonium and nitrate status and inflorescence necrosis in Pinot noir grapevine. Am J Enol Viticult 47:173–180
Huglin P, Schneider C (1998) In: Lavoisier (eds) Biologie et écologie de la vigne. Tech and Doc, Paris
Iglesias DJ, Tadeo FR, Primo-Millo E, Talon M (2003) Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiol 23:199–204
Jackson DI (1991) Environmental and hormonal effects on development of early bunch stem necrosis. Am J Enol Viticult 42:290–294
Jean D, Lapointe L (2001) Limited carbohydrate availability as a potential cause of fruit abortion in Rubus chamaemorus. Physiol Plant 112:379–387
Lillecrapp AM, Wallwork MA, Sedgley M (1999) Female and male sterility cause fruit set in a clone of the ‘Trevatt’ variety of apricot (Prunus armeniaca). Sci Hortic 82:255–263
Miyazawa SI, Terashima I (2001) Slow development of leaf photosynthesis in an evergreen broad-leaved tree, Castanopsis sieboldii: Relationships between leaf anatomical characteristics and photosynthetic rate. Plant Cell Environ 24:279–291
Nishizawa T, Shishido Y (1998) Changes in sugar and starch concentrations of forced June-bearing strawberry plants as influenced by fruiting. J Am Soc Hortic Sci 123:52–55
Prymakowska-Bosak M, Przewloka MR, Slusarczyk J, Kuras M, Lichota J, Kilianczyk B, Jerzmanowski A (1999) Linker histones play a role in male meiosis and the development of pollen grains in tobacco. Plant Cell 11:2317–2329
Rodrigo J, Herrero M (1998) Influence of intraovular reserves on ovule fate in apricot (Prunus armeniaca L.). Sex Plant Reprod 11:86–93
Rodrigo J, Hormaza JI, Herrero M (2000) Ovary starch reserves and flower development in apricot (Prunus armeniaca). Physiol Plant 108:35–41
Ruiz R, Garcia-Luis A, Honerri C, Guardiola JL (2001) Carbohydrate availability in relation to fruitlet abscission in Citrus. Ann Bot 87:805–812
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Saladin G, Magné C, Clément C (2003) Impact of flumioxazin herbicide on growth and carbohydrate physiology in Vitis vinifera L. Plant Cell Rep 21:821–827
Sato M, Kanbe K, Nakagawa S, Yuda E, Fukunaga S (1988) Studies on development of the embryo sac and its abnormality in the triploid apple cultivar ‘Mutsu’. J Jpn Soc Hortic Sci 57:366–372
Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V (2000) The dyad gene is required for progression through female meiosis in Arabidopsis. Development 127:197–207
Truernit E (2001) Plant physiology: the importance of sucrose transporters. Curr Biol 11:169–171
Vemmos SN, Goldwin GK (1994) The photosynthetic activity of Cox’s orange pippin apple flowers relation to fruit setting. Ann Bot 73:385–391
Voznesenskaya EV, Franceschi VR, Pyankov VI, Edwards GE (1999) Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae). J Exp Bot 50:1779–1795
Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol 35:253–289
Yu TS, Lue WL, Wang SM, Chen JC (2000) Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiol 123:319–325
Zapata C, Deléens E, Chaillou S, Magné C (2004). Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.). J Plant Physiol (in press)
Acknowledgements
The authors thank Mumm-Perrier-Jouet Vignobles et Recherches (Epernay, France) and the RVVS (Réseau Vigne et Vins Septentrionaux) for their financial support, and F. Gimenez and E. Perrin for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebon, G., Duchêne, E., Brun, O. et al. Flower abscission and inflorescence carbohydrates in sensitive and non-sensitive cultivars of grapevine. Sex Plant Reprod 17, 71–79 (2004). https://doi.org/10.1007/s00497-004-0217-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-004-0217-9