Abstract
This study evaluated the impact of combined stressors (heat and nutritional stresses) on the growth and adaptive capability of Sahiwal (SW) and Karan Fries (KF) calves during the summer season. Calves in each breed were randomly divided into four groups. In SW breed the groupings were as follows: SWC (n = 4; Sahiwal Control); SWHS (n = 4; Sahiwal Heat Stress); SWNS (n = 4; Sahiwal Nutritional Stress) and SWCS (n = 4; Sahiwal Combined Stresses). Likewise, in the KF breed, KFC (n = 4; Karan Fries Control); KFHS (n = 4; Karan Fries Heat Stress); KFNS (n = 4; Karan Fries Nutritional Stress), and KFCS (n = 4; Karan Fries Combined Stresses). Control (C) and Heat Stress (HS) calves were fed ad libitum while Nutritional Stress (NS) and Combined Stresses (CS) calves were fed restricted feed (50% of C calves of respective breed) to induce nutritional stress in both the breeds. SWHS, SWCS, KFHS, and KFCS were exposed to summer heat stress from 1000 to 1600 h. All growth and adaptation variables were recorded at fortnightly intervals. Respiration rate, pulse rate, and rectal temperature during the afternoon were significantly (P < 0.01) higher in the CS group in both breeds. Further, CS had significantly (P < 0.05) higher plasma growth hormone and cortisol levels. Insulin-like growth factor-1, Triiodothyronine, and Thyroxine levels significantly decreased (P < 0.05) in the CS group in both breeds. Interestingly, heat stress didn’t affect SWHS and KFHS bodyweight, however, a significant (P < 0.05) decrease in body weight of SWCS and KFCS was observed when compared with C. Hepatic mRNA expression of growth hormone, insulin-like growth factor-1, and growth hormone receptor significantly (P < 0.05) varied when compared between C and CS groups in both the breeds. The overall magnitude of stress was more pronounced in KF compared to the SW breed. This study concludes that when two stressors occur concurrently, they may have a greater influence on the adaptive capability of calves. Further, SW had better tolerance levels than KF, confirming the indigenous breed's superiority over cross-bred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By 2050, the world's population is projected to grow from 7.2 billion to 9.6 billion (UN 2013). To fulfill the global food demand by 2050, few estimates anticipate an 50 to 70 percent increase in global food productivity (Ingram 2011). Likewise, global demand for agricultural products is also anticipated to increase dramatically during the same period, by roughly 70% (FAO 2009a), owing primarily to an increase in living standards. Besides, the need for animal-based consumables to fulfill the global food demand necessitates an increase in animal production on a global scale over the next few decades. But, according to the intergovernmental group of experts, an increase in global temperature will impact the present ecosystems, various agricultural animals, and lastly, global food security (Bernabucci 2019). We can therefore expect that all animal production systems, whether they are based on pasture, mixed farming, or industrialized methods, will be greatly impacted by climate change, especially global warming.
Cattle reared in a tropical environment, alongside heat stress, are also subjected to multiple environmental stresses like nutrition stress, walking stress, overcrowding stress, and many more in an extensive rearing system (Sejian et al. 2013). When animals are subjected to a single stressor, the effects can be dramatic, depending on its intensity, duration, and the state of the animals. It can overwhelm their ability to cope. However, if animals are subjected to multiple stressors, they are more likely to experience detrimental effects. Multiple environmental stresses deplete the stored animal reserve eventually, which are necessary for maintaining homeostasis, hence resulting in hindered growth and impaired adaptive capabilities in animals (Sejian et al. 2012).
With the current climate change scenario, temperature fluctuations enhance the requirement for energy for sustenance and survival; yet, the insufficient supply and quality of fodder in grazing areas restricts the energy intake, causing an energy crisis, and loss of productivity in livestock (Shilja et al. 2016). In addition, in plants, cellular damage and cell death can occur due to long-term exposure to high temperatures (Hu et al. 2020), which might hamper pasture availability including both the quality and quantity of available feed resources. Further, it also leads to an increase in lignin content in plants (Polley et al. 2013), which reduces the digestibility and degradation rate in cattle. This suggests that the decline in livestock productivity during extreme summers is due in part to nutritional stress in addition to heat stress. The crucial consideration here is that these two stressors do not occur separately but rather simultaneously, influencing an animal’s adaptive behavior (Shilja et al. 2016).
Multiple stressors cause dairy cattle to adapt metabolically by modulating physiological variables, behavioral reactions, and genotypic variables; however, this reduces productivity. Unfortunately, most of the research on dairy cattle is concentrated only on heat stress rather than multiple stresses. Particularly in tropical countries, it is imperative that the animals are commonly exposed to multiple environmental stressors, and therefore it is vital to quantify the cumulative impacts of these stressors together on dairy cattle. Very limited information is available on the influence of multiple stressors on growth and adaptive responses in calves because it is very difficult to manage conventional experiments covering multiple stressors. Further, little information available on this line was restricted to small ruminants (Sejian et al. 2012, 2015). Therefore, it is essential to design a study in dairy cattle to generate baseline information on the multiple stressors concept as this could play a vital role in determining the intervening points for amelioration. Such an effort would be very valuable for defining future policies for dairy cattle. With this background, a study was designed with the primary objective to quantify the cumulative impacts of heat and nutritional stress on growth performance and adaptive capabilities in dairy calves. Efforts were also made to comparatively assess the response mechanisms of indigenous and crossbred animals for these cumulative stress impacts.
Materials and methods
Study site
The study was conducted at the Climate Resilient Livestock Research Centre (CRLRC), ICAR-NDRI, Haryana, India, which is located in the arid to semi-arid region of the country with a latitude and longitude of 29° 41' N and 76° 59' E and, at an elevation of 243 m above mean sea level. The annual average maximum and minimum ambient temperature range between 0 °C and 45 °C. Relative humidity in the region ranges between 31 and 82%. The annual rainfall in this area is around 600-750 mm.
Animals and accommodation
The study was conducted on healthy Sahiwal (SW) (Indigenous breed) (n = 16) and Karan Fries (KF) (Holstein Friesian X Tharparkar) (n = 16) female calves of 10–12 months of age, weighing between 91 and 95 kg. The animals were acclimatized for 30 days before the actual experiment in the study area (NICRA modern state of art shelter shed). The animals were housed in a well-ventilated shed (east-to-west orientation) of insulated roofing at a height of 7.62 m at the center and 3.81 m at the sides and partly open from the side and maintained under proper hygienic conditions. The side openings were covered with wet gunny bags to prevent entry of hot air in the shed. Further, ceiling fans were installed in shed to improve air circulation and reduce the heat load on animals. The animals were housed in head to head housing system arranged with the sand beds. The experimental animals had ad libitum access to good-quality drinking water. Before the study, prophylactic measures against cattle diseases were carried out as prescribed by the health calendar of the CRLRC, ICAR-NDRI, Karnal to ensure that the animals were in a healthy condition throughout the study.
Technical details
The study was conducted for a period of 90 days during the summer season (May–July). Calves in each breed were randomly distributed into four groups. In SW breed the groupings were as follows: SWC (n = 4; Sahiwal Control); SWHS (n = 4; Sahiwal Heat Stress); SWNS (n = 4; Sahiwal Nutritional Stress) and SWCS (n = 4; Sahiwal Combined Stresses) (Thermal and Nutritional Stress). Likewise, in the KF breed, the groupings were as follows: KFC (n = 4; Karan Fries Control); KFHS (n = 4; Karan Fries Heat Stress); KFNS (n = 4; Karan Fries Nutritional Stress), and KFCS (n = 4; Karan Fries Combined Stresses) (Thermal and Nutritional Stress). The calves were stall-fed individually with a diet consisting of roughage (maize) and concentrate mixture in the ratio 60:40 as per routine practices. Table 1 describes the feed ingredients and the chemical composition of the feed offered to the animals. SWC, SWHS, KFC, and KFHS groups were fed with ad libitum feeding while SWNS, SWCS, KFNS, and KFCS groups were provided with restricted feed (fifty percent of intake of C calves in each breed) to induce nutritional stress. The SWHS, SWCS, KFHS, and KFCS groups were exposed to outside summer heat stress between 1000 to 1600 h, whereas the SWC, SWNS, KFC, and KFNS groups were maintained under the shed. All animals were fed and watered individually throughout the study period. All growth and adaptation variables were recorded at fortnightly intervals.
Variables studied
Meteorological data
Micro and macro environment climatic data viz., dry and wet bulb temperature in degrees Celsius (ºC) was measured at 0730 h and 1430 h by analog hygrometer (Zeal, UK) throughout the experimental period both outside and inside the shed. Temperature-Humidity Index (THI) was calculated from the dry bulb and wet bulb temperature using the following formula (McDowell 1972):
where Tdb = Dry bulb temperature (ºC) and Twb = Wet bulb temperature (ºC).
Physiological variables
All physiological variables were recorded at fortnightly intervals during both morning and afternoon. The respiration rates (RR) of each animal were recorded by counting the inward and outward movement of the flank while all the calves were in a standing position. Immediately after RR, pulse rates (PR) of the calves were measured by palpating the pulsation of the middle coccygeal artery at the base of the tail head. The rectal temperature (RT) was recorded using a clinical digital thermometer by keeping the thermometer in contact with the rectal mucosa for almost a minute.
Plasma variables
Blood samples were collected at fortnightly intervals from all the groups at 0800 h (before offering feed) in sterile heparinized vacutainer tubes (BD, Franklin Lakes NJ, USA) through jugular vein puncture, posing minimal disturbance to the animal. Blood samples were immediately centrifuged at 2,500 rpm for 25 min to separate the plasma, which was stored at -20 °C for further analysis of plasma hormonal parameters.
Plasma variables like Growth hormone (GH), Insulin-like Growth Factor-1 (IGF-1), Cortisol, Triiodothyronine (T3), and Thyroxine (T4) were estimated by enzyme-linked immunosorbent assay (ELISA) using a microplate reader (Thermo Scientific, Finland) by ELISA kits (Bioassay Technology Laboratory, Shanghai, China). The analytical sensitivity of the kits were; GH-0.026 ng/ml; IGF1-0.53 ng/ml; Cortisol-0.02 ng/ml; T3- 0.01 ng/ml and T4- 2.61 ng/ml. The intra-assay and inter-assay coefficients of variations of all the ELISA kits were < 8% and < 10%.
Body weight and measurements
Body weights (BW) and body measurements of experimental animals were recorded at fortnightly intervals using a computerized weighing bridge (Leotronic Scales Pvt. Ltd) and measuring tape during the morning before offering feed. Body measurements like body length (BL) were measured considering the oblique distance from the point of shoulder to pin bone; heart girth (HG) was measured considering the circumference of the thorax just behind the point of the elbow; Height at withers (HW) was measured considering the vertical distance from the point of the hoof of the foreleg to the top of the withers (highest point of withers) and height at rump (HR) was measured considering the vertical distance from the hoof of the hind leg to the pelvis tuber sacrale.
Liver biopsy
Liver biopsies were performed on all calves at end of the experiment with a 14G × 6″ disposable Clear needle ™ biopsy needle (NewTech Medical devices, New Delhi, India) according to the surgical and post-surgical procedures mentioned by Singh et al. (2019). Liver biopsies samples were rinsed in saline and transferred to a micro-centrifuge tube containing 3 mL of Trizol reagent (Invitrogen, Corp., CA, USA), frozen in liquid nitrogen and stored at -80 °C pending mRNA extraction and analysis.
RNA isolation, cDNA synthesis, and qPCR from liver samples
Total RNA was isolated from liver samples (n = 4 in each group) using Trizol reagent according to the manufacturer’s instructions (Invitrogen, Corp., CA, USA). RNA concentration and purity were checked using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, USA) and Experion Bio-analyzer (Bio-Rad, USA). The OD 260/280 absorption ratio for different samples range was 1.92 to 2.10. Further, cDNA was synthesized using the Revertaid First strand cDNA synthesis kit (Thermo Scientific, CA, USA), using a total of 200 ng RNA for each sample. The primers for gene expression analysis were designed using Primer 3.0 software. The details of the primer sequences are provided in Table 2. Before using them as templates for qPCR, each of the cDNA samples was diluted 1:5 (v:v) with DNase/RNase-free water. The qPCR reactions were performed with a total reaction volume of 10 µL consisting of 4 µL of diluted cDNA, 5 µL (2X) Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Thermo Fisher Scientific), 0.4 µL each of forward and reverse primers (10 µM) and 0.2 µL DNase/RNase-free water. The reactions were performed in duplicates and qPCR amplification reaction conditions were: 10 min at 95ºC, 40 cycles of 15 s at 95ºC (denaturation), and 1 min at 60ºC (annealing + extension). The relative gene expression data was analyzed using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
The data were analyzed by the general linear model (GLM) repeated measurement analysis of variance (SPSS 20.0). The effect of fixed factors, namely breed (SW and KF) and treatments (C, HS, NF, and CS), was considered as between-subjects factors and days (day 0, 15, 30, 45, 60, 75, and 90) were considered as within-subjects factor and also the interaction between breed, treatments and days was analyzed on various parameters studied. Comparison of means of the different subgroups was made by Duncan's multiple range tests as described by Kramer (1957). The changes in the relative expression of hepatic GH, IGF-1, and GHR mRNA to GAPDH were analyzed by Two-way analysis of variance (ANOVA). The results are shown as mean ± standard error (SE). The significant level was set at P < 0.05.
Results
Meteorological data
Figure 1 presents the average THI values during the study period. Experimental calves were exposed around mean values of 84.88 and 77.65 units outside and inside the installation during the afternoon, indicating severe stress load outside the shed. Whereas, in the morning, THI values were 77.82 and 71.09 units, outside and inside the installation, respectively, indicating no stress or mild stress. The greatest THI was recorded during the month of June, with 79.39 and 86.04 units during morning and afternoon outside the installation, respectively. According to McDowell (1972), THI exceeding 78 is considered as severe stress in cattle.
Physiological responses
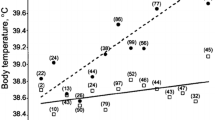
The physiological responses across the experimental groups during both morning and afternoon are described in Table 3. All factors namely breed, treatment, and experimental days significantly (P < 0.01) influenced respiration rate morning (RRM), respiration rate afternoon (RRA), pulse rate morning (PRM), pulse rate afternoon (PRA), and rectal temperature afternoon (RTA), during the study period. During the afternoon, there was a significant (P < 0.05) difference in the RR between groups of the same treatment; the values were highest in HS, followed by CS, C, and NS. At a cursory look, both PRM and PRA had a significant (P < 0.05) difference in values between groups of the same treatment; further, a similar (P < 0.05) trend like RR was observed in PR (HS > CS > C > NS). RTM in SWNS was significantly (P < 0.05) lower than the rest of the groups. However, during the afternoon HS group of both breeds had significantly (P < 0.05) higher RT. The breed x treatment x days (BxTxD) interaction did not influence RRM, RRA, PRA, and rectal temperature morning (RTM). However, PRM and RTA were significantly (P < 0.05) influenced by BxTxD interaction. To conclude the magnitude of the impact of the stressors on physiological responses was higher in KF compared to SW calves.
Endocrine responses
The endocrine responses across the experimental groups during the study period are summarized in Table 4. The breed, treatment, and experimental days factors significantly (P < 0.01) influenced plasma GH, plasma IGF-1, plasma cortisol, plasma T3, and plasma T4 levels in experimental animals. When compared between groups of the same treatment, plasma GH levels were significantly (P < 0.05) higher in KF, particularly in the CS group, whereas SWC had the lowest plasma GH value. Parallelly, plasma IGF-1 in all the groups differed significantly (P < 0.05) within breeds and between breeds of the same treatment. Especially C group had the highest plasma IGF-1 levels followed by HS, NS, and CS in declining order in both the breeds. Both individual stresses (HS & NS) as well as cumulative stressors (CS) up-surged the plasma cortisol levels in KF when compared with SW. A significant (P < 0.01) trend of plasma cortisol levels was observed in the CS group with lowering levels of plasma cortisol recorded in KF after the 45th day while in SW the plasma cortisol levels kept increasing during the experimental days. Further, to compare between groups of the same treatment, all the experimental groups of SW had significantly (P < 0.05) higher plasma T3 and T4 levels than the KF groups. However, NS did not influence the plasma T4 levels in the SW and KF breeds. BxTxD interaction was significantly (P < 0.01) evident in plasma IGF-1, cortisol, and T3, respectively.
Body weight and measurements
The impact of stressors on body weight and body measurements during the study period is described in Table 5. The breed, treatment, and experimental day factors significantly (P < 0.01) influenced the BW of animals. Among the individual stresses, HS did not influence BW in both breeds while NS, followed by CS significantly (P < 0.05) decreased BW in both breeds. Treatment (P < 0.01), experimental days (P < 0.01), and interaction between BxTxD (P < 0.05) factors significantly influenced HG. SWNS had significantly (P < 0.05) lower HG compared to SWC. Likewise, the CS significantly (P < 0.05) decreased HG in both breeds when compared with controls of the respective breed. Similar to HG, a significant (P < 0.01) influence of the HS, NS, and CS on BL was observed in both the breeds in factors like treatment and experimental days. A major (P < 0.05) impact of CS on BL was observed only in KFCS when compared to KFC. Both individuals as well as cumulative stressors significantly (P < 0.05) lowered HW when compared to C in KF while the HW remained intact in all SW groups. In addition, the experimental days also significantly (P < 0.01) influenced the HW during the study period. The breed and experimental days factors significantly (P < 0.01) influenced HR in both breeds. The HR remained intact in both the breeds across all the individual as well as combined stressors groups. With the numerically higher in SW compared to KF.
Molecular parameters
Figure 2 presents the impact of individual stresses (HS and NS) and cumulative stressors (HS + NS) on hepatic mRNA expression during the study period. A significant difference (P < 0.01) was observed in the hepatic mRNA expression pattern of Growth hormone (GH), Insulin-like growth factor-1 (IGF-1), and Growth hormone receptor (GHR) between group and breed factors however, there was no significant difference between the breed and group interactions. There was a significant down regulation of GH, IGF-1, and GHR genes in the CS group in both breeds compared to C, respectively.
Discussion
Climate change has a direct effect on the reliability of food supply networks, both locally and internationally. Particularly in a tropical environment, climate change is a common cause of multiple stresses that impair the performance of livestock. However, better comprehension of the interplay between livestock and multiple stressors is required to enhance the resilience of livestock (Shashank et al. 2021). The present experiment establishes the impact of combined stresses on the growth and adaptive capability of Karan Fries and Sahiwal calves in the tropical environment.
Physiological responses are significantly exhibited primarily in response to increased core body temperature in heat-stressed animals as an attempt to re-establish homeostasis (Indu and Pareek 2015). In the present study, RR was considerably higher in both the breeds' of HS and CS groups, especially during the afternoon. It is consistent with the findings of Kovács et al. (2018), where RR peaked at noon as opposed to the morning in calves. Further, when ewes were exposed to multiple stressors, Sejian et al. (2012) observed a similar elevation in RR. Resting RR in calves is approximately 30 breaths per minute, as reported by Jackson and Cockcroft (2002). However, our data shows that RR in calves had up surged to 2–3 times the typical RR, except for SWNS. Notably, the KFHS's RR (101.92 ± 11.99) is far higher than that of comparable groups. However, in contrast to KF, SW calves were able to maintain the RR, demonstrating their superiority in heat dissipation. Further, the HS group had higher RR than the CS group in both breeds, which might be due to increased metabolic heat production as HS groups were fed ad libitum, coupled with the environmental heat load. The amount of metabolic heat production in animals is determined by the volume of feed intake (Ando et al. 1997). Conversely, the NS group in both breeds had lower RR than other groups might be due to less metabolic heat production. Bharti et al. (2018) recorded similar RR results in restricted-fed cows. The PR reflects the homeostasis of general circulation and metabolic status (Sejian et al. 2010a). PR positively correlates with RT, per Mishra et al. (1995). HS and CS groups in both breeds exhibited higher PR, especially in the afternoon, suggesting heat load dissipation by augmented peripheral circulation. Similar results were observed by Shilja et al. (2016) in Osmanabadi goats subjected to combined (heat and nutritional stressors). The average PR of KF heifers in the thermal comfort zone ranges between 69.90 to 85.71 beats/min (Banerjee and Ashutosh 2011). In contrast, our results indicated a higher PR, validating the Karan Fries calves' poor response to the thermal environment by boosting circulation to dissipate more heat. This mechanism proposes the commitment of heat loss mechanism for poorly adapted breeds (Gaughan et al. 1999). The NS group had lower PR. Similar results were observed by Samad et al. (2014) when animals were fed a 50% limited concentrate diet.
The core body temperature results from all thermal regulation processes in animals and RT is an excellent thermal regulation index (Yousef 1985). Theurer et al. (2014) confirm a significant positive correlation between RT and THI in heifers. The average rectal temperature in calves ranges between 38.5–39.5ºC (101.3–103.1ºF) (Jackson and Cockcroft 2002). Compared to that reference, KFHS and KFCS survived 41.66–42.22ºC, whereas SWHS and SWCS maintained 39.44–40.00ºC, validating the efficiency of indigenous animals thriving in tropical environments. This might be due to differences in sweat glands' number (and activity) and hair coat characteristics (Olson et al. 2006). Furthermore, according to Gaughan et al. (1999), genetic adaptation enables Bos indicus cattle to have less RT than Bos taurus undergoing heat stress. Based on physiological results, fluctuations in RR, RT, and PR may indicate the calves' metabolic condition when subjected to multiple stressors.
Reduced feed intake is thought to be the primary mechanism by which heat stress affects production in animals (Collier and Beede 1985). Hyperthermically induced physiological and endocrine responses and elevated maintenance requirements in animals (Collier et al. 2005) may divert energy from growth to maintaining homeostasis. Animal metabolism and physiology are regulated by homeorhetic hormones like GH and IGF-1. When an animal's biological reserves are depleted during various physiological processes, uncoupling of somatotropic axis hormones (GH and IGF-1) facilitates homeorhetic modifications to maintain homeostasis (Bauman and Vernon 1993). In our study, plasma GH concentrations in the CS groups have increased significantly; conversely, plasma IGF-1 concentrations were reduced. NS groups tailed the CS groups. This uncoupling mechanism might be due to reduced hepatic GH receptors or reduced binding of GH to its receptors (Pulina et al. 2012). According to Schams et al. (1989), the normal concentration of plasma GH in cattle ranges around 3 to 30 ng/ml, depending upon age sex, and lactation stage. Parallelly, plasma IGF-1 concentrations of heifers in our study are in accordance with the range recorded by Kerr et al. (1991) in heifers from birth to 18 months. In our studies, though the plasma GH and IGF-1 concentration are in the vicinity of the normal range, elevated plasma GH and decreased IGF-1 concentrations can be appreciated in KFNS and KFCS when compared with SWNS and SWCS indicating lipolysis and energy mobilization towards combating severe negative energy balance.
IGF-1 synthesis relies on the number of GH receptor binding sites in the liver. During feed restriction, the liver has refractory effects on GH, resulting in reduced IGF-1 levels and conversely increased GH production due to IGF-1's negative feedback on hypothalamic growth production (Breier and Gluckman 1991). Additionally, the drop in plasma IGF-1 levels may be attributable to decreased GH receptors through which STAST-5 signaling occurs, resulting in reduced hepatic IGF-1 mRNA synthesis (Bernabucci et al. 2010). Further, negative energy balance promotes GH production, subsequently increasing lipolysis in response to β-adrenergic receptors in adipocytes and inhibiting insulin-mediated lipogenesis and glucose utilization by enhancing free fatty acid generation from adipose tissue (Baumgard and Rhoads 2013). The degree of negative energy balance and lipid mobilization in calves exposed to CS may account for weight loss. Particularly in KF, the production of GH was greater than that of SW, especially in CS groups representing the state of severe negative energy balance and the process of energy utilization leading to mobilization of body reserves to maintain survival functions.
CS had a considerable decrease in metabolic hormones such as T3 and T4 compared to C due to reduced metabolic activity to reduce heat production during cumulative stresses. Calves are likely adapting to cumulative stress by reducing their thyroid function, which minimizes metabolic heat output (Sejian et al. 2010a, b). The thyroid gland regulates protein and energy metabolism along with the production of hormones (Sejian et al. 2010a, b). Thus, thyroid hormone concentrations reflect the animal's metabolic and nutritional state and are positively correlated with growth (Aleena et al. 2016). Glucocorticoid levels can increase in part because of a reduction in thyroid hormones in calves. Particularly in the KF breed, T3 and T4 concentrations in the CS group were significantly lower than SWCS. According to Doornenbal et al. (1988), the normal T3 and T4 levels of young cattle around 12.5 months were 2.3 ng/ml and 87.94 ng/ml, respectively. On comparing these values with our results, it validates KFCS had very less T3 and T4 values; however, these values are subjected to breed variation. Additionally, this explains the superior adaptive capability of SW even on exposure to cumulative stresses the animals were able to sustain metabolic hormones production. T3 was drastically reduced in KFNS on about the 45th day, but in SWNS it decreased gradually. According to Cassar-Malek et al. (2001) maintaining the concentrations of T3 during feed restriction could be metabolically necessary for growing steers. This is consistent with our findings that SWNS attempted to maintain T3 levels throughout the trial, indicating that even on feed restriction, SW can sustain metabolism. SW outperformed KF in thyroid hormones even in HS groups, along with NS. SW's heat dissipation mechanism may allow metabolic activity to remain unaffected by heat stress. It also confirms that an optimal diet for indigenous animals may maintain growth and metabolism even during heat stress.
Cortisol is considered one of the primary stress hormones in ruminants which provokes physiological modifications in animals to tolerate stress. During stress, cortisol works on tissues to ensure they have a steady source of energy. Cortisol stimulates adipose tissue to produce fatty acids, which serve as energy for the tissues. The importance of these metabolic changes is to restore the blood glucose level and improve glycogen stores to combat stress in animals. This mechanism was evident in SWCS. KFCS had a reduction in plasma cortisol levels after the 45th-day exposure, whereas, this pattern was not observed in SWCS. This portrays the superior adaptability of SW to multiple stressors. SW calves were able to regulate plasma cortisol levels even when exposed to combined stresses, whereas KFCS started undermining cortisol's basic function, depicting the impact of multiple stressors on them. These findings contradict the results of Sejian et al. (2010ab); however, this may be attributed to the species' adaptability. In our study calves produced plasma cortisol to provide energy to tissues, while sheep in the CS group lowered plasma cortisol to regulate body temperature. According to Henricks et al. (1984), the normal ranges of cortisol in heifers of 7 to 12 months were between 2.9 to 6.0 ng/ml. This coincides with the control values in our study. However, on comparing SWC and KFC with SWCS and KFCS, we can appreciate a significant (P < 0.05) variation depicting stress load on CS groups. Feed-restricted calves also showed a progressive increase in plasma cortisol levels. This is per the results of Ronchi et al. (2001), where thermal comfort calves fed a restricted diet showed greater plasma cortisol levels than thermal comfort ad libitum calves. As noted, NS groups were maintained under the shed, thus they did not need the energy to cope with heat stress, unlike HS and CS groups. Compared to SWNS, KFNS showed greater plasma cortisol levels, depicting the impact of feed restriction.
Body weight and measurements are positively correlated (Ozkaya and Bozkurt 2009). Combined stressors significantly impacted experimental calves’ body weight and measurements. Interestingly, in both breeds, there was no significant difference between the C and HS groups. These findings are consistent with Nonaka et al. (2008), where body weight was not affected by heat stress. Similar results were obtained by Sejian et al. (2010ab), where there was no significant difference between the control and heat stress groups of Maplura ewes. It might be attributed to the reduction in the passage rate in the gastrointestinal tract as the environmental temperature increases (Christopherson 1985; Beede and Collier 1986). In addition, during heat stress, contraction amplitude, and frequency decreases leading to reduced rumen motility (Bernabucci 2012). Further HS groups were not exposed to the consumption of straw unlike NS and CS groups, which reduces the palatability and digestibility. This is in line with the results of Mathers et al. (1989), where dry matter digestibility in Ayrshire cattle was similar at 33ºC and 20ºC, providing a good quality diet. On the other hand, body weights in restricted groups were either maintained or reduced. This might be mediated by an increase in growth hormone in cattle (Bauman and Currie 1980), which is evident in our study. However, the mechanism(s) by which body weight is reduced in growing ruminants exposed to thermal load has not been established clearly (O’Brien et al. 2010).
The liver carries outs various vital roles in the body such as the expression of genes encoding plasma proteins, clotting factors, enzymes involved in detoxification, gluconeogenesis, glycogen synthesis, and the metabolism of glucose, lipids, and cholesterol (Jungermann and Katz 1989). Narrowing down to growth, in cattle and other livestock species, genes encoding GH, IGF-1, and GHR have been associated with physiological growth pathways (Angel et al. 2018). In this line, the results of this study might provide some essential preliminary data on the expression of various genes related to growth in calves subjected to combined stressors. As mentioned previously, the number of GHR binding sites in the liver available is critically essential for the production of hepatic IGF-1. According to Collier et al. (2008), heat stress decreased the abundance of GHR in the liver, further due to a reduction in GH signaling through the STAT-5 pathway, hepatic IGF-1 mRNA abundance was found to be reduced in animals exposed to heat stress and underfed thermo neutral animals. Similar results were validated in our study, where CS and NS groups in both the breed had reduced GH mRNA production further leading to a reduction in GHR mRNA abundance which resulted in reduced IGF-1 mRNA production. To further validate, Rhoads et al. (2010) observed a positive relationship between GHR and IGF-1 gene expression in animals exposed to heat stress, where all the gene expressions had reduced to a similar extent. In the same experiment, they observed a reduction in intracellular GH signaling through a reduction in both GHR mRNA and protein abundance. The mechanism(s) for the fluctuations in GHR gene expression during heat stress and nutritional deficiency has been not properly resolved. However, one possibility might be related to increased levels of stress hormones (e.g., cortisol) observed during heat stress and the nutritional deficiency (Collier and Beede 1985), which are in accordance with our results. We witnessed a slight reduction in GHR, IGF-1, and GH gene expressions in HS groups, as they were well-fed; however, the magnitude was higher in NS and CS groups. Further studies might help in solving the mysteries behind the GHR, IGF-1, and GH gene expressions and associated genes during heat stress and nutritional deficiency in calves.
Conclusion
The study established that calves subjected to CS had a more detrimental effect on growth and adaptive capability compared to other stresses. Hence, it is very appropriate to conclude that when two stressors occur simultaneously, the total impact on biological functions necessary to adapt to stressful conditions may be severe. Various studies validate the heat tolerance (individual stress) superiority of indigenous animals over crossbred or exotic animals; additionally, this study confirms the ability of indigenous animals to counter multiple stressors with minimal production losses over cross bred animals. This study also suggests an alternative to counter the heat stress in calves by providing optimum nutrition so that growth can be achieved, thus reducing farmers' financial losses during heat stress conditions.
References
Aleena J, Pragna P, Archana PR et al (2016) Significance of Metabolic Response in Livestock for Adapting to Heat Stress Challenges. Asian J Anim Sci 10:224–234. https://doi.org/10.3923/ajas.2016.224.234
Ando S, Mundia MC, Nakamura Y, Yamamoto S (1997) Effect of Feed Intake Levels on Heart Rate, Heat Production, and Mean Body Temperature of Holstein Heifers. Nihon Chikusan Gakkaiho 68:177–184. https://doi.org/10.2508/chikusan.68.177
Angel SP, Bagath M, Sejian V et al (2018) Expression patterns of candidate genes reflecting the growth performance of goats subjected to heat stress. Mol Biol Rep 45:2847–2856. https://doi.org/10.1007/s11033-018-4440-0
Banerjee D, Ashutosh (2011) Circadian changes in physiological responses and blood ionized sodium and potassium concentrations under thermal exposure in Tharparkar and Karan Fries heifers. Biol Rhythm Res 42:131–139. https://doi.org/10.1080/09291011003729411
Bauman DE, Bruce Currie W (1980) Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J Dairy Sci 63:1514–1529. https://doi.org/10.3168/jds.S0022-0302(80)83111-0
Bauman DE, Vernon RG (1993) Effects of Exogenous Bovine Somatotropin on Lactation. Annu Rev Nutr 13:437–461. https://doi.org/10.1146/annurev.nu.13.070193.002253
Baumgard LH, Rhoads RP (2013) Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci 1:311–337. https://doi.org/10.1146/annurev-animal-031412-103644
Beede DK, Collier RJ (1986) Potential Nutritional Strategies for Intensively Managed Cattle during Thermal Stress. J Anim Sci 62:543–554. https://doi.org/10.2527/jas1986.622543x
Bernabucci U (2019) Climate change: impact on livestock and how can we adapt. Anim Front 9:3–5. https://doi.org/10.1093/af/vfy039
Bernabucci U, Lacetera N, Baumgard LH et al (2010) Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 4:1167–1183. https://doi.org/10.1017/S175173111000090X
Bernabucci U (2012) Impact of Hot Environment on Nutrient Requirements. In: Collier RJ, Collier JL (eds) Environmental Physiology of Livestock, 1st edn. Wiley, 101–128. https://doi.org/10.1002/9781119949091.ch7
Bharti M, Pandey S, Singh G et al (2018) Effect of Feed Restriction on Physio-Biochemical Changes, Milk Yield and Expression Profile of Leptin in Crossbred Cow. Int J Livest Res 8:21–32. https://doi.org/10.5455/ijlr.20180521073544
Breier BH, Gluckman PD (1991) The regulation of postnatal growth: nutritional influences on endocrine pathways and function of the somatotrophic axis. Livest Prod Sci 27:77–94. https://doi.org/10.1016/0301-6226(91)90047-T
Cassar-Malek I, Kahl S, Jurie C, Picard B (2001) Influence of feeding level during postweaning growth on circulating concentrations of thyroid hormones and extrathyroidal 5’-deiodination in steers. J Anim Sci 79:2679–2687. https://doi.org/10.2527/2001.79102679x
Christopherson RJ (1985) The thermal environment and the ruminant digestive system. In: Yousef MK (ed) Stress Physiology in Livestock. CRC Press, Boca Raton, Florida, pp 163–180
Collier RJ, Collier JL, Rhoads RP, Baumgard LH (2008) Invited Review: Genes Involved in the Bovine Heat Stress Response1. J Dairy Sci 91:445–454. https://doi.org/10.3168/jds.2007-0540
Collier RJ, Beede DK (1985) Thermal stress as a factor associated with nutrient requirements and interrelationships. In: McDowell L (ed) Nutrition of grazing ruminants in warm climates. Academic Press, Inc., Orlando, FL, pp 59–71
Collier RJ, Baumgard LH, Lock AL, Bauman DE (2005) Physiological limitations, nutrient partitioning. In: R Sylvester-Bradley R, Wiseman J (eds) Yield of farmed species. Constraints and opportunities in the 21st Century, Nottingham University, Nottingham, pp 351–377
Doornenbal H, Tong AK, Murray NL (1988) Reference values of blood parameters in beef cattle of different ages and stages of lactation. Can J Vet Res 52:99–105
FAO (Food and Agriculture Organization of the United Nations) (2009a). Global agriculture towards 2050. High-Level Expert Forum Issues Paper. FAO, Rome.
Gaughan JB, Mader TL, Holt SM et al (1999) Heat tolerance of Boran and Tuli crossbred steers1. J Anim Sci 77:2398–2405. https://doi.org/10.2527/1999.7792398x
Henricks DM, Cooper JW, Spitzer JC, Grimes LW (1984) Sex Differences in Plasma Cortisol and Growth in the Bovine1. J Anim Sci 59:376–383. https://doi.org/10.2527/jas1984.592376x
Hu S, Ding Y, Zhu C (2020) Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front Plant Sci 11:375. https://doi.org/10.3389/fpls.2020.00375
Indu S, Pareek A (2015) A Review: Growth and Physiological Adaptability of Sheep to Heat Stress under Semi-Arid Environment. Int J Emerg Trends Sci Technol 2:3188–3198. https://doi.org/10.18535/ijetst/v2i9.09
Ingram J (2011) A food systems approach to researching food security and its interactions with global environmental change. Food Secur 3:417–431. https://doi.org/10.1007/s12571-011-0149-9
Jackson PGG, Cockcroft PD (eds) (2002) Clinical Examination of Farm Animals: Jackson/Clinical. Blackwell Science Ltd., Oxford, UK
Jungermann K, Katz N (1989) Functional specialization of different hepatocyte populations. Physiol Rev 69:708–764. https://doi.org/10.1152/physrev.1989.69.3.708
Kerr DE, Manns JG, Laarveld B, Fehr MI (1991) Profiles of serum IGF-I concentrations in calves from birth to eighteen months of age and in cows throughout the lactation Cycle. Can J Anim Sci 71:695–705. https://doi.org/10.4141/cjas91-085
Kovács L, Kézér FL, Ruff F et al (2018) Heart rate, cardiac vagal tone, respiratory rate, and rectal temperature in dairy calves exposed to heat stress in a continental region. Int J Biometeorol 62:1791–1797. https://doi.org/10.1007/s00484-018-1581-8
Kramer CY (1957) Extension of multiple range tests to group correlated adjusted means. Biometrics 13:13–18
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Mathers JC, Baber RP, Archibald RF (1989) Intake, digestion and gastro-intestinal mean retention time in Asiatic buffaloes and Ayrshire cattle given two contrasting diets and housed at 20° and 33 °C. J Agric Sci 113:211–222. https://doi.org/10.1017/S0021859600086792
McDowell RE (1972) Improvement of livestock production in warm climates. W. H. Freeman and Co., San Francisco
Mishra L, Mohanty A, Nayak M, Prusty BM, Misra MS (1995) Effects of climatic stress on the physiological reactions of crossbred and purebred animals. Indian Vet J 72:929–934
Nonaka I, Takusari N, Tajima K et al (2008) Effects of high environmental temperatures on physiological and nutritional status of prepubertal Holstein heifers. Livest Sci 113:14–23. https://doi.org/10.1016/j.livsci.2007.02.010
O’Brien MD, Rhoads RP, Sanders SR et al (2010) Metabolic adaptations to heat stress in growing cattle. Domest Anim Endocrinol 38:86–94. https://doi.org/10.1016/j.domaniend.2009.08.005
Olson TA, Chase CC, Lucena C et al (2006) Effect of hair characteristics on the adaptation of cattle to warm climates. In Proceedings of the 8th world congress on genetics applied to livestock production, Belo Horizonte, Minas Gerais, Brazil, 13–18 August, 2006 16–07
Ozkaya S, Bozkurt Y (2009) The accuracy of prediction of body weight from body measurements in beef cattle. Arch Anim Breed 52:371–377. https://doi.org/10.5194/aab-52-371-2009
Polley HW, Briske DD, Morgan JA et al (2013) Climate Change and North American Rangelands: Trends, Projections, and Implications. Rangel Ecol Manage 66:493–511. https://doi.org/10.2111/REM-D-12-00068.1
Pulina G, Nudda A, Battacone G et al (2012) Effects of short-term feed restriction on milk yield and composition, and hormone and metabolite profiles in mid-lactation Sarda dairy sheep with different body condition score. Ital J Anim Sci 11:e28. https://doi.org/10.4081/ijas.2012.e28
Rhoads ML, Kim JW, Collier RJ et al (2010) Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J Dairy Sci 93:170–179. https://doi.org/10.3168/jds.2009-2469
Ronchi B, Stradaioli G, Verini Supplizi A et al (2001) Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17β, LH, FSH, prolactin and cortisol in Holstein heifers. Livest Prod Sci 68:231–241. https://doi.org/10.1016/S0301-6226(00)00232-3
Samad HA, Anuraj KS, Shyma K, Latheef MV (2014) Effect of nutritional stress on physiological responses of nondescript Indian buck (Capra Hircus). Vet Sci 3:2277–2280
Schams D, Winkler U, Theyerl-Abele M, Prokopp A (1989) Variation of BST and IGF-I concentration in blood plasma of cattle. In: Sejrsen K, Vestergaard M, Neimann-Sorensen A (eds) Use of somatotropin in livestock production. Elsevier Applied Science, New York, NY, p 18
Sejian V, Maurya VP, Naqvi SMK (2010a) Adaptability and growth of Malpura ewes subjected to thermal and nutritional stress. Trop Anim Health Prod 42:1763–1770. https://doi.org/10.1007/s11250-010-9633-z
Sejian V, Maurya VP, Naqvi SMK (2010b) Adaptive capability as indicated by endocrine and biochemical responses of Malpura ewes subjected to combined stresses (thermal and nutritional) in a semi-arid tropical environment. Int J Biometeorol 54:653–661. https://doi.org/10.1007/s00484-010-0341-1
Sejian V, Maurya VP, Kumar K, Naqvi SMK (2012) Effect of multiple stresses on growth and adaptive capability of Malpura ewes under semi-arid tropical environment. Trop Anim Health Prod 45:107–116. https://doi.org/10.1007/s11250-012-0180-7
Sejian V, Iqbal Hyder IH, Malik PK et al (2015) Strategies for alleviating abiotic stress in livestock. In: Malik PK, Bhatta R, Takahashi J et al (eds) Livestock production and climate change. CABI, Wallingford, pp 25–60
Sejian V, Indu S, Naqvi M (2013) Impact of short-term exposure to different environmental temperature on the blood biochemical and endocrine responses of Malpura ewes under semi-arid tropical environment. The Indian Journal of Animal Sciences 83:1155–1160. Retrieved from https://epubs.icar.org.in/index.php/IJAnS/article/view/34756. Accessed 18 Feb 2023
Shashank CG, Silpa MV, Ezhil Vadhana P, et al (2021) Climate Change and Livestock Production: Significance of Studying Multiple Stressors Impact in Cattle in the Changing Climate Scenario. In: Sejian V, Chauhan SS, Devaraj C, et al. (eds) Climate Change and Livestock Production: Recent Advances and Future Perspectives. Springer Singapore, Singapore, pp 59–72
Shilja S, Sejian V, Bagath M et al (2016) Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level, and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. Int J Biometeorol 60:1311–1323. https://doi.org/10.1007/s00484-015-1124-5
Singh S, Golla N, Sharma D et al (2019) Buffalo liver transcriptome analysis suggests immune tolerance as its key adaptive mechanism during early postpartum negative energy balance. Funct Integr Genomics 19:759–773. https://doi.org/10.1007/s10142-019-00676-1
Theurer ME, Anderson DE, White BJ et al (2014) Effects of weather variables on thermoregulation of calves during periods of extreme heat. Am J Vet Res 75:296–300. https://doi.org/10.2460/ajvr.75.3.296
UN (United Nations) (2013) World population projected to reach 9.6 billion by 2050. United Nations Department of Economic and Social Affairs. <http://www.un.org/en/development/desa/news/population/un-report-world-population-projected-to-reach-9-6-billion-by-2050.html>. Accessed 24 Jan 2023
Yousef MK (1985) Measurement of heat production and heat loss. In: Book Series Title Stress physiology in livestock (ed. MK Yousef), CRC Press 1:35–46.
Acknowledgements
Authors are thankful to the Director, ICAR-National Dairy Research Institute (NDRI), Karnal, Haryana for providing the financial grant for the study and the National Innovations in Climate Resilient Agriculture, Indian Council of Agricultural Research (NICRA-ICAR), New Delhi for providing necessary facilities to conduct this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Declaration
The experiment was approved by the Institutional Animal Ethics Committee (IAEC) constituted as per article no. 13 of the CPCSEA rules, laid down by Govt. of India (Reference No. 44-IAEC-19–21).
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shashank, C.G., Prashant, R.G., Kumar, P. et al. Comparative assessment of growth performance of indigenous and cross-bred calves subjected to combined stressors (heat and nutritional). Int J Biometeorol 67, 1435–1450 (2023). https://doi.org/10.1007/s00484-023-02511-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-023-02511-6