Abstract
Heat stress (HS) causes oxidative stress and cellular changes in an attempt to detoxify the harmful effects of reactive oxygen species (ROS). However, how ROS affect different organs in chickens under acute and chronic HS is relatively unknown. We investigated the cellular enzyme activity and biomarker changes in the liver and Pectoralis (P) major muscle in broiler chickens subjected to both acute and chronic HS. Forty-eight broiler chickens at 14 days old were randomly assigned to either 25 °C (control) or 35 °C (heat-stressed) for 12 days. Five birds per treatment at 1 and 12 days post-HS were euthanized, and the liver and P. major muscle were sampled. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione (GSH), glutathione reductase (GR), glutathione S-transferase (GST) activity as well as 8-hydroxy-2′-deoxyguanosine (8-OHdG), advanced glycation end product (AGE), malondialdehyde (MDA), and protein carbonyl (PCO) were analyzed as biomarkers for DNA, carbohydrate, lipid, and protein oxidation, respectively. The SOD, CAT, and GSH-GPx activity levels in the liver and the P. major muscle changed under HS; however, some of the changes were tissue-specific or dependent on the duration of the HS. There were increased liver 8-OHdG during chronic HS and also increased liver AGE levels during both acute and chronic HS indicating significant carbohydrate and DNA oxidations. In the P. major muscle, we observed significant increases in lipid peroxidation and protein oxidation which may reflect that this tissue is less resilient to oxidative damage under heat stress. We show that heat stress caused tissue-specific changes to levels of oxidation biomarkers in chicken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat stress (HS) in growing birds affects performance (Temim et al. 1999), amino acid digestibility (Habashy et al. 2017a), dietary protein retention (Geraert et al. 1996; Habashy et al. 2017b), and mRNA expression of genes that encode for oxidants and antioxidants (Mujahid et al. 2007; Habashy et al. 2018) causing an imbalance between oxidant and antioxidant defense systems. HS promotes the generation of reactive oxygen species (ROS) which causes oxidative stress (Lu et al. 2010).
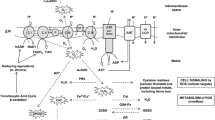
ROS in general causes oxidative damage to DNA, proteins, and lipids (Dröge 2002). ROS represent partially reduced oxygen metabolites such as superoxide anions, hydrogen peroxide, and hydroxyl radicals (Thannickal and Fanburg 2000). There are a number of both enzymatic and non-enzymatic systems that are involved in the elimination of ROS (Lu et al. 2010). One of the non-enzymatic antioxidants is glutathione (GSH) and the enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Cadenas and Davies 2000). GSH serves as an electron donor for GPx which reduces hydrogen peroxide to water (Hayes and McLellan 1999). By donating an electron, GSH is oxidized to glutathione disulfide (GSSG). Glutathione disulfide can be reduced back to GSH by glutathione reductase (GR) (Srinivasan et al. 1997). Therefore, the cellular GSH to GSSG ratio plays an essential role in the detoxification of ROS and maintaining redox homeostasis. Glutathione S-transferase (GST) plays an important role in protecting cells and tissues from oxidative damage (Hayes et al. 2005) by accelerating the reaction between GSH and different electrophilic xenobiotics (Zhu et al. 2013).
Biomarkers have been used as proxies to determine the extent of oxidation stress in tissues. Protein carbonyl (PCO), malondialdehyde (MDA), 8-hydroxy-2′-deoxyguanosine (8-OHdG), and advanced glycation end product (AGE) have been used as indicators of protein, lipid, DNA, and carbohydrate oxidation, respectively (Levine et al. 1994; Mujahid et al. 2009; Ismail et al. 2013; Sultana et al. 2013). Since oxidative stress is caused by several factors, the relative importance of various responses to the type of causal factor will be important in designing mitigation strategies to reduce its effect. Understanding the relative sensitivity of tissues to oxidative stress may assist in the design of mitigation strategies to reduce this effect on that particular tissue. There are several reports on short-term response to HS, but limited data on long-term effects of HS (Geraert et al. 1996; Azad et al. 2010; Sun et al. 2015; Habashy et al. 2017a, b). Our objective was to study the cellular enzyme activity and biomarker changes in the liver and Pectoralis major muscle in meat-type chickens exposed to both short- and long-term heat stress.
Material and methods
Experimental design and animals
Research on live chickens met the guidelines approved by the institutional animal care and use committee of the University of Georgia. A total of 48 male broilers (Cobb500) were divided into two treatments and raised under either constant normal (25 °C; 40–50% humidity) or high temperature (35 °C; 40–50% humidity) from day 14–26 of age in individual cages (L = 30.48 cm × B = 60.96 × H = 45.72). The birds were fed ad libitum on a diet containing 18.7% crude protein (CP) and 3560 kcal ME/kg. At day 15 (after 1 day of HS exposure) and day 26 (after 12 days of HS exposure) of age, birds were humanely euthanized by cervical dislocation and the P. major muscle and liver tissue were collected from five birds from each treatment and were immediately placed in liquid nitrogen and later stored at − 86 °C.
Determination of superoxide dismutase (U/mL)
Approximately 0.2 g of the P. major muscle and liver tissue were homogenized in 1 mL of cold 20 mM HEPES buffer, pH 7.2, containing 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose. After centrifugation at 1500×g for 5 min at 4 °C, the supernatants were used for determining superoxide dismutase (SOD) activity level (Cayman Chemical Company, Ann Arbor, MI, USA, item no.706002). Activity was measured using Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 460 nm.
Determination of catalase (nmol/min g tissue), glutathione S-transferase (nmol/min/mL), and glutathione reductase (nmol/min/mL)
About 0.2 g of the P. major muscle and liver tissue were homogenized in 1 mL of cold buffer of 50 mM potassium phosphate buffer, pH 7, containing 1 mM EDTA. After centrifugation (10,000×g per 15 min) at 4 °C, the supernatants were removed and stored frozen at − 86 °C until the time of analysis of CAT, GST, and GR. The activity of CAT (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 707002), GST (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 703302), and GR (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 703202) were measured with a Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA) at 540, 340, and 340 nm, respectively.
Determination of glutathione peroxidase (nmol/min/mL)
Approximately 0.2 g of the P. major muscle and liver tissue were homogenized in 1 mL of cold 50 mM Tris-HCL, pH 7.5, 5 mM EDTA, and 1 mM DTT, and the supernatant was centrifuged at 10000×g for 15 min at 4 °C and used for determination of GPX activity (nmol/min/mL) (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 703102). Activity was determined by the Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 340 nm.
Determination of glutathione (μM) and glutathione disulfide (μM)
About 0.2 g of the P. major muscle and liver tissue were homogenized in 1 mL of cold 50 mM phosphate buffer, pH 7, containing 1 mM EDTA. After centrifugation (10,000×g for 15 min) at 4 °C, the supernatants were removed and stored frozen at − 86 °C until the time of analysis of GSH and GSSG. The activities of GSH and GSSG (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 703002) were determined by the Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 405 nm.
Determination of DNA oxidative damage (pg/mL)
DNA was extracted from the P. major muscle and liver tissue using Trizol reagent (Invitrogen Corp., Carlsbad, CA, USA) according to manufacturer’s instructions and stored at − 20 °C until used. After that, DNA samples were converted to single-stranded DNA by incubation at 95 °C for 5 min and rapidly chilled at 4 °C. Then, DNA samples were digested using five units nuclease P1 (Sigma-Aldrich, Saint Louis, MO, USA, item no. 8630). One molar of Tris was used to adjust the pH to 7.5–8.5, and 5 μL alkaline phosphatase was added and the product was incubated for 45 min at 37 °C. The lysate was then boiled for 10 min and stored in 4 °C until used. The DNA/RNA oxidative damage (Cayman Chemical Company, USA, item no. 589320) was determined by the Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 405 nm.
Determination of advanced glycation end product (μg/mL)
Approximately 0.1 g of the P. major muscle and liver tissue were homogenized in 1 mL of phosphate-buffered saline (PBS), pH 7.4, containing 10 μL of a protease inhibitor. After centrifugation at 12,000×g for 10 min at 4 °C, the supernatants were removed and used for measuring AGE activity (Cell Biolabs, San Diego, CA, USA, item no. STA-817). AGE activity was measured by the Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm.
Determination of malondialdehyde (μM)
About 25 mg of the P. major muscle and liver tissue were homogenized in 250 μL of RIPA buffer containing 1 μL protease inhibitors) and then centrifuged at 1,600×g for 10 min at 4 °C. The supernatants were then used for measuring the malondialdehyde (MDA) activity (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 10009055). MDA activity was measured by the Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 540 nm.
Determination of protein carbonyl (nmol/mL)
Approximately 0.2 g of the P. major muscle and liver tissue were homogenized in 1 mL of cold 50 mM potassium phosphate buffer, pH 7, containing 1 mM EDTA. After centrifugation (10,000×g for 15 min) at 4 °C, the supernatants were removed and incubated with 10% streptomycin sulfate stock solution for 15 min at room temperature and then centrifuged at 6,000×g for 10 min at 4 °C. Following centrifugation, the supernatants were used for determining the protein carbonyl content (Cayman Chemical Company, Ann Arbor, MI, USA, item no. 10005020). Activity was determined by the Spectra Max 5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 360 nm.
Statistical analysis
Statistical analysis was performed separately for acute and chronic HS and each tissue. 8-OHdG, AGE, MDA, PCO, SOD, CAT, GPX, GSH, GR, GST, GSSG, and GSH/GSSG as dependent variable between treatment (HS and control groups) were analyzed using SAS 9.4 (2011), and the differences among treatments were tested using the Tukey test.
Results
Cellular antioxidant enzyme activity
The cellular levels of SOD, CAT, GPx, GSH, GR, GST, GSSG, and GSH/GSSG ratio are presented in Figs. 1, 2, 3, 4, 5, 6, 7, and 8. The SOD levels increased (P < 0.0001) in the liver only at 12 days post-HS. There were no changes (P > 0.05) in the SOD levels in the P. major muscle both at 1 and 12 days post-HS (Fig. 1). Also, heat-stressed birds showed a decrease (P < 0.05) in the CAT level at 1 day post-HS in the P. major muscle, but there were no changes (P > 0.05) in the CAT levels in the liver when birds were subjected to HS (Fig. 2). The liver levels of GPx did not change when birds were subjected to 1 day of HS; however, at 12 days post-HS, there was an increase (P < 0.0029) in the liver GPx level. In the P. major muscle, the level of GPx was similar between the heat-stressed birds and the controls both at 1 and 12 days post-HS (Fig. 3). The GSH levels in both the liver and P. major muscle were similar between the heat-stressed birds and control birds at 1-day treatment, but at 12 days post-treatment, the GSH levels decreased (P < 0.001) in the liver, but increased (P < 0.0023) in the P. major muscle of birds subjected to HS (Fig. 4). Tissue GR levels are shown in Fig. 5. Among heat-stressed birds, the GR activity increased (P < 0.05) at 1 day post-HS; however, by 12 days post-HS, the GR levels in the liver had decreased (P < 0.01) compared with the control birds. In the P. major muscle, GR increased (P < 0.05) only at 1 day post-HS. The GST level did not change in the liver and P. major at 1 d post-HS, but decreased (P < 0.0001) only in the liver at 12 days post-HS (Fig. 6). At 1 day post-treatment, GSSG levels increased (P < 0.01) in both the liver and P. major muscle in heat-stressed birds compared with the controls. The opposite effect was observed at 12 days post-treatment where the GSSG levels decreased (P < 0.01) in the heat-stressed birds compared with the control birds (Fig. 7). The GSH/GSSG ratio did not change in the liver under both acute and chronic heat stress; however, in the P. major muscle, the ratio decreased in the heat-stressed birds at 1 day post-HS, but increased at 12 days post-HS (Fig. 8).
Biomarkers
The biomarkers for DNA, carbohydrate, lipid, and protein oxidations in the liver and P. major muscle are presented in Figs. 9, 10, 11, and 12. Figure 9 shows that 8-OHdG was significantly increased (P < 0.0007) in the liver at 12 days post-HS compared with their control counterparts, but in the P. major muscle, 8-OHdG did not change between the control and heat-stressed chickens. Chickens exposed to HS had significant increase in the activities of AGE in the liver at 1 (P < 0.005) and 12 days (P < 0.008) post-HS; however, in the P. major muscle, AGE did not change between the groups at 12 days post-HS (Fig. 10). The MDA activities in the liver did not differ between the treatment and control groups (Fig. 11) at both 1 and 12 days post HS. Meanwhile, in the P. major muscles, the levels of MDA were significantly increased after 1 (P < 0.0075) and 12 days (P < 0.05) post-HS (Fig. 11). The PCO levels in the liver increased significantly at 1 day post-HS, but not at 12 days post-HS, but in the P. major muscle, PCO levels significantly increased both at 1 (P < 0.0040) and 12 days (P < 0.0002) post-HS (Fig. 12).
Discussion
Heat is one of the major stressors affecting the poultry industry, and several studies have indicated that HS has a negative impact on productivity (Habashy et al. 2017a), immune competence (Mashaly et al. 2004), and reproductive performance (Rozenboim et al. 2007). Based on physiological responses to heat stress, several mitigation strategies have been tried with varying success. An understanding of the cellular responses to HS could offer additional strategies to curtail the effects of HS in growing birds. There are several reports that demonstrate that HS causes oxidative stress. Habashy et al. (2018) have shown that mRNA expression of genes that encode for oxidants is altered by heat stress in a way that it putatively increases the cellular ROS activity.
Cellular antioxidant enzyme activity
The main cellular antioxidant enzymatic defense systems against ROS are SOD, GPx, and GR. They scavenge ROS and convert them to less reactive species. Heat stress has been shown to elevate the cellular level of ROS in several animal models (Kikusato and Toyomizy 2013). Reactive species comprise of superoxide, hydroxyl, hydrogen peroxide, nitric oxide, and nitrogen dioxide (Halliwell 2001). SOD catalyzes the conversion of the superoxide radical to hydrogen peroxide (H2O2) (Fridovich 1997). The liver SOD levels increased under chronic heat stress but not under acute stress. Other researchers have also demonstrated the changes in liver SOD levels under HS (Lin et al. 2000; Yang et al. 2010; Tan et al. 2010). Huang et al. (2015) also showed that chronic heat stress led to increased plasma SOD levels in chickens. The SOD response to HS may depend on the duration of the stress and also the tissue, as SOD levels in the P. major muscle did not change under both acute and chronic HS. The tissue SOD level under heat stress may reflect the tissue’s role in detoxification and tolerance for cellular oxidation. Increase in liver SOD observed in the current study may be due to the need to detoxify increased free radicals resulting from the heat stress. Both protein and lipid oxidation levels were also significantly elevated in the P. major muscle compared with the liver under chronic HS. Based on these observations, it seems that the liver tissue plays a larger role in scavenging free radicals. Hydrogen peroxide is an ROS that needs to be converted to water. Catalase enzymatically converts H2O2 to water and O2 to protect the cells from the destructive effect of H2O2 (Halliwell 2001; Finkel and Holbrook 2000; Altan et al. 2003; Seven et al. 2009). In the current study, liver CAT levels did not change when birds were subjected to HS. However, when Japanese quails were subjected to cyclical heat stress, liver CAT and GPx levels were reduced (Sahin et al. 2010). Baud et al. (2004) reported that increased H2O2 levels corresponded with decreased CAT activity in rat oligodendrocytes.
One of the principal redox pathways is the GSH-GPx enzyme system. Glutathione peroxidase and GSH coordinate to eliminate H2O2 and peroxynitrite as well as acting as a buffer to protect certain proteins against pathological modifications (Chen et al. 2015). GPX enzymes scavenge H2O2 by utilizing two hydrogens from two GSH molecules, thereby oxidizing GSH to GSSG. GSSG is reduced to GSH by using electrons donated by NADPH and catalyzed by GR (El-Bahr 2013). Liver GPx levels increased at 12 days post-HS, but there was no change observed in the P. major muscle. The GSH levels did not change in both tissues 1 day post-HS; however, at 12 days post-HS, the levels depended on the tissue. It appears that the activity levels of CAT, GPx, GR, and GSH are coordinated differently among tissues, possibly due to the tissue’s relative tolerance to the damage caused by oxidative stress. Murphy and Kehrer (1989) reported that higher activities of GSH in the muscle of dystrophic chickens may reflect a higher degree of continuing oxidative stress in this tissue. In the current study, we have shown that there is a negative relationship between GSH and 8-OHdG, AGE in the liver tissue at day 12 post-HS, whereas the relationship between GSH and AGE, MDA, and PCO in the P. major muscle tissue at day 12 post-HS was positive. GSH can also bind to byproducts of lipid oxidation in a reaction catalyzed by GST (Cheng et al. 2001). From the current study, GST levels increased slightly in the liver at 1 day post-HS, but decreased when the HS continued up to 12 days. It is possible that the reduced GST at 12 days post-HS in the liver may be due to concurrent depletion in GSH. The GSH:GSSG ratio, often referred to as redox potential, is used as a measure of oxidative stress (Zitka et al. 2012). The level of GSSG increased in both the liver and P. major muscle with acute HS; however, under chronic stress, GSSG levels decreased probably due to increased detoxification of ROS. The redox potential was reduced under acute HS in both tissues because of the significant increase in GSSG. Under chronic HS, we observed an increase in the redox potential in the P. major muscle which may imply a relatively different enzymatic steady state aiming at detoxifying the muscle from ROS. Flohé (2013) cautioned that a GSH/GSSG equilibrium does not exist, but the ratio is a reflection of a steady state resulting from enzymatic use and regeneration of GSH which could be useful as a tool to unearth shifts in redox metabolism.
Biomarkers
Oxidative stress changes the balance between oxidants and antioxidants either by accumulation of ROS and/or depletion of antioxidants. Increased levels of ROS can alter DNA and RNA structures and gene expression (Wautier et al. 2001; Habashy et al. 2018). Several types of base damage by ROS have been demonstrated, but guanine is the most reactive of the nucleic acid bases (Barciszewski et al. 1999). Thus, the oxidized base 8-OHdG is the most abundant among the oxidized bases and therefore used as a biomarker for DNA oxidation (Helbock et al. 1999). In the current study, liver 8-OHdG levels increased when chickens were subjected to chronic HS; however, no such increase was observed in the P. major muscle. Studies have demonstrated the direct relationship between increased hydroxyl radicals and DNA damage (Zhang et al. 2004; Kojima et al. 2010). The product of glucose oxidation when bound to proteins or ROS oxidation of glycated residues in proteins is irreversible and produces AGEs (Jenkins and Lyons 1997). AGEs have been shown to be associated with some diseases including diabetes mellitus and neurodegenerative diseases (Bucala and Cerami 1992; Ahmed 2005). During oxidative stress, there is rerouting of glucose metabolism via the pentose phosphate pathway in an attempt to maintain redox balance and ROS clearance (Kuehe et al. 2015). Therefore, heat waves can lead to glycation which may render poultry vulnerable to other pathological diseases.
ROS can also damage the cellular membrane lipid bilayer arrangement and in the process may inactivate membrane-bound receptors and increase tissue permeability (Girotti 1985). The process generates relatively stable end products such as MDA and isoprostanes which can be measured in plasma or tissues as markers of oxidative stress. The stable end products are also capable of inactivating many cellular proteins by forming protein cross-linkages (Siu and Draper 1982). We observed a significant increase in MDA levels in the P. major muscle when chickens were subjected to both acute and chronic heat stress. The MDA levels in the liver did not change under heat stress; however, Ramnath et al. (2008) reported that plasma MDA levels were elevated when chickens were subjected to heat stress. Lipid peroxidation in the P. major muscle may affect meat quality, as Zhang et al. (2011) showed that oxidative stress in live chickens accelerated post-mortem glycolysis and increased drip loss in broiler breast muscles.
Oxidative stress also modifies protein function resulting in protein carbonylation and protein nitration which are used as biomarkers (Dalle-Donne et al. 2003, 2006; Sultana et al. 2013). Protein oxidation leads to increased vulnerability to proteolysis by degradation of specific proteases (Kelly and Mudway 2003). PCO levels in the liver increased only under acute heat stress but not under chronic heat stress; however, the P. major muscle exhibited high levels of protein oxidation when the chickens were exposed to both acute and chronic heat stress. It has been demonstrated that methionine and cysteine residues in proteins are the most susceptible to oxidation (Dean et al. 1985; Levine et al. 2000; Zhang et al. 2013), and oxidation of methionine causes conformation changes, protein unfolding, and degradation (Lyras et al. 1997). Recently, Habashy et al. (2017a, b) showed that the amount of cysteine per unit of growth was the highest among all amino acids under heat stress further signifying the relative importance of cysteine in chickens under heat stress. Protein oxidation in the muscle increases the muscles’ susceptibility to proteolysis and this could be a major factor contributing towards reduced growth under heat stress. Protein oxidation in the muscle could also change the muscle pH and that could affect meat quality.
Conclusion
Heat stress causes oxidative stress in chickens and subsequently elicits changes in the oxidant/antioxidant cellular mechanisms to respond to the oxidative stress. The principal redox pathways including SOD, CAT, and GSH-GPx respond sometimes differently in the liver and the P. major muscle according the tissue’s need to detoxify ROS or tolerance for ROS. Biomarker changes show that heat stress causes carbohydrate and DNA oxidation in the liver. Lipid peroxidation and protein oxidation significantly occur in the P. major muscle of chickens under heat stress.
References
Ahmed N (2005) Advanced glycation end products: role in pathology of diabetic complications. Diabetes Res Clin Pract 67:3–21
Altan O, Pabuccuoglu A, Altan A, Konyalioglu S, Bayraktar H (2003) Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br Poult Sci 44:545–550
Azad MAK, Kikusato M, Maekawa T, Shirakawa H, Toyomizu M (2010) Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Comp Biochem Physiol A Mol Integr Physiol 155:401–406
Barciszewski J, Barciszewski MZ, Siboska G, Rattan SI, Clark BF (1999) Some unusual nucleic acid bases are products of hydroxyl radical oxidation of DNA and RNA. Mol Biol Rep 26:231–238
Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA (2004) Glutathione peroxidase–catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurol Sci 24:1531–1540
Bucala R, Cerami A (1992) Advanced glycation end products: role in pathology of diabetic complications. Adv Pharmacol 23:1–34
Cadenas E, Davies KJA (2000) Mitochondrial free radical generation, oxidative stress and aging. Free Radic Biol Med 29:222–230
Chen J, Bhandar B, Kavdia M (2015) Interaction of ROS and RNS with GSH and GSH/GPX systems. FASEB J 29:636–637
Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC (2001) Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem 276:41213–41223
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006) Protein carbonylation, cellular dysfunction and disease progression. J Cell Mol Med 10:389–406
Dean RT, Roberts CR, Jessup W (1985) Fragmentation of extracellular and intracellular polypeptides by free radicals. Prog Clin Biol Res 180:341–350
Dröge W (2002) Free radicals in physiological control of cell function. Physiol Rev 82:47–95
El-Bahr SM (2013) Biochemistry of free radicals and oxidative stress. Sci Int 1:111–117
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Flohé L (2013) The fairytale of the GSSG/GSH redox potential. Biochim Biophys Acta 1830:3139–3142
Fridovich I (1997) Superoxide anion radical, superoxide dismutases, and related matters. J Biol Chem 272:18515–18517
Geraert PA, Padilha CF, Guillaumin S (1996) Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr 75:195–204
Girotti AW (1985) Mechanisms of lipid peroxidation. J Free Radic Biol Med 1:87–95
Habashy WS, Milfort MC, Adomako K, Attia YA, Rekaya R, Aggrey SE (2017a) Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult Sci 96:2312–2319
Habashy WS, Milfort MC, Fuller AL, Attia YA, Rekaya R, Aggrey SE (2017b) Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int J Biometeorol 61:2111–2118
Habashy WS, Milfort MC, Rekaya R, Aggrey SE (2018) Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol Biol Rep 45:389–394
Halliwell B (2001) Free radicals and other reactive species in disease. Encyclopedia of life sciences. Nature Publishing Group /http://www.els.net. Accessed 15 Dec 2018
Hayes JD, McLellan LI (1999) Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31:273–300
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Helbock HJ, Beckman KB, Ames BN (1999) 8-hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol 300:156–166
Huang C, Jiao H, Song Z, Zhao J, Wang X, Lin H (2015) Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J Anim Sci 93:2144–2153
Ismail IB, Al-Busadah KA, El-Bahr SM (2013) Oxidative stress biomarkers and biochemical profile in broilers chicken fed zinc bacitracin and ascorbic acid under hot climate. Am J Biochem Mol Biol 3:202–214
Jenkins AJ, Lyons TF (1997) Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: a carbonyl stress hypothesis. Diabetes Rev (Alex) 5:365–391
Kelly FJ, Mudway IS (2003) Protein oxidation at the air-lung interface. Amino Acids 25:375–396
Kikusato M, Toyomizy M (2013) Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS One 8:e64412
Kojima T, Norose T, Tsuchiya K, Sakamoto K (2010) Mouse 3T3-L1 cells acquire resistance against oxidative stress as the adipocytes differentiate via the transcription factor Foxo. Apoptosis 15:83–93
Kuehe A, Emmert H, Soehle J, Winnefeld M, Fischer F, Wenck H, Gallinat S, Terstegen L, Lucius R, Hildebrand J, Zamboni N (2015) Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin cells. Mol Cell 59:359–371
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Levine RL, Moskovitz J, Stadtman ER (2000) Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life 50:301–307
Lin H, Du R, Zhang ZY (2000) Peroxide status in tissues of heat stressed broilers. Asian-Australas J Anim Sci 13:1373–1376
Lu T, Piao XL, Zhang Q, Wang D, Piao XS, Kim SW (2010) Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food Chem Toxicol 48:764–770
Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B (1997) An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem 68:2061–2069
Mashaly MM, Hendricks GL 3rd, Kalama MA, Gehad AE, Abbas AO, Patterson PH (2004) Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 83:889–894
Mujahid A, Akiba Y, Toyomizu M (2007) Acute heat stress induces oxidative stress and decrease adaptation in young White Leghorn cockerels by down regulation of avian uncoupling protein. Poult Sci 86:364–371
Mujahid A, Akiba Y, Toyomizu M (2009) Progressive changes in the physiological responses of heat-stressed broiler chickens. J Poult Sci 46:163–167
Murphy ME, Kehrer JP (1989) Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem J 260:359–364
Ramnath V, Rekha PS, Sujatha KS (2008) Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by Brahma Rasayana. Evid Based Complement Alternat Med 5:77–84
Rozenboim I, Tako E, Gal-Garber O, Proudman JA, Uni Z (2007) The effect of heat stress on ovarian function of laying hens. Poult Sci 86:1760–1765
Sahin K, Orhan C, Tuzcu M, Ali S, Sahin N, Hayirli A (2010) Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult Sci 89:2251–2258
SAS Institute (2011) SAS/ SAT user guide: statistics ver, 9.3 edn. AS Inst. Inc., Cary
Seven PT, Yilmaz S, Seven I, Cerci IH, Azman MA, Yilmaz M (2009) Effects of propolis on selected blood indicators and antioxidant enzyme activities in broilers under heat stress. Acta Vet Brno 78:75–83
Siu GM, Draper HH (1982) Metabolism of malonaldehyde in vivo and in vitro. Lipids 17:349–355
Srinivasan U, Mieyal PA, Mieyal JJ (1997) pH profiles indicative of rate limiting nucleophilic displacement in thioltransferase catalysis. Biochem 36:3199–3206
Sultana R, Cenini G, Butterfield DA (2013) Biomarkers of oxidative stress in neurodegenerative diseases. In: Villamena FA (ed) Molecular basis of oxidative stress: chemistry, mechanisms, and disease pathogenesis. John Wiley and Sons, Inc., New York, pp 359–376
Sun X, Zhang H, Sheikhahmadi A, Wang Y, Jiao H, Lin H, Song Z (2015) Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Int J Biometeorol 59:127–113
Tan GY, Yang L, Fu YQ, Feng JH, Zhang MH (2010) Effect of different acute ambient temperatures on function of hepetic mitochondria respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult Sci 89:115–122
Temim S, Chagneau AM, Guillaumin S, Michel J, Peresson R, Geraert PA, Tesseraud S (1999) Effects of chronic heat exposure and protein intake on growth performance, nitrogen retention and muscle development in broiler chickens. Reprod Nutr Dev 39:145–156
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Phys Lung Cell Mol Phys 279:L1005–L1028
Wautier M, Chappey O, Corda S, Stern DM, AM, Wautier JL (2001) Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280:E685–E694
Yang L, Tan GY, Fu YQ, Feng JH, Zhang MH (2010) Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol 151:204–208
Zhang X, Wu RS, Fu W, Xu L, Lam PK (2004) Production of reactive oxygen species and 8-hydroxy-2'deoxyguanosine in KB cells co-exposed to benzo[a]pyrene and UV-A radiation. Chemosphere 55:1303–1308
Zhang W, Ahn DU, Lee EJ, Xiao S (2011) Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J Agric Food Chem 59:969–974
Zhang W, Xiao S, Ahn DU (2013) Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr 53:1191–1201
Zhu H, Wang J, Santo A, Li Y (2013) Downregulation of antioxidants and phase 2 proteins. In: Villamena FA (ed) Molecular basis of oxidative stress: chemistry, mechanisms, and disease pathogenesis. John Wiley and Sons, Inc., New York, pp 113–121
Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R (2012) Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett 4:1247–1253
Acknowledgments
Walid Habashy was supported by the Missions Sector of the Egyptian Ministry of Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habashy, W.S., Milfort, M.C., Rekaya, R. et al. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. Int J Biometeorol 63, 1569–1584 (2019). https://doi.org/10.1007/s00484-019-01769-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-019-01769-z