Abstract
Adaptation is a relevant characteristic to be understood in livestock animals in order to maintain and raise productivity. In Brazil, the Nellore beef cattle are widely disseminated and well-adapted breed that present good thermoregulatory characteristics for tropical environment conditions. Conversely, the physiological and cellular mechanisms required for thermoregulation and thermotolerance in this breed are still limited. The aim of this study was to comprehend the heat loss efficiency at the whole animal level and heat shock response at the cellular level of Nellore cows in tropical climate conditions. Healthy purebred Nellore cows were classified according to their capacity to lose body heat as Efficient or Inefficient based on vaginal temperature which was continuously monitored by data-loggers. Rectal, tail, and ocular temperatures, sweating rate, and respiratory frequency were collected to assess other thermoregulatory responses. Peripheral mononuclear cells were used for gene expression of heat shock proteins 60, 70, and 90 induced by in vitro heat treatments at 38, 40, and 42 °C. In our findings, the Efficient cows presented higher sweating rates compared to Inefficient cows that presented higher rectal temperature with greater amplitude of vaginal temperature profile. Transcription of the HSP genes was stable at 38 and 40 °C and decreased for all HSP genes at 42 °C. In conclusion, the Nellore efficiency to lose heat was mainly associated with their sweating capacity and cellular thermotolerance confirmed by the maintenance of heat shock proteins transcripts under heat stress. Taken together, this knowledge contributes as a future key for genetic selection of adapted animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans and animals are recently experiencing climate changes that affect health, welfare, agriculture, and livestock production. Different compensatory mechanisms are recruited in order to minimize the perturbations caused by a stressor and enabling return to normality (Dobson and Smith 2000). Adaptation is a crucial characteristic for animals to maintain homeostasis within its environment. For this reason, it is necessary to comprehend the dynamics between animal-environment and which are the important features to overcome adverse situations.
In a long adaptive process in tropical climates, Bos taurus indicus were able to better regulate the rectal temperature in response to heat stress than Bos taurus taurus (Deb et al. 2013). This capacity is related to low metabolic heat production and high heat flow capability (Hansen 2004); arteriovenous anastomoses in the skin (Hales et al. 1978); thick, dense, smooth and shiny hair coat (Hutchinson and Brown 1969; Finch et al. 1984); and a high sweat glands density (Nay and Hayman 1956). The Nellore breed originated from Indian Ongole cattle and the purebred and its crosses are widely disseminated in Brazil, contributing to 80% of the beef cattle herds (Carvalho et al. 2014). The Nellore breed presents as phenotype characteristics a hump behind the neck; pigmented skin, nose, and hoof; white/gray or red coat; physical strength; high maternal ability; ectoparasite resistance; hardiness; and adaptability to food availability and to climate conditions; they like affection and become extremely docile with good handling (Mason 1988).
The knowledge related to physiological body temperature regulation and thermotolerance of this breed in tropical climate conditions still remains unknown. An organism’s capacity to perform work even if in heat stress by improving heat dissipation due to moderate and repeated elevation of temperature is part of the acclimatization process (Moseley 1997). According to the same author, cells are able to survive to a lethal heat stress by the previous synthesis and/or accumulation of heat shock proteins (HSPs); this adaptation ability is denominated thermotolerance. The genes involved in the synthesis of HSPs can be used as genetic markers in the study of stress in cattle and buffalo and their expression varies across breeds and species (Kumar et al. 2015). Additionally, there are different profiles of HSPs expression related to animal heat tolerance, adaptation capability, and the climate conditions.
The investigation of thermoregulatory and thermotolerance mechanisms will elucidate characteristics related to Nellore adaptation and this knowledge may contribute as a future key for genetic selection or molecular markers to improve productive and reproductive performance in the face of heat stress. Hence, the aim of this study is to gain insights into heat loss efficiency and the cellular heat shock response of Nellore cows (Bos taurus indicus) in tropical climate conditions.
Material and methods
Experimental site
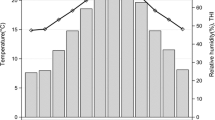
The Institutional Ethics Committee of Animal Use approved all animal care, handling and procedures in this project with the protocol number no. 13.1.2186.74.2. The study was conducted during the months of October and November in the Biometeorology and Ethology Laboratory, Faculty of Animal Science and Food Engineering (FZEA), University of São Paulo, Pirassununga (21° 80′ latitude South and 47° 25′ 42″ longitude West). The local climate is classified as a subtropical humid (Cwa) according to Köppen-Geiger classification (Kottek et al. 2006). The qPCR analyses were performed in the Physiology and Molecular Endocrinology Laboratory, Faculty of Veterinary Medicine and Animal Science, University of São Paulo. Nellore cows with 73 ± 15 days post-partum, 5.3 ± 0.6 years old, body condition score of 5.65 ± 1.27 (scale 1 to 9), and live weight of 677 ± 17 kg were used in this project. They were kept in Urochloa (syn. Brachiaria) pastures, with mineral supplementation and water ad libitum. All cows were held in a shaded area with a water trough before measurement and after they were returned to pasture. The air temperature (Ta), black globe temperature (Tbg) and relative humidity (RH) were continuously measured during 6 days of experiment by data-loggers HOBO U12-013 (Onset HOBO® temp/RH/2 ext. channels) (Table 1). On the last four days of the experiment, the heat condition was also assessed by the temperature and humidity index (THI), as described by Thom (1958): THI = Ta + 0.36 × Tdp + 41.2, where Ta is the air temperature and Tdp is the dew point temperature, both in degrees Celsius (Table 2).
Experiment 1: heat loss efficiency
The animals were classified as Efficient (EF) and Inefficient (NEF) using the vaginal temperature (Tvag) continuously monitored from day 1 to 6 by data-loggers. In this experiment, 16 healthy lactating purebred Nellore cows (Bos taurus indicus) were used. The data-loggers (HOBO Water Temperature Pro v2) used were housed in a blank progesterone implant to allow better placement in the reproductive tract, with the dimensions 11.5 cm × 3.0 cm × 43 g (L×C×W). This methodology is described in the conference abstract of Hooper et al. 2017b.
The methodology used to understand the heat loss efficiency was based on the studies of Fisher et al. (2008) and Hillman et al. (2009). From the 6 days of vaginal temperature profiles measurement, 2 days with similar climatic conditions were chosen. On these 2 days, the vaginal temperatures were separated in heat gain period, from 12:00 to 19:00, and heat loss period, from 19:00 to 07:00. To represent heat loss and heat gain, two regression equations were calculated. The angular coefficients were determined to each animal for the heat gain, ascending slope (positive β), and heat loss, downward slope (negative β). The EF were those that quickly dissipate heat with higher downward slope and the NEF were those below the mean of downward slope. Rectal (Tr), tail (Tt), and ocular area temperatures (Toc), sweating rate (SR), and respiratory frequency (RF) were collected to understand the thermoregulatory responses and compare with the heat loss classification, on days 3, 4, 5, and 6, in the morning at 07:00 and afternoon at 15:00.
The Tr and Tt were measured using clinical and contact thermometers. The Toc was taken by thermal images (IRT) using the IR camera Testo® 875-2i (Testo Ltd., Altom Hampshire, UK). The camera featured a temperature range from − 30 °C to + 100 °C with a resolution of 2.1 mrad, accuracy of ± 2% of reading, and thermal sensitivity of < 50 mK. The emissivity was adjusted to 0.98 in accordance with the emissivity for mammals and livestock skin (Kastberger and Stachl 2003). The thermal images were taken on animals’ face at 1-m distance and processed in the camera software where a circle on the ocular area was drawn to get the surface mean temperature (Fig. 1). The SR was obtained by the Schleger & Turner (1965) method, in which three paper discs containing cobalt chloride are fixed with adhesive tape in a rectangular area without fur at the left upper thorax between the 10th and 11th ribs. After fixation, the discs’ color change (blue to light pink) are timed. The SR is obtained with the following formula: SR (g m−2 h−1) = (22 × 3.600)/(2.06 × mean of time in seconds for discs’ color change). The RF was measured by counting the breath movements per minute (bpm).
Experiment 2: HSPs gene expression
Blood sampling and in vitro heat treatment
At 7:00 h on day 6 of experiment 1 (Ta = 26.33 °C), non-coagulated whole blood samples were taken to in vitro heat treatment in three different water bath devices calibrated for 38 °C (control), 40 °C (heat stress), and 42 °C (heat stroke) for two consecutive hours (Wang et al. 2003). Six sodium heparin tubes (10 IU/ml, total volume of 8 ml) were sampled by cow in the jugular vein, in which each water bath receipt two tubes (16 ml of blood per treatment). All blood tubes were kept at room temperature after sampling and were immediately subjected to heat treatment.
Isolation of peripheral blood mononuclear cells
At the end of heat treatment, the PBMCs were isolated by gradient centrifugation. The samples were centrifuged at 1.174g for 20 min at room temperature. The leucocyte layer was transferred to a 1.5-mL microfuge and the tube filled with hemolysis solution. Successive washes were done until the leucocyte pellet was depleted of plasma and lysed RBCs. One last wash was performed with PBS, the final supernatant was discarded and the cell pellet rapidly stored (less than 2 min) at − 80 °C for RNA preservation. The cells were kept on that condition for 1 year until RNA extraction. The PBMCs were used as a model to understand the systemic heat shock response without the interference of other external factors (McClung et al. 2008; Deb et al. 2013; Kishore et al. 2013; Mohanarao et al. 2014). Five EF and five NEF cows were randomly selected for the study of HSPs gene expression in PBMCs. The molecular analysis presented here are also described in the conference abstract of Hooper et al. 2017a.
Total RNA extraction and cDNA synthesis
The total RNA was isolated by Trizol Reagent method in 1.5-mL tubes (Invitrogen, Carlsbad, CA). The RNA obtained was treated with DNAse I (Invitrogen, Carlsbad, CA) following the manufacturer’s procedures to exclude genomic DNA contamination. The RNA concentration and purity were obtained by NanoVue Plus spectrophotometer (GE Life Sciences) and analyzed by the absorbance ratios of 260/280 and 260/230 respectively. Samples were stored at − 80 °C until cDNA synthesis by reversed transcription (RT-PCR).
The RT-PCR was performed using the Superscript III kit (Invitrogen, Carlsbad, CA). The RNA was diluted in DEPC water to a concentration of 1 μg in 10 μL water. In 0.2 mL microtubules free of DNase and RNase were added 10.0 μL of RNA (1 μg) and 1 μL of pd. (T) 12–18 (0.5 μg μLˉ1). The tubes were then incubated at 65 °C for 5 min and then cooled to 4 °C. A mix solution containing 4.0 μL 5× buffer, 2.0 μL DTT, 1.0 μL dNTP (10 mM each), 1.0 μL RNase Out, and 1.0 μL Superscript III. 9.4 μL of the mix solution was added to each tube and incubated for 1 h at 50 °C, followed by 15 min at 70 °C. Subsequently, the tubes were stored at − 20 °C.
Quantitative real-time PCR
The gene sequences were sourced from NCBI database (http://www.ncbi.nlm.nih.gov/gene) masked by RepeatMasker, designed by PrimerQuestQM and specificity was tested using the BLAST1 software (Table 3) (Bettegowda et al. 2006). The primers were synthesized by a commercial supplier. Each primer was validated by concentration test and standard curve. The concentration test was performed with final concentrations of 150:150 nM, 300:300 nM, and 600:600 nM (forward and reverse primers) in duplicate with a pool of cDNA. To select the concentration of the primers to be used, the data provided by the StepOnePlus software was analyzed by the LinRegPCR software. The choice was made considering the concentration of primers that generated: reaction efficiency closer to 100%, dissociation curve with single amplification peak for cDNA reactions, without peaks for negative control reactions, and with the lowest threshold concentration (Ct). After choosing the primer concentration for each transcript analyzed, a standard curve was elaborated. Serial dilutions of the cDNA: 1:20, 1:40, 1:80, 1:160, and 1:320 were tested in duplicate. The concentration of primers used was chosen according to the result of the concentration test. For acceptance of the curves, slope values, coefficient of determination (r2) and efficiency provided by the StepOnePlus software were analyzed. Curves with efficiency between 90 and 110% and r2 near 1 were accepted.
In order to select the reference genes, GeNorm Microsoft Excel which provides a measure of gene expression stability (M) was used (Vandesompele et al. 2002). The genes beta-actin (ACTβ), ribosomal protein L15 (RPL-15), histone 2, and cyclophilin A (PPIA) were converted to a quantitative expression scale using the delta Ct method and were processed in GeNorm. These genes have been classified based on M values, in which the genes with the most stable expression had the smallest M values. The three most stable genes were ACTβ, RPL15, and PPIA. The target genes Bos taurus heat shock protein family D member 1 (HSP60), Bos taurus heat shock 70-kDa protein 1A (HSP70), and Bos taurus heat shock protein 90 alpha family class A member 1 (HSP90) were normalized with the geometric mean of the most stable reference genes. The efficiency of qPCR and the quantification cycle (Cq) were determined for each sample using the Software LinRegPCR.
The qPCR analyzes were performed in Applied Biosystem equipment StepOnePlus® (software version 2.3, Invitrogen, Carlsbad, CA) with SYBR® Green (Power SYBR® Green PCR Master Mix, Invitrogen, Carlsbad, CA) as the fluorescent label (Power SYBR® Green PCR Master Mix). Each reaction had the total volume of 20 μL (16 μL of SYBR® Green, primers, water with 4 μL of cDNA). MicroAmp® Optical Adhesive Film (MicroAmp® Fast Optical 96-Well Reaction Plate, catalog number: 4346907) and transparent adhesive tape were used to seal them (MicroAmp® Optical Adhesive Film, catalog number: 4360954).
The thermocycling condition of qPCR is characterized by waiting stage: 95 °C for 10 min; 40 cycles: denaturation, temperature increase and separation of the double strand (95 °C for 15 s), annealing, primers bind to their homologous region in the cDNA (60 °C for 1 min); dissociation curve. In all plates, samples and control were tested in triplicate and the negative control was performed only with ultrapure water. The specificity of the amplified portion, the PCR products were purified using QIAquick® PCR Purification Kit (Qiagen Laboratories). The samples were diluted, added with the respective primers and were sent to the Center for Human Genome Studies at the University of São Paulo. The sequences of the results were accessed by the Chomas software and tested for their specificity using the BLAST1 software.
Data analysis
The environmental data were analyzed by the F test and Student’s t test. The Ta, Tbg, RH, and THI were analyzed as dependent variables and the day and period as independent variables, as well as the interaction within days and periods, with GLM procedure of SAS 9.2 software (SAS Inst., Inc., Cary, NC).
For experiment 1, to classify the animals by heat management, logistic regressions using Tvag as dependent variable and time of day as independent variable were made, with repeated measures in time, setting times to obtain the angular coefficients (β) for heat gain and heat loss. The physiological variables were analyzed as repeated measures in time using the animal as a random effect and the date as a block. The means were compared by the F test and Student’s t test. In the multiple comparisons procedure, PDIFF of MIXED procedure of SAS 9.2 software (SAS Inst., Inc., Cary, NC) was used. No interaction was obtained from period and loss efficiency. Pearson correlation coefficients were estimated within physiological and meteorological variables.
In experiment 2, gene expression was analyzed by MIXED procedure of SAS 9.2 software (SAS Inst., Inc., Cary, NC) taking in consideration the fixed effects the heat shock treatments (38, 40, and 42 °C), classification (EF and NEF), and the interaction within heat treatment and classification. Means were compared by F test and Student’s t test (PDIFF). All tests were performed at 5% probability and the values presented by mean and standard error of the mean. The structure used in repeated measures in time analysis was chosen based on the lower Akaike Information Criterion value.
Results
Meteorological data
The highest values of Ta were found at days 1, 2, and 6, of Tbg on days 1, 2, 5, and 6, of RH on days 2, 5, and 6, respectively (Table 1). To analyze the Tvag, days 5 and 6 were selected as a 2 days’ period with similar high load conditions. On these days, the mean of Ta and Tbg observed from 12:00 to 19:00 were 33.67 and 41.82 °C, and from 19:00 to 07:00 were 20.48 and 20.09 °C, respectively. The thermal conditions on the morning (7:00 to 8:00) and afternoon (15:00 to 16:00) during physiological measurements are presented in Table 2. There was difference for all variables between morning and afternoon in days, especially for Tbg and THI. The days 5 and 6 chosen for heat loss classification also showed similarity in the afternoon. This result is described in the conference abstract of Hooper et al. (2017b).
Experiment 1: heat loss efficiency
The maximum, minimum, and angular coefficients for the heat gain (β1) and heat loss (β2) for Tvag in the selected periods are shown in Table 4. There was no difference within EF (0.09 ± 0.006) and NEF (0.16 ± 0.09) for β1 (P value = 0.379). Otherwise, there was statistical difference for β2 (P < 0.001) within EF (0.06 ± 0.005) and NEF (0.03 ± 0.002). The profile of Tvag for EF and NEF cows is illustrated on Fig. 2 under the same environmental condition. The continuous measurement of the Tvag on days 5 and 6 showed body temperature increase from 12:00 to 19:00 and gradual decrease from 19:00 to 7:00. In our study, it was observed that the Tvag reached the peak in both groups at around 16:00. According to our classification, the Tvag variation for heat gain period was similar between EF and NEF; however, in the heat loss period, a greater amplitude of Tvag for NEF was observed. The Tr, Tt, Toc, RF, and SR were lower in the morning than in the afternoon (Table 5). Complementarily, the EF cows presented higher SR than NEF cows that presented higher Tr. No statistical difference for the other physiological variables between groups was observed.
Tr showed moderate and positive correlation (P < 0.0001) with Tvag and Tt (r = 0.63), Toc (r = 0.56) and RF (r = 0.35). Tr exhibited weak and positive correlation with SR (r = 0.22), high and positive (P < 0.0001) with Ta (r = 0.71), and Tbg (r = 0.7), and moderate and negative (P < 0.0001) with RH (r = − 0.64). The Ta showed strong or moderate positive correlations (P < 0.0001) with other physiological variables. The strong correlation was observed between Ta and Toc (r = 0.87). This result is described in the conference abstract of (Hooper et al. 2017b).
Experiment 2: HSPs gene expression
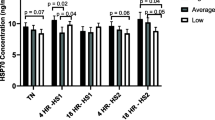
No difference of RNA concentration within treatments was found (38 °C: 1.19 ± 0.20 μg/μL, 40 °C: 0.82 ± 0.09 μg/μL, 42 °C: 1.45 ± 0.28 μg/μL, P = 0.12). Gene expression for all HSPs from 38 to 40 °C was stable. There was a decrease of transcripts’ abundance from 38 to 42 °C and from 40 to 42 °C (Table 6). (The descending order of HSPs is as follows: HSP70 > HSP60 > HSP90 in the treatments at 38 and 40 °C, and at 42 °C was HSP60 > HSP70 > HSP90). There was no difference of gene expression between EF and NEF; however, for both, the HSP70 presented higher expression, followed by HSP60 and HSP90. No effect for the interaction within heat treatment and classification was found.
Discussion
The environmental conditions were within the thermal comfort zone for cattle. The comfort zone for cattle ranges from 10 to 27 °C for air temperature and from 60 to 70% for relative humidity (Titto et al. 2011). Titto (1999) considered for Marchigiana, Nellore, and Simental breeds, the air temperature of stress from 28 to 35 °C. However, in certain hours of the day, the cows were exposed to stress conditions mostly because of high solar radiation, expressed in Tbg. Therefore, the evaluation of black globe temperature is considered a good tool for biometeorology analyses because it combined the effects of air temperature, radiant temperature, and wind speed, providing an indirect measure of radiant heat in degrees Celsius (Kelly and Bond 1971).
The heat stress level can be evaluated according to the air temperature, relative humidity, solar radiation, wind speed, and the combination of them in indexes. The Livestock Weather Safety Index (LWSI) commonly assigns heat stress following THI classification (Mader et al. 2006). The THI values ≤ 74 are classified as normal; alert between 75 and 78; danger, 79–83; and emergency, ≥ 84 (Hahn 1995). In the morning, the THI values were considered normal; however, the risk of heat stress was observed in the afternoon with THI values classified as alert and emergency. Domiciano et al. (2018) working with Nellore steers on integrated systems found THI values from 79 to 85 depending on the dry-rainy season, and health and weight loss problems during the experimental period were not observed even with THI values. Navarini et al. (2009) reported mean THI values of 76, 78, and 80 for small forests, isolated trees, and not shaded conditions, with higher values at 15:00. The same authors suggested that THI around 80 for Nellore cows characterize heat stress condition that in our experiment was observed in the afternoon during physiological measurements.
The continuous measure of internal body temperature has been used to understand animal responses over time rather than single time point measures. It can be performed in different ways, the most common methods are the tympanic (Hahn 1999), rectal (Reuter et al. 2010), intraruminal (Alzahal et al. 2011), and vaginal (Burdick et al. 2012). Hillman et al. (2009) and Burdick et al. (2012) observed a high correlation (r = 0.92) and a difference of 0.06 ± 0.015 °C within Tr and Tvag. In our study, we decided to evaluate vaginal temperature as a continuously measurement to understand heat loss efficiency in time. This methodology allowed us to distinguish cows, in a feasible and reproducible way, in more or less capable to dissipate heat in the same environmental condition.
The influence exerted by the environment on the physiological responses should be evaluated considering changes in day heating and night cooling (West et al. 2003). In our findings, the cows presented an increase on body temperature on day heating, with low influence in the morning and higher in the afternoon. The diurnal rhythm of body temperature is monophasic and cyclic with a maximum at the end of the afternoon and minimum in the morning (Hahn 1999). The same author underlines that the variations of the body temperature depend on the animal and are related to environmental conditions and may alter the rhythm and amplitude.
The Tr was in the physiological normality proposed by Kolb and Ketz (1987), 38.5 to 39.3 °C. The Tvag was slightly above that observed for Tr, nonetheless within physiological normality. The influence of environmental temperature on animals also can be noticed on surface temperatures (Tt and Toc). We anticipated that NEF would have higher Tt and Toc; however, there was no difference in these parameters in the EF and that animals with lower skin surface temperature are more efficient to dissipate heat to the environment (Montanholi et al. 2008, 2009). Martello et al. (2016) emphasize that in thermal stress, the association of infrared thermography traits (IRT) with physiological parameters may be altered according to the environment; that in our case all cows were in the same conditions. The Toc had a positive and strong correlation with Ta (0.81) reflecting the impact of environment on this superficial temperature; moreover, Church et al. (2009) suggest that when the animal is under stress, the activation of the hypothalamic-pituitary adrenal axis occurs with subsequent increase of catecholamine and cortisol levels as well as changes in blood flow and ocular temperature.
The EF presented higher mobilization of evaporative mechanism by increased sweating rate to lose heat than NEF. Probably, the NEF had difficult to manage heat loss using evaporative mechanism (SR) and consequently presented a higher variation in Tvag profile. Jessen (2001) affirms that both sweating and panting are relevant for homeothermia maintenance. Barbosa et al. (2014) found sweating rates of 259.8 and 228.7 g.m−2 h−1 respectively for white and red Nellore. McManus et al. (2009) observed in Nellore the sweating rate from 224.6 to 258.61 g.m−2 h−1. Our findings are higher than that found in the literature. Silva (2000) stated that Zebu cattle are more efficient in using sweating rate than other types. Moreover, it reflects a characteristic related to adaptation in hot climate conditions and its increment promotes the reduction of heart rate and rectal temperature (Cheung and McLellan, 1998).
In both groups, respiratory rate was within the normal range from 24 to 55 bpm (McManus et al. 2014) and all body temperatures studied were positively associated with respiratory rate; hence, the increase in body temperature led to the increase of respiratory frequency. The cows did not recruit panting to lose heat, because the thermal environment was in the thermocomfort zone. The knowledge of these thermoregulatory profiles points out traits in the breed related to good adaptation in our climate condition, majority related to sweating rate capacity and low resistance to heat loss, and would be a good strategy for genetic improvement in tropical regions (Finch 1985).
At the cellular level, the heat treatments did not affect the RNA yield or quality. At control (38 °C) and heat stress (40 °C), HSP gene expression was stable. However, there was a decrease in the amount of transcripts of all HSPs at heat stroke (42 °C). HSP70 had the highest gene expression on control and heat stress treatments, followed by HSP60 and HSP90, respectively. The HSP70 is a highly induced chaperone and is very important in stabilizing native protein conformation, maintaining cell survival and integrity when thermal stressed (Moseley 1997). Although in the heat stroke the total amount of transcripts reduced for all HSPs, the HSP60 was the most expressed chaperone; that is, because it participates in mitochondrial activity and bidirectional mitochondrial membrane transport (Baraja-Vásquez et al. 2005). In our study, the thermal stress at 42 °C leads to a decrease in cell protection mechanisms by the reduction on all HSPs, but among them, the HSP60 was the one with higher expression. This may be explained by the fact that HSP60 is associated with the mitochondria with higher activity during heat stroke than compared to the other treatments. Both HSP70 and HSP90 form a complex of co-chaperone in the cytoplasm to restructure protein conformation, and at heat stroke, both decreased their amount of transcripts. These chaperones collaborate in protein folding to restructure conformation, to promote stabilization, and turnover by their grouping in multichaperone complexes (Pratt et al. 2010). Comparing the amount of transcripts within treatments, the HSP90 was the most constant HSP among the others analyzed. This indicates that it is a good indicator of the existence of heat stress, but it is not as discriminatory in relation to the intensity of heat stress such as HSP70.
In this way, the existing and de novo synthesis of HSPs helps the cell to survive in the face of stress, especially for HSP70. Besides, as observed in other studies, the heat stress promoted cell instability by protein denaturation and degradation, compromising cytoskeletal components, membrane permeability, and inhibition of protein synthesis (Sonna et al. 2002). Controversially, our finding did not corroborate with the results observed by Bañuelos-Valenzuela and Sánchez-Rodríguez (2005); Agnew and Colditz (2008); Mishra et al. (2011) and Kishore et al. (2013).
The acclimatization process not only reduces body temperature by increasing the heat flow to skin and heat dissipation but also allows the body to tolerate high temperatures, and the HSPs are involved in this process (Moseley 1997). Although, EF and NEF cows did not present changes in gene expression, in warm conditions, a different pattern of HSP accumulation can be observed between species and this characteristic is associated with more or less thermotolerance. Liver cells of lizards has greater amounts of HSP70 when the animals were in high-temperature ecological niches, even in non-stressful conditions (Ulmasov et al. 1992). The same was described in brain cells of ants (Cataglyphis bombycina) inhabiting the Sahara Desert (Gehring and Wehner 1995) and with fibroblast cells of humans that inhabit Asia and Europe (Lyashko et al. 1994).
However, as elucidated by Lepock (2005), the cellular response to stress is related to the amount of proteins denatured and aggregated by the effect of thermal stress. In our findings, the conservation of HSPs at 40 °C compared to the control was a protection mechanism used by cells against damage. We know that HSPs and their regulation in PBMCs is one adaptation component of the heat stress response, and heat tolerance in the highly adapted Nellore breed also is encouraged in vivo studies of thermotolerance for future researches.
Conclusions
In conclusion, the method used to discriminate the Nellore cows by their heat loss capacity is reproducible and feasible. In this breed, the efficiency to lose heat was mainly associated with sweating capacity and the cellular adaptation was confirmed by the cellular maintenance of HSPs even at 40 °C. The knowledge to understand the animal’s thermoregulation and thermotolerance is long and requires more studies of cell signaling, in vitro and in vivo. The information achieved in this study contributes as a future key for genetic selection of adapted animals in tropical conditions, when it is important to consider the relation within the environment and productivity.
Abbreviations
- cDNA:
-
Complementary DNA
- EF:
-
Efficient cows
- FZEA:
-
Faculty of Animal Science and Food Engineering
- HSPs:
-
Heat shock proteins
- NEF:
-
Inefficient cows
- IRT:
-
Infrared thermography trait
- L×C×W:
-
Length × circumference × weight
- m:
-
Meter
- PBMCs:
-
Peripheral blood mononuclear cells
- qPCR:
-
Quantitative polymerase chain reaction
- RBCs:
-
Red blood cells
- RF:
-
Respiratory frequency
- RH:
-
Relative humidity
- RT-qPCR:
-
Reverse transcription
- SR:
-
Sweating rate
- T a :
-
Air temperature
- T bg :
-
Black globe temperature
- T dp :
-
Dew point temperature
- THI:
-
Temperature humidity index
- T oc :
-
Ocular temperature
- T r :
-
Rectal temperature
- T t :
-
Tail temperature
- T vag :
-
Vaginal temperature
References
Agnew LL, Colditz IG (2008) Development of a method of measuring cellular stress in cattle and sheep. Vet Immunol Immunopathol 123:197–204. https://doi.org/10.1016/j.vetimm.2008.01.038
Alzahal O, Alzahal H, Steele MA et al (2011) The use of a radiotelemetric ruminal bolus to detect body temperature changes in lactating dairy cattle. J Dairy Sci 94:3568–3574. https://doi.org/10.3168/jds.2010-3944
Bañuelos-Valenzuela R, Sánchez-Rodríguez SH (2005) La proteína de estrés calórico Hsp70 funciona como un indicador de adaptación de los bovinos a las zonas áridas. Redvet VI:1–18
Baraja-Vásquez GE, Baldwin-Sevilla C, Barbosa Cisneros OY, Sánchez-Rodríguez SH (2005) Las proteínas de estrés calórico Hsp60, 70 y 90 participan en la adaptación de los caprinos a las zonas áridas (heat shock proteins 60, 70 and 90 participate in the adaptation of goats to the arid zones). Redvet VI:3–16
Barbosa BRP, Santos SA, UGP A, Egito AA, Comastri Filho JA, Juliano RS, Paiva SR, McManus C (2014) Tolerância ao calor em bovinos das raças Nelore branco, Nelore vermelho e Pantaneira. Rev Bras Saúde Prod Anim 15:854–865
Bettegowda A, Patel OV, Ireland JJ, Smith GW (2006) Quantitative analysis of messenger RNA abundance for ribosomal protein L-15, cyclophilin-A, phosphoglycerokinase, β-glucuronidase, glyceraldehyde 3-phosphate dehydrogenase, β-actin, and histone H2A during bovine oocyte maturation and early embryogenesis. Mol Reprod Dev 73:267–278. https://doi.org/10.1002/mrd.20333
Burdick NC, Carroll JA, Dailey JW, Randel RD, Falkenberg SM, Schmidt TB (2012) Development of a self-contained, indwelling vaginal temperature probe for use in cattle research. J Therm Biol 37:339–343. https://doi.org/10.1016/j.jtherbio.2011.10.007
Carvalho ME, Gasparin G, Poleti MD, Rosa AF, Balieiro JCC, Labate CA, Nassu RT, Tullio RR, Regitano LCA, Mourão GB, Coutinho LL (2014) Heat shock and structural proteins associated with meat tenderness in Nellore beef cattle, a Bos indicus breed. Meat Sci 96:1318–1324. https://doi.org/10.1016/j.meatsci.2013.11.014
Cheung SS, McLellan TM (1998) Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol 84:1731–1739
Church JS, Cook NJ, Schaefer AL (2009) Recent applications of infrared thermography for animal welfare and veterinary research: everything from chicks to elephants. In: InfraMation Proceedings. p 10
Deb R, Sajjanar B, Singh U, Kumar S, Singh R, Sengar G, Sharma A (2013) Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus × Bos taurus) breed of cattle: a comparative study. Gene 536:435–440. https://doi.org/10.1016/j.gene.2013.11.086
Dobson H, Smith RF (2000) What is stress, and how does it affect reproduction? Anim Reprod Sci 60–61:743–752
Domiciano LF, Mombach MA, Carvalho P, da Silva NMF, Pereira DH, Cabral LS, Lopes LB, Pedreira BC (2018) Performance and behaviour of Nellore steers on integrated systems. Anim Prod Sci 58:920. https://doi.org/10.1071/AN16351
Finch VA (1985) Body temperature in beef cattle: its control and relevance to production in the tropics. J Anim Sci 62:531–542. https://doi.org/10.2134/jas1986.622531x
Finch VA, Bennett IL, Holmes CR (1984) Coat colour in cattle: effect on thermal balance, behaviour and growth, and relationship with coat type. J Agric Sci 102:141. https://doi.org/10.1017/S0021859600041575
Fisher AD, Morton R, Dempsey JMA, Henshall JM, Hill JR (2008) Evaluation of a new approach for the estimation of the time of the LH surge in dairy cows using vaginal temperature and electrodeless conductivity measurements. Theriogenology 70:1065–1074. https://doi.org/10.1016/j.theriogenology.2008.06.023
Gehring WJ, Wehner R (1995) Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl Acad Sci U S A 92:2994–2998
Hahn GL (1995) Environmental management for improved livestock performance, health and well-being. Japanese J Livest Manag 30:113–127. https://doi.org/10.20652/kachikukanri.30.3_113
Hahn GL (1999) Dynamic responses of cattle to thermal heat loads. J Anim Sci 77(Suppl 2):10–20
Hales JR, a a F, Bennett JW, Needham a D (1978) Thermal control of blood flow through capillaries and arteriovenous anastomoses in skin of sheep. Pflugers Arch 378:55–63. https://doi.org/10.1007/BF00581958
Hansen PJ (2004) Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci 82–83:349–360. https://doi.org/10.1016/j.anireprosci.2004.04.011
Hillman PE, Gebremedhin KG, Willard ST et al (2009) Continuous measurements of vaginal temperature of female cattle using a data logger encased in a plastic anchor. Appl Eng Agric 25:291–296
Hooper HB, Gonella-Diaza ÂM, Binelli M, et al (2017a) Heat shock response in PBMCs of Nellore cows after in vitro thermal stress. In: VII Brazilian Congress of Biometeorology, Ambience, Behaviour and Animal Welfare (Conference proceeding)
Hooper HB, Henrique FL, Rodríguez LFP, et al (2017b) Heat loss efficiency and thermoregulation responses of Nellore cows. In: VII Brazilian Congress of Biometeorology, Ambience, Behaviour and Animal Welfare (Conference proceeding)
Hutchinson JC, Brown GD (1969) Penetrance of cattle coats by radiation. J Appl Physiol 26:454–464
Jessen C (2001) Temperature regulation in human and other mammals, 1st edn. Springer, Verlag, Berlin
Kastberger G, Stachl R (2003) Infrared imaging technology and biological applications. Behav Res Methods Instrum Comput 35:429–439
Kelly CF, Bond TE (1971) Bioclimatic factors and their measurement. Natl Acad Sci A Guid Environ Res Anim 123
Kishore A, Sodhi M, Kumari P, Mohanty AK, Sadana DK, Kapila N, Khate K, Shandilya U, Kataria RS, Mukesh M (2013) Peripheral blood mononuclear cells: a potential cellular system to understand differential heat shock response across native cattle (Bos indicus), exotic cattle (Bos taurus), and riverine buffaloes (Bubalus bubalis) of India. Cell Stress Chaperones 19:613–621. https://doi.org/10.1007/s12192-013-0486-z
Kolb E, Ketz AHG (1987) Fisiologia Veterinária, 4a. Rio de Janeiro
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World Map of the Köppen-Geiger climate classification updated. Meteorol Zeitschrift 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Kumar A, Ashraf S, Goud TS, Grewal A, Singh SV, Yadav BR, Upadhyay RC (2015) Expression profiling of major heat shock protein genes during different seasons in cattle (Bos indicus) and buffalo (Bubalus bubalis) under tropical climatic condition. J Therm Biol 51:55–64. https://doi.org/10.1016/j.jtherbio.2015.03.006
Lepock JR (2005) How do cells respond to their thermal environment? Int J Hyperth 21:681–687. https://doi.org/10.1080/02656730500307298
Lyashko VN, Vikulova VK, Chernicov VG, Ivanov VI, Ulmasov KA, Zatsepina OG, Evgen’ev MB (1994) Comparison of the heat shock response in ethnically and ecologically different human populations. Proc Natl Acad Sci U S A 91:12492–12495
Mader TL, Davis MS, Brown-Brandl T (2006) Environmental factors influencing heat stress in feedlot cattle. J Anim Sci 84:712–719
Martello LS, da Luz e Silva S, da Costa Gomes R, da Luz e Silva S, da Costa Gomes R, da Silva Corte RRP, Leme PR (2016) Infrared thermography as a tool to evaluate body surface temperature and its relationship with feed efficiency in Bos indicus cattle in tropical conditions. Int J Biometeorol 60:173–181. https://doi.org/10.1007/s00484-015-1015-9
Mason IL, World Dictionary of Livestock Breeds (1988) Third Edition. C.A.B International
McClung JP, Hasday JD, He J-R et al (2008) Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 294:R185–R191. https://doi.org/10.1152/ajpregu.00532.2007
McManus C, Prescott E, Paludo GR, Bianchini E, Louvandini H, Mariante AS (2009) Heat tolerance in naturalized Brazilian cattle breeds. Livest Sci 120:256–264. https://doi.org/10.1016/j.livsci.2008.07.014
Mcmanus CM, Louvandini H, Paim TP, Silva FCP, Bernal FEM (2014) Factors affecting heat tolerance in crossbred cattle in central Brazil. Ciência Anim Bras 15:152–158. https://doi.org/10.1590/1809-6891v15i28726
Mishra A, Hooda OK, Singh G, Meur SK (2011) Influence of induced heat stress on HSP70 in buffalo lymphocytes. J Anim Physiol Anim Nutr (Berl) 95:540–544. https://doi.org/10.1111/j.1439-0396.2010.01082.x
Mohanarao GJ, Mukherjee A, Banerjee D et al (2014) HSP70 family genes and HSP27 expression in response to heat and cold stress in vitro in peripheral blood mononuclear cells of goat (Capra hircus). Small Rumin Res 116:94–99. https://doi.org/10.1016/j.smallrumres.2013.10.014
Montanholi YR, Odongo NE, Swanson KC, Schenkel FS, McBride BW, Miller SP (2008) Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J Therm Biol 33:468–475. https://doi.org/10.1016/j.jtherbio.2008.09.001
Montanholi YR, Swanson KC, Schenkel FS, McBride BW, Caldwell TR, Miller SP (2009) On the determination of residual feed intake and associations of infrared thermography with efficiency and ultrasound traits in beef bulls. Livest Sci 125:22–30. https://doi.org/10.1016/j.livsci.2009.02.022
Moseley PL (1997) Invited review: heat shock proteins and heat adaptation of the whole organism. J Appl Physiol 83:1413–1417
Navarini FC, Klosowski ES, Campos AT, Teixeira RA, Almeida CP (2009) Conforto térmico de bovinos da raça nelore a pasto sob diferentes condições de sombreamento e a pleno sol. Eng Agríc [online] 29:508–517. https://doi.org/10.1590/S0100-69162009000400001
Nay T, Hayman R (1956) Sweat glands in Zebu (Bos indicus L.) and European ( B. taurus L.) cattle. I. Size of individual glands, the denseness of their population, and their depth below the skin surface. Aust J Agric Res 7:482. https://doi.org/10.1071/AR9560482
Pratt WB, Morishima Y, Peng H-M, Osawa Y (2010) Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp Biol Med 235:278–289. https://doi.org/10.1258/ebm.2009.009250
Reuter RR, Carroll JA, Hulbert LE, Dailey JW, Galyean ML (2010) Technical note: development of a self-contained, indwelling rectal temperature probe for cattle research. J Anim Sci 88:3291–3295. https://doi.org/10.2527/jas.2010-3093
Silva RG (2000) Introdução à bioclimatologia animal. Ed. Nobel, São Paulo
Sonna LA, Fujita J, Gaffin SL, Lilly CM (2002) Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92:1725–1742. https://doi.org/10.1152/japplphysiol.01143.2001
Thom EC (1958) Measuring the need for air conditioning. Air Cond Heat Vent 53:68–70
Titto EAL (1999) Estudo da tolerância ao calor em tourinhos das raças Marchigiana, Nelore e Simental. In: Congresso de Zooecnia. Porto, Portugal,
Titto CG, Titto EAL, Titto RM, Mourão GB (2011) Heat tolerance and the effects of shade on the behavior of Simmental bulls on pasture. Anim Sci J 82:591–600. https://doi.org/10.1111/j.1740-0929.2011.00872.x
Ulmasov KA, Shammakov S, Karaev K, Evgen’ev MB (1992) Heat shock proteins and thermoresistance in lizards. Proc Natl Acad Sci U S A 89:1666–1670
Vandesompele J, De Preter K, Pattyn F et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:34
Wang S, Diller KR, Aggarwal SJ (2003) Kinetics study of endogenous heat shock protein 70 expression. J Biomech Eng 125:794–797. https://doi.org/10.1115/1.1632522
West JW, Mullinix BG, Bernard JK (2003) Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J Dairy Sci 86:232–242. https://doi.org/10.3168/jds.S0022-0302(03)73602-9
Acknowledgements
This research was supported by São Paulo Research Foundation, no. 2011/09282-3. We would also like to show our gratitude to Prof. Ed Hoffman Madureira from University of São Paulo, who provided insight and expertise that greatly assisted the research. This article contains parts derived from two conference abstract presented in the VII Brazilian Congress of Biometeorology, Ambience, Behavior and Animal Welfare and are stated in Figshare proceedings (DOIs: https://doi.org/10.6084/m9.figshare.5176753; https://doi.org/10.6084/m9.figshare.5176756).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving animals were in accordance with the current Federal Law no. 11.794, sanctioned by the President of the Republic on November 8, 2008. The law is available in full at http://planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm, and is known as the Arouca Law, which regulates the scientific use of animals in Brazil. The local committee certifies the protocol number CEUA 13.1.2186.74.2, which involves the production, maintenance and/or use of animals belonging to the phylum Chordata, subphylum Vertebrata (except human beings), for scientific research purposes (or teaching)—it is in accordance with Law 11.794, of October 8, 2008, Decree 6899, of July 15, 2009, with the rules issued by the National Council for Control of Animal Experimentation (CONCEA), and was approved by the Ethics Committee on Animal Use of the School of Animal Science and Food Engineering of São Paulo University.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The animals used in the experiment had prior approval of the Ethics Faculty Committee, receiving the protocol number no. 13.1.2186.74.2.
Rights and permissions
About this article
Cite this article
Hooper, H.B., Titto, C.G., Gonella-Diaza, A.M. et al. Heat loss efficiency and HSPs gene expression of Nellore cows in tropical climate conditions. Int J Biometeorol 63, 1475–1486 (2019). https://doi.org/10.1007/s00484-018-1576-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-1576-5