Abstract
We aimed to test the anti-inflammatory and angiogenic properties of two different thermal waters at the cellular level in human keratinocyte cells in the present study. Two different thermal waters, thermo-mineral BJ1 (Bursa, Turkey) and oligomineral BG (Bolu, Turkey), were tested in human keratinocyte (HaCaT) cell line. HaCaT cells were incubated for 3 days with thermal waters; RNA isolation was carried out in the treated and untreated cells. The gene expressions of TNFα, IL-1α, and VEGF were measured using the RT-qPCR. The tested thermal waters significantly decreased the expression of IL-1α (BJ1 93% p = 0.0024 and BG 38% p = 0.0303). BJ1 and BG thermal waters downregulated the expression of TNFα (59% p = 0.0001 and 23% p = 0.0238 respectively). Furthermore, BJ1 and BG significantly downregulated the gene expression of VEGF (98% p = 0.0430 and 15% p = 0.0120). The observed decrease in the gene expression of TNFα and IL1α could be interpreted as an anti-inflammatory effect of mineral waters on HaCaT cells. Moreover, the suppressed VEGF expression might be an indicator of the antiangiogenic effect on human keratinocytes. Therefore, we hypothesized that depending on their specific chemical composition such as silica (128 mg/L) in BJ1 and hydrogen sulfide (1.2 mg/L) in BG, thermal waters suppress pro-inflammatory cytokines and angiogenic growth factor. These preliminary findings might give insight on the underlying mechanisms of the therapeutic benefits observed in some skin diseases such as rosacea and psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural thermal waters have been used for centuries as part of the healing process and the treatment of the diseases. Although there is evidence supporting that thermal mineral water treatment was clinically effective in a variety of dermatological conditions such as atopic dermatitis, psoriasis, and urticaria, there is still a lack of knowledge on the mechanism of effectiveness at the cellular and molecular level. Researchers have recently published an increasing number of in vitro studies providing some insight into the external application of thermal waters on the skin (Joly et al. 2014). Recent in vitro studies with the cell cultures and protein imaging revealed information regarding anti-inflammatory, antioxidant, UVB-protective, anti-allergic, and antiangiogenic properties of these waters (Jung et al. 2009; Varga et al. 2015). The French cosmetic industry has introduced dermocosmetic products formulated with thermal mineral waters (Beauvais et al. 1998; Ghersetich et al. 2001; Ferreira et al. 2010). Researchers reported a thermo-mineral water with known anti-allergic and anti-inflammatory properties which was claimed to be due to inhibition of the mast cell activation (Joly et al. 1998). The anti-inflammatory effect of another natural water on keratinocyte function of human Langerhans cells was also reported (Staquet et al. 2002). The treatment with a mineral water from Korea decreased the expression of pro-inflammatory cytokines associated with the Toll-like receptor stimulation to the HaCaT cells (Lee et al. 2012). A commercial thermal water from France was reported to protect the skin from dehydration based on TauT and SVCT1 expression (Verdy et al. 2012). Thermo-mineral waters were reported to suppress ROS in the keratinocytes after UVB exposure (Joly et al. 2014).
Although the above studies partially revealed the interaction between mineral waters and the skin at cellular level, there is still a lack of understanding of this interaction. Therefore, there is a need for further research on the mechanisms of beneficial effects of external application of thermo-mineral waters. The use of cell cultures is a fast, reliable, and precise method for determining the mechanism of these effects. Therefore, the purpose of the present study was to investigate the anti-inflammatory and anti-angiogenic effects of two different thermal mineral waters from Turkey in human keratinocytes (HaCaT).
Materials and methods
Chemical analysis of thermal waters
Thermal mineral waters were taken from the two traditional and historical thermal resorts of Turkey: Bursa and Bolu. Bursa is a well-known thermal town located in the north west of Turkey south coast of Marmara Sea. The thermal mineral sources of Bursa are being used for treating mostly rheumatic diseases for centuries. Bolu is located in the central Anatolia where one of the thermal water hospitals is being in service for rehabilitation of musculoskeletal. The physicochemical analyses of the thermal mineral waters (BJ1, Bursa, Turkey; BG, Bolu, Turkey) were carried out at the balneology laboratory of Istanbul University. All the analyses were performed based on the Standard Methods for the Examination of Water and Wastewater (Federation WE 2005).
Cell culture
The human keratinocyte cells (HaCaT) were maintained in high-glucose Dulbecco’s Modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/mL gentamicin in an incubator at 37 °C in a humidified atmosphere at 5% CO2 (Supplements and media, Sigma Aldrich, USA).
Cytotoxicity assay
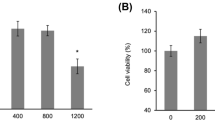
The Cell Proliferation Kit II (XTT) (Roche Diagnostics, Germany) was used for cell viability. 104 cells were seeded 96-well culture plates. After 24 h, the medium was replaced and cells were exposed to different concentrations of thermal waters (50, 25, and 10%) for 48 h. Fifty microliters XTT reagent and activator reagents were added and cells were incubated at same condition for 4 h in order that XTT reagent was reduced to formazan compound. The absorbance was read in microplate reader (BIO-RAD, Japan) at 450 nm with 620 nm reference wavelength. Cell survival was calculated from the equation:
Reverse transcription
HaCaT cells were incubated for 3 days with 10% concentration of thermal waters before total RNA isolation. TRI reagent (Sigma Aldrich) was used for RNA isolation. The concentration and purity of isolated RNA samples were determined using BioSpec-nano (Japan). Transcriptor First Strand cDNA Synthesis Kit was used for reverse transcriptase (Roche Molecular Diagnostics, Germany). Total volume of reaction mixture was 13 μl containing 1 μg total RNA, 2 μM sequence-specific primer, and water. The mixture was heated for 10 min at 65 °C. The mixture volume was completed to 20 μl by adding 10 U of transcriptor reverse transcriptase, 20 U of protector RNase inhibitor, 1× buffer, and 1 mM deoxy nucleotide, and the mixture was incubated at 50 °C for 60 min and then 85 °C for 5 min.
Real-time quantitative polymerase chain reaction
The experiments were conducted with Light Cycler 96 (Roche Diagnostics, USA) and Fast Start DNA Green Master Kit (Roche Molecular Diagnostics, Germany). Each 20 μL reaction contained 10 μL SYBR Green Master Mix (2×), 0.5 μM of reverse and forward primers (Table 1), 2.5 ng cDNA, and appropriate amount of nuclease-free water. All samples were run as triplicates in each run including a non-template control and four standards (1:10, 1:100, and 1:1000). All reactions subjected to initial denaturation step at 95 °C for 10 min and 45 cycle of 3-step amplification. Melting curve analysis was performed to confirm specificity of the amplified products. PCR reaction was carried out in triplicate. For quantitation of RT-qPCR results, ΔΔCt method was used.
Statistical analysis
All data are representative of three experiments and expressed as mean ± standard deviation. Statistical evaluation was performed by non-parametric Mann–Whitney test (GraphPad prism 6) and statistical significance was defined as p < 0.05.
Results
Chemical analysis of thermal waters
The chemical analysis of thermo-mineral waters is given in Table 2.
Cytotoxicity analysis (XTT cell proliferation assay)
The concentration of the tested thermal waters showed no toxic effect for HaCaT cells (Fig. 1).
RT-PCR
The tested thermal waters decreased the expression of IL-1α (BJ1 0.07 ± 0.043 p = 0.0024 and BG 0.623 ± 0.125 p = 0.0303) when compared with the untreated control cells. However, a significant downregulation of TNF-α was found only with BJ1 and BG thermal waters (0.4123 ± 0.013 p = 0.0001 and 0.7745 ± 0.262 p = 0.0238 respectively). BJ1 and BG waters showed downregulation of the gene expression of VEGF significantly (0.018 ± 0.031 p = 0.043 and 0.8526 ± 0.115 p = 0.012 respectively) (Table 3).
Discussion
The biological effects of thermal waters at cellular level have been reported since early 1990s (Beauvais et al. 1998; Joly et al. 1998; Celerier et al. 1995). The most significant effect of thermo-mineral waters was the anti-inflammatory activity. Among the studied anti-inflammatory factors were IL-1α, IL-2, IL-6, IL-8, TNFα, and other cytokines (Jung et al. 2009; Lee et al. 2012; Seite 2013), and also, the anti-angiogenic effect of VEGF suppression (Chiarini et al. 2006). The cells were incubated with the thermal waters at different concentrations to determine the cell viability. Based on our results, thermal waters were not cytotoxic at any concentration. Fluctuations in cell viability were not significant in this test. Therefore, cell viability results could not be correlated with factor levels such as VEGF at this stage. The only purpose of this stage was to determine the optimum concentration of thermal waters for further studies. One of the studied waters was BJ1 which significantly suppressed IL-1α, TNFα, and VEGF (Fig. 2). Whereas, the other thermo-mineral water BG suppressed TNFα and IL-1α, although significant but less pronounced effect on the VEGF expression. This observed difference might be attributed to their specific mineral content. The main difference of BG from BJ1 was sulfur content (1278 mg/L) (Table 2). Sulfur, which is found as H2S form in thermal mineral waters, has a well-known anti-inflammatory and anti-oxidative effect in HaCaT cells (Carbajo and Maraver 2017; Yang et al. 2011); therefore, the anti-inflammatory effect of BG water might be attributed to its sulfur content. On the other hand, silicium, zinc, sodium bicarbonate, and boron ingredients which are common elements in both thermal waters might be also responsible for the suppression of pro-inflammatory cytokines. In the previous studies, researchers attributed the suppression of interleukins to selenium and strontium (Chiarini et al. 2006). Therefore, a specific element in thermo-mineral water may induce a biological effect at least in the cell cultures representing the skin. High zinc content of both waters (BJ1 = 0.130 mg/L and BG = 0.1 mg/L) may be responsible for the anti-inflammatory effect. The anti-inflammatory effect of zinc was previously reported (Prasad 2014). Furthermore, researchers have also demonstrated boron as a component of a thermo-mineral water acted on keratinocytes as a wound-healing agent (Chebassier et al. 2004). Therefore, one of our thermal waters, BG has high boron content (51.5 mg/L); BG could be a good candidate on keratinocyte proliferation. The other common chemical components of BJ1 and BG were sodium, calcium, and bicarbonate. Calcium was found to be essential in wound healing and keratinocyte differentiation in the skin (Lansdown 2002; Elsholz et al. 2014). The BJ1 thermal water contains a higher level of calcium (88 mg/L) with respect to BG (0.9 mg/L). We have discussed the individual elements and their effects in HaCaT cells; however, it must be born in mind that the total chemical composition of the studied waters might be responsible for the observed overall effect. Finally, there are some limitations in this study. First of all, it is a cell culture study. The changes in the cells do not necessarily reflect themselves at the tissue or organ level. Secondly, our conclusions relating to the minerals of the thermal waters with the measured factor levels do not cover the entire range of elements dissolved in water. In addition, we did not use primary cells as control. We only used standardized HaCaT cells for the repeatability and reproducibility of the tests. We finally underline the fact that the findings of this study were based on selected gene levels not based on the protein synthesis in the cell.
Conclusion
In conclusion, BJ1 and BG thermo-mineral waters, when incubated with HaCaT cells, caused significant downregulation of TNFα, IL-1α, and VEGF gene expressions. These findings might give some insight on the underlying mechanisms of the therapeutic benefit of balneotherapy and topical applications of mineral waters. Clinical studies are needed for confirmation of the possible benefits of these waters in the management of some skin diseases such as rosacea and psoriasis where inflammation and angiogenesis are involved.
References
Beauvais F, Garcia-Mace JL, Joly F (1998) In vitro effects of Uriage spring water on the apoptosis of human eosinophils. Fundam Clin Pharmacol 12(4):446–450
Carbajo JM, Maraver F (2017) Sulphurous mineral waters: new applications for health. Evid Based Complement Alternat Med 2017:8034084
Celerier P, Litoux P, Dreno B, Richard A (1995) Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res 287(7):680–682
Chebassier N, Ouijja el H, Viegas I, Dreno B (2004) Stimulatory effect of boron and manganese salts on keratinocyte migration. Acta Derm Venereol 84:191–194
Chiarini A, Dal Pra I, Pacchiana R, Menapace L, Zumiani G, Zanoni M, Armato U (2006) Comano’s (Trentino) thermal water interferes with the expression and secretion of vascular endothelial growth factor-A protein isoforms by cultured human psoriatic keratinocytes: a potential mechanism of its anti-psoriatic action. Int J Mol Med 18(1):17–25
E.W. Rice Federation, WE and American Public Health Association (2012) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
Elsholz F, Harteneck C, Muller W, Friedland K (2014) Calcium-a central regulator of keratinocyte differentiation in health and disease. Eur J Dermatol 24(6):650–661
Federation WE, American Public Health Association (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
Ferreira MO, Costa PC, Bahia MF (2010) Effect of São Pedro do Sul thermal water on skin irritation. Int J Cosmet Sci 32(3):205–210
Ghersetich I, Brazzini B, Hercogova J, Lotti TM (2001) Mineral waters: instead of cosmetics or better than cosmetics? Clin Dermatol 194:478–482
Joly F, Charveron M, Aries MF, Bidault J, Kahhak L, Beauvais F, Gall Y (1998) Effect of Avène spring water on the activation of rat mast cell by substance P or antigen. Skin Pharmacol Physiol 11(2):111–116
Joly F, Branka JE, Lefeuvre L (2014) Thermal water from Uriage-les-Bains exerts DNA protection, induction of catalase activity and Claudin-6 expression on UV irradiated human skin in addition to its own antioxidant properties. J Cosmet Dermatol Sci Appl 4:99–106
Jung SH, Seo YK, Youn MY, Park CS, Song KY, Park JK (2009) Anti-aging and anti-inflammation effects of natural mineral extract on skin keratinocytes. Biotechnol Bioprocess Eng 14(6):861–868
Lansdown AB (2002) Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen 10(5):271–285
Lee HP, Choi YJ, Cho KA, Woo SY, Yun ST, Lee JT, Kim HJ, Lee KH, Kim JW (2012) Effect of spa spring water on cytokine expression in human keratinocyte HaCaT cells and on differentiation of CD4+ T cells. Ann Dermatol 24.3:324–336
Prasad AS (2014) Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol 28:364–371
Seite S (2013) Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example. Clin Cosmet Investig Dermatol 6:23
Staquet MJ, Peguet-Navarro J, Richard A, Schmitt D, Rougier A (2002) In vitro effect of a spa water on the migratory and stimulatory capacities of human Langerhans calls. Eur J Dermatol 12.4:LIX–LXI
Varga C, László M, Gerencsér G, Gyöngyi Z, Szendi K (2015) Natural UV-protective organic matter in thermal water. J Photochem Photobiol B Biol 144:8–10
Verdy C, Branka JE, Lefeuvre L (2012) Modulation of sodium-dependent transporters expression in normal human keratinocytes by a sodium rich isotonic thermal water. J Cosmet Dermatol Sci Appl 2:254–262
Yang C, Yang Z, Zhang M, Dong Q, Wang X, Lan A, Zeng F, Chen P, Wang C, Feng J (2011) Hydrogen sulfide protects against chemical hypoxia-induced cytotoxicity and inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2 pathway. PLoS One 6(7):e21971
Acknowledgments
We would like to thank the provider of the samples of thermal waters: Bursa Jeotermal A.Ş., Bursa, Turkey, and Erpilic A.Ş., Bolu, Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karagülle, M.Z., Karagülle, M., Kılıç, S. et al. In vitro evaluation of natural thermal mineral waters in human keratinocyte cells: a preliminary study. Int J Biometeorol 62, 1657–1661 (2018). https://doi.org/10.1007/s00484-018-1565-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-1565-8