Abstract
Assessing diet composition of White Storks (Ciconia ciconia) breeding under North African conditions provides key information to understanding its trophic niche for conservation purpose. Since, climate controls productivities of foraging habitats and thus food availability for predators, this study examines how Storks’ diet parameters varied following a climate gradient along with rural-to-urban landscapes in north-eastern Algeria. Feeding strategies to cope with severe conditions were discussed in light of climate aridity and urbanization and how these influence reproduction, population dynamics and distribution. While invertebrate prey accounted for 94 % of ingested individuals, the biomass intake was dominated by chicken remains scavenged from rubbish dumps (67 %) and small mammals (14 %). Generalized linear models revealed that prey numbers varied significantly between climatic regions and landscapes types, but no significant differences were observed for other dietary parameters, including prey biomass. The study showed high dietary similarity between study climates and landscapes, mainly among rural and urban colonies located in semi-arid and sub-humid areas, which differed from those in suburban and arid climate. Rarefaction and extrapolation curves indicated that prey species richness in White Stork diets was expected to be higher in urban colonies located in sub-humid climate. Despite low prey species diversity in arid regions, the White Stork demonstrates a broad trophic niche, which could be due to supplementary feeding from human refuse. This study suggests that regardless of the climate or landscape, White Storks ensure a constant food intake, despite prey biomass fluctuations, by adapting their diet. Foraging in diverse habitats, including trash dumps, ensures a sufficiently balanced diet to meet nutritional requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modifications of climate and landscape due to urbanization affect birds through modifications in the habitat and food supply, which change ecosystem processes, including changes in biotic interactions like intra- and interspecific competitions, predation, and illnesses (Chace and Walsh 2006; Fuller et al. 2012). Urbanization changes local climates through modifications of clouds and precipitation, but also through changes in atmospheric radiation and precipitation as initiated by anthropogenic aerosols. These climate modifications are connected to population density, land use, level of industrialization, building compactness, seasons, and predominating environmental forcing (Jin et al. 2005). Moreover, urban areas provide also additional and permanent foraging and nesting opportunities (Alberti 2005; Mainwaring 2015; Chenchouni 2016; Chenchouni et al. 2016), which positively affect urban-adapted and urban-exploiter species (Chace and Walsh 2006; Fuller et al. 2008).
Because local climatic conditions are critical in controlling food resources, which in turn affect reproductive performance, the effects of climate on the diet and reproduction of the White Stork have been explored (Jovani and Tella 2004; Tryjanowski et al. 2009). Studies have investigated the influence of climate factors and variability in food availability at foraging/breeding habitats on reproductive patterns (Tortosa et al. 2003; Tryjanowski et al. 2005a; Denac 2006; Djerdali et al. 2008; Kosicki 2012; Si Bachir et al. 2013). In addition, Si Bachir et al. (2013) reported that levels of urbanization affected the breeding success and population densities of the species.
Several studies of the impacts of climate conditions on White Stork have started to explore how climate patterns affect breeding success and individual survival through changes in habitat primary production (Schaub et al. 2005) and prey availability (Tryjanowski and Kuzniak 2002) following severe climatic/weather events (Tryjanowski et al. 2005a, b, 2009; Tobolka et al. 2015). These studies document how climate/weather modifies breeding parameters and population dynamics including nestling survival through change in climate, food supply and nest sites. Most studies have not asked directly questions on the effects of climate patterns on diet composition and feeding ecology, but instead they investigate how weather conditions affect White Stork productivity and nestling survival and their inferences for population dynamics (Tryjanowski et al. 2009; Kosicki 2012) while insinuating that modifications in feeding/foraging ecology are interlocutors in the process. With this in view, climate change also induces an uphill shift in the distribution and nesting of the White Stork (Tryjanowski et al. 2005b), which causes changes in foraging niches and opportunities between different climatic regions (Chenchouni 2016) and thus influences breeding success (Kosicki 2010; Chenchouni et al. 2016).
Research on food composition plays a vital role in assessing foraging habitats and defining the trophic niches of species for conservation purposes (Alonso et al. 1991). Birds in general, and waterbirds in particular, are good indicators of rapid changes in environmental conditions due to climate change, urbanization or changes in landscape configuration and function (Durant et al. 2007; Gregory and Strien 2010; Bensizerara et al. 2013). The abundance of available food resources appears to be one of the main factors limiting the densities of animal populations (Karasov 1996; Janiszewski et al. 2014), especially in birds (Brasher et al. 2007; Tryjanowski et al. 2009; Crampton et al. 2011).
Studies of the diet of White Storks (Ciconia ciconia Linnaeus 1758) have shown that it is an opportunistic predator (Vrezec 2009). Most of these studies are based on the analysis of digested remains found in regurgitated pellets (Mužinić and Rašajski 1992). Even though the analysis of pellets has some caveats, this technique has been successfully used in the past and is a less invasive technique than other options such as direct analysis of gut contents (Chenchouni 2014). However, it is as relevant as some techniques that force regurgitation using chemical components (Zduniak 2005).
The White Stork has been extensively studied in Europe (Tryjanowski et al. 2009). However, in North African breeding grounds, information on its diet is scarce and mostly limited to the northern areas of Algeria (Boukhemza 2001; Sbiki 2009; Djerdali et al. 2016). This makes data from the African range of this species very useful ecologically in terms of establishing a comprehensive conservation plan since data from other North African countries is scarce (Ciach and Kruszyk 2010). Previous studies have mainly focused on (1) dietary composition based on pellet analyses (Boukhemza et al. 1995); (2) food resource availability and composition (Boukhemza et al. 2004, 2006); (3) foraging habitat use (Boukhemza et al. 2006; Chenchouni 2016); and (4) feeding strategies during different breeding stages (Chenchouni et al. 2015). White storks have been reported to feed and rest at rubbish dumps in North Africa (Morocco, Egypt), as well as in the Middle East and the Arabian Peninsula (Ciach and Kruszyk 2010). This behaviour is also common in Algeria (Chenchouni et al. 2015; Djerdali et al. 2016). To my knowledge, there is no study addressing variation in White Stork dietary patterns along a climatic gradient, including arid and semi-arid areas, nor comparing rural to urban landscapes. Furthermore, the influence of urbanization on wildlife, including birds, remains poorly investigated at the North African scale.
In a global warming scenario (Durant et al. 2007), mismatches can occur between local peaks in food abundance and the timing of breeding, with severe consequences for reproductive success (Van Der Jeugd et al. 2009; Thomas et al. 2001). Since, climatic conditions are subject to spatial and temporal variations that affect foraging habitats and food resources, White Storks are likely to modify their feeding efforts to maintain high levels of energy consumption and fitness whatever the climate, thereby ensuring high reproductive success. Under different climatic conditions, the White Stork is expected to adjust its food intake according to dietary characteristics (quantity, quality and availability), e.g. either by consuming a large number of prey under mesic climate, targeting prey of higher biomass in xeric climate areas where prey are scarce, or selecting prey higher in nutritional value in particular foraging habitat with high productivity.
The climate along with landscape type and structure, affects the productivity and breeding numbers of birds, through changes in water availability and agricultural conditions (Tryjanowski et al. 2005a, b; Ramo et al. 2013; Si Bachir et al. 2013). Moreover, birds select heterogeneous productive habitats (Johst et al. 2001; Tsachalidis and Goutner 2002; Boukhemza et al. 2006; Janiszewski et al. 2014) that offer a range of food resources varying in quality, abundance and availability (Kosicki et al. 2006), which affects breeding success (Tryjanowski and Kuźniak 2002; Tortosa et al. 2003; Massemin-Challet et al. 2006). To properly understand whether aridity and urbanization influence White Stork diet, it is essential to describe dietary composition across climates and landscapes. Here, I assess if diversity in White Stork diets decrease in urban environments compared to natural-rural habitats and if dietary composition (in terms of diversity and quantity) varies according to habitat productivity (as influenced by climate). This study examines the variation in biodiversity and similarity of prey communities found in White Stork diet across a mesic-xeric climatic gradient and along rural-to-urban areas.
Material and methods
Study area and colonies
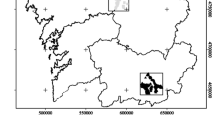
The diet of nesting pairs of White Storks in rural and urban areas of Batna (north-eastern Algeria), where the bulk of the White Stork population lives (Si Bachir et al. 2013), was studied using pellet analysis. Eight White Stork colonies located in different climatic areas (sub-humid, semi-arid and arid) were surveyed (Fig. 1): (1) Merouana, (2) Oued El Ma, (3) Seriana, (4) K’sar Belezma all experience a cold sub-humid climate; (5) Ferme Riche, (6) Bouachaoune, (7) Chemora have a cool semi-arid climate; and (8) N’Gouas presents a cool arid climate. These broad climatic differences in the study area are mainly due to the large range in elevations and the specific location between the Mediterranean Sea (~115 km to the north) and Sahara Desert (~90 km to the south) (Chenchouni et al. 2010).
Climate information for each colony site was generated based on interpolations of long-term weather data from the nearest ten meteorological stations around the study colony in question. Interpolations were carried out using the nearest neighbour method of the climate software package New_LocClim version 1.10 (http://www.fao.org/nr/climpag/pub/en3_051002_en.asp). Although these interpolations are more suited for broad-scale areas, some climatic differences were observed between some of these White Stork study sites, as follow:
Climate characteristics in Merouana, Oued El Ma, K’sar Belezma and Seriana were quite similar. A second group of climatically similar colonies includes the Ferme Riche, Bouachaoune and Chemora sites. Finally, these two sets of colonies have different climate characteristics than those of the colony of N’Gouas. The Budyko’s radiation index of dryness (Budyko 1974) (range, 2.88–2.94) indicated a semi-arid climate in the first set of colonies, whereas the rest of colonies are classified as desert with Budyko’s radiation index of dryness varied between 3.48 and 3.86. However, the De Martonne’s index (De Martonne 1925) revealed that the climate in Merouana, Oued El Ma, K’sar Belezma and Seriana is dry sub-humid, whereas it is semiarid in Ferme Riche, Bouachaoune, Chemora and N’Gouas (Appendix Table 6). In all study colonies, the estimated precipitation deficit showed values greater than 688 mm/year, which is higher than the annual rainfall recorded in the region. The climatic net primary production (NPP) of these sites ranges between 582 and 741 g DM/m2/year, with precipitation, rather than temperatures, thought to be the main factor limiting NPP in such North African habitats (Bradai et al. 2015).
The colonies of Bouachaoune, K’sar Belezma, and Ferme Riche are located in rural landscapes, the colonies of Oued El Ma and Seriana lie within suburban landscapes, while the colonies of Chemora, Merouana and N’Gouas are situated in urban areas. This classification was based on the extent of urban areas, human population size, and adjacent agricultural and natural landscapes. Urbanized areas consisted of built-up conurbations with a population of 20,000 or more. The population sizes of Merouana, N’Gouas and Chemora was about 41,125, 31,920 and 20,369 inhabitants, respectively, at the time of study (data provided by DPAT, Direction de Planification et d’Aménagement du Territoire, Batna City) (Appendix Table 6). Rural areas were considered any place with less than 1,000 inhabitants living in scattered houses situated within a matrix of natural and/or agricultural landscapes. Suburban territories were those that did not fit either of these latter two classifications.
Collection and analysis of pellets
In late January, old pellets were removed from nesting sites constructed on top of different support-types (e.g. roofs, electricity pylons) at each study colony before the arrival of breeding White Storks. Regurgitated pellets were then collected at regular intervals (every 2 weeks) throughout the reproductive period from early February 2011 to late July 2011. Out of more than 200 total pellets collected during that period, only 87 regurgitated pellets from breeding adults were analysed during this study. Only non-fragmented pellets were analysed, which were selected based on their size (large length and breadth) and oval shape. Small pellets from chicks were not analysed for this study. Of these 87 pellets, 36 were from the sub-humid zone, 40 from the semi-arid region and 11 from arid areas. Similarly, divided among landscapes, these 87 pellets were represented by 14 from suburban landscapes 37 from rural and 36 from urban areas. We employed sample-based rarefactions to compare diet richness (Colwell 2013) (see ‘Extrapolation of species richness’ section).
Pellets were separated and analysed using standard methods (Duffy and Jackson 1986; Rosenberg and Cooper 1990). Prey remains were identified to genus and/or species level (or at least to family level) using a binocular stereomicroscope and identification guides/keys.
Biodiversity analyses
Observed prey biodiversity
The pellet was used as a unit of measure for the following dietary parameters. The number of individual ‘prey-items’ (ni) was determined for each prey species found in each pellet, and the prey frequency ‘PF’ was calculated as the proportion of the prey species i of the total number of individuals (N). Observed species richness ‘S’ was estimated as the total number of prey species identified (Magurran 2004). Mean N per pellet (Nm) and mean S per pellet (Sm) were the average numbers of individuals and prey species, respectively, observed in pellets. Prey biomass (B) was evaluated as the weight of ‘ni’ individuals of a prey species divided by the total weight of all prey individuals (Vivien 1973). Prey biomass was obtained from previous studies conducted under the same ecological conditions in the study area (Ghenam and Si Bachir 2011; Chetara and Mechouma 2012; Chenchouni 2014). For both invertebrate and vertebrate prey species, fresh weight was used to determine the biomass of the identified prey species. Prey frequency (PF) and prey biomass (B) were calculated for pellets pooled according to climate zone or landscape type. For these latter two parameters, data are presented at the level of order to facilitate comparisons between studies (Duffy and Jackson 1986). Pearson’s Chi-square tests (χ 2) were used to compare values of PF and B of the main prey groups (defined as taxonomic orders of prey) among study climatic areas and landscape types.
The diversity of White Stork diets was measured using Shannon’s index (H′ = −∑(pi × log2pi)) and Shannon’s evenness (E = H′/H max × 100, with H max = log2 S), where pi is the proportion of the prey species i in the diet (Magurran 2004). Evenness values vary from 0 (representing a dietary specialist) to 100 % (indicating a dietary generalist). In addition, the Food Niche Breadth (FNB): FNB = 1/∑pi2 was applied to assess prey diversity and the trophic niche of the species. The values of this index range from 1 to S (i.e. the total number of observed prey species), with larger values indicating a wider feeding niche dimension (Levins 1968). A new ratio (FNB/S) was adapted to express accurately the food niche breath of the species. The ratio FNB/S varies between 0 (representing a narrow trophic niche) and 1 (indicating a broader feeding niche).
Estimates of prey species richness
The program EstimateS 9.1.0 (Colwell 2013) was used to compute estimated prey species richness (S est). S est was assessed using the most precise and the least biased estimators (Brose and Martinez 2004). The following asymptotic species richness estimators were applied: (1) S est (analytical) that provides the expected number of prey species among a given number of samples (Colwell et al. 2012); (2) the first-order Jackknife estimator of species richness (S (Jack 1) = S + Q 1((m–1)/m)) (Heltshe and Forrester 1983); and (3) Chao 2 estimated species richness (S (Chao 2) = S+((m–1)/m)(Q 1 2/2Q 2)) (Chao 1987); where S is the total number of prey species observed in all pellets pooled, m is the total number of pellets analysed, and Q 1 and Q 2 are the frequencies of uniques (prey species that occur in only one pellet) and duplicates (species that occur in only two pellets), respectively. The numbers of uniques and duplicates, as well as values of species richness estimators, are given as mean (±standard deviation ‘SD’) based on 100 runs of randomizations (Colwell et al. 2012). Moreover, four indices of species diversity were simultaneously computed using EstimateS: (1) Fisher’s alpha (the alpha parameter of a fitted logarithmic series distribution); (2) Shannon diversity index (H′); (3) the inverse form of Simpson diversity index (1/I s) where I s = (∑ni(ni − 1))/N(N − 1); and (4) Hill numbers = (1/I s )/exp.(H′) (Magurran 2004).
Extrapolation of species richness
In order to deal with the varying sample size between climates and landscapes and to equalize the information content of samples, rarefaction and extrapolation curves were linked based on a set of appropriate statistical sampling models (Colwell et al. 2012; Colwell 2013). Species richness was extrapolated to facilitate comparison of the richness of rarefied reference samples (i.e. the numbers of pellets). Extrapolations were conducted to a sample size of 300 pellets for the entire study area and for each climate and landscape. Interpolations of S est are given with the lower and upper bounds of a 95 % confidence interval.
Model of raw data is multiple-sample-based-data (batch input, including climates and landscapes of colony sites: see Table S2: https://dx.doi.org/10.6084/m9.figshare.2008671) used in species richness estimations and interpolations. More details on rarefaction, extrapolation, species richness estimators, and indices of species diversity are available in Colwell (2013).
Spatial similarities of prey communities
In order to estimate shared species richness between climatic regions and landscape types, several similarity indices were computed. These included qualitative similarity estimates using the classic Jaccard and Sørensen indices, and also quantitative approaches such as raw and estimated Chao’s Jaccard indices, raw and estimated Chao’s Sørensen indices, the Morisita–Horn index and the Bray–Curtis index (Magurran 2004; Chao et al. 2005). The free software EstimateS was applied in calculation of all shared species richness and similarity statistics (Colwell 2013). The model of raw data used for the analysis of similarities was Format 1 of Single-Sample-Based-Data ‘Filetype 1’ (see Table S3 at https://dx.doi.org/10.6084/m9.figshare.3463310 for climatic regions and Table S4 at https://dx.doi.org/10.6084/m9.figshare.3463313 for landscape types).
Statistical analyses
Generalized linear models (GLMs) were performed to model the effects of climate and landscape types on each dietary parameter (N, B, S, FNB, FNB/S, H′, H max, E) recoded within each pellet. The effect of the interaction ‘climate × landscape’ was included in every GLM. Parameters represented by count data (N, S) were modelled using a Poisson distribution error and log link function, whereas the remaining dietary parameters (B, FNB, FNB/S, H′, H max, E) were tested with a Gaussian distribution and Identity link (Myers et al. 2012). Type-II analysis-of-variance was used for each GLM to emphasize the effects of factors (climate, landscape, climate × landscape) and to downplay the analysis of deviance (likelihood-ratio tests ‘LR’). Type-II tests were applied because all of the predictors in the GLMs were factors. The software R was used for statistical modelling (R Core Team 2015).
Results
Diet composition and prey richness
Chi-squared tests revealed that prey frequencies of taxonomic prey orders differed significantly, both between climatic areas (χ 2 = 45.41, df = 24, P = 0.005) and between landscape types (χ 2 = 70.15, df = 24, P < 0.0001). However, analysis of contingency tables showed no significant difference in biomass values for these prey groups either among climatic areas (χ 2 = 7.39, df = 24, P = 0.999) or among landscape types (χ 2 = 4.91, df = 24, P = 0.999). Two thousand one hundred thirty-eight prey items were identified in White Stork diets within the study area, comprising 61 prey species distributed among 7 classes, 13 orders, 30 families and 54 genera. Twelve orders of prey were consumed in semi-arid colonies, and nine in both sub-humid and arid colonies. Invertebrate prey accounted for 95 % of all prey items, while vertebrate prey constituted the remainder. Insects were the most consumed prey for all climate zones (PF = 94 % of all prey items), of which the Coleoptera were the best represented, with 1,728 individuals (PF = 80.82 %, S = 35 species), followed by Dermaptera (PF = 8.79 %, S = 5 species) and Orthoptera (PF = 3.41 %, S = 5 species). Chicken remains were the most constant food item in the studied pellets. This food category also dominated dietary biomass (B = 68.97 %), followed by Rodentia (B = 14.41 %) and Coleoptera (B = 7.70 %; Table 1).
Dietary characteristics and food niche
The average number of prey items per pellet ranged between 30.1 ± 15.4 (mean ± standard deviation) in arid areas and 23.2 ± 14.4 in the semi-arid climate (where the maximum number of prey observed was 927, PF = 43 %). The highest value of species richness was recorded in semi-arid colonies, having 56 prey species (Sm = 7.7 ± 2.4 prey species per pellet) and in colonies located in rural and urban landscapes (both S = 54 prey species; Sm = 8.4 ± 3.0 and Sm = 8.2 ± 2.4, respectively), followed by 52 prey species for sub-humid colonies (Sm = 4.6 ± 5.6). Food niche breadth was broader in arid colonies (FNB/S = 0.36), although the highest values of FNB and S were recorded for semi-arid colonies (FNB/S = 0.25). Regarding prey species diversity and evenness, the diet of White Storks was more diversified in semi-arid and sub-humid climates (H′ = 4.5 and H′ = 4.3, respectively), compared to the arid climate sites (H′ = 3.9). Rural and urban colonies exhibited higher values of Shannon’s index (H′ = 4.6 and H′ = 4.5, respectively), compared to suburban colonies (H′ = 3.6). Prey species were equally distributed under different climate types (evenness = 75.7–78.8 %), but they were slightly more evenly distributed in rural colonies (E = 79.7 %) compared to suburban colonies (E = 70.6 %) (Table 2).
Variation of diet across climates and landscapes
The generalized linear model revealed that the number of prey individuals (N) varied significantly between climatic areas (P = 0.01) and between landscape types (P < 0.01). In addition, the GLMs showed that the remaining dietary parameters measured in each pellet did not vary significantly either between climates or between landscape types (P > 0.05). The effect of the interaction of these two factors (climate × landscape) on all dietary characteristics was not significant (Table 3).
Estimates of prey species richness
For the entire study area, i.e. all pellets pooled irrespective of the climatic region or landscape type, rarefaction curves increased slightly with the number of pellets before reaching a plateau (Fig. 2). Expected species richness curves calculated using the Chao 2 richness estimator ‘S (Chao 2)’ and the first-order Jackknife richness estimator ‘S (Jack 1)’ both proved to be stable and reached a plateau for the entire study area. Even though the shapes of the rarefaction curves for these two estimators are similar, S (Jack 1) is probably the most appropriate for determining expected prey species richness in the White Stork diet. This is due to its correctness and robustness (Brose et al. 2003) and because its curve displayed higher values than that of S (Chao 2). Moreover, this estimator presented an interval that covered all values of the estimated species richness generated with the other asymptotic estimators used in this analysis (Table 4).
Asymptotic richness estimation, diversity indices and rarefaction of prey species in White Stork diets in North Africa: Top left: sample-based rarefaction curves of three nonparametric richness estimators (analytical ‘S (est)’, first-order Jackknife ‘S (Jack 1)’ with SD represented by shaded area, and Chao 2 ‘S (Chao 2)’) and uniques ‘Q 1’ and duplicates ‘Q 2’ with SD as bars . Bottom left: diversity indices (Fisher’s alpha, Shannon, Simpson and Hill) are given for the entire study area based on the reference sample of 87 pellets. Values represented in the curves are means with standard deviations (SD) as vertical bars. Only every fourth data point of the diversity indices and richness estimators are shown. Means and SD are computed based on 100 randomized sample ‘runs’ (see details in Colwell 2013). Top right: species richness estimated with the first-order Jackknife ‘S (Jack 1)’ for different climates and landscapes. Bottom left: rarefied and expected species richness for the whole study area. Violet area represents lower and upper bounds of 95 % confidence intervals (CI) for the analytical estimated species richness S (est)
Total prey species richness was estimated to be 66 prey species, while S (Chao 2) indicated a value of 62 prey species for the White Stork diet (Fig. 2). The first-order Jackknife richness estimator revealed that the expected prey species richness in the diet decreased with increasing aridity. Total prey species richness was estimated to be 68.5 for sub-humid colonies, 64.8 for semi-arid colonies and 38.2 for the arid colony. Expected prey species richness was lower for colonies located in suburban landscapes with 44.1 prey species compared to urban and rural colonies where prey species richness was estimated to be 69.9 and 61.8, respectively (Table 4). Values for accumulated species, richness estimators, and diversity indices are reported in Table S5 (https://dx.doi.org/10.6084/m9.figshare.3463316) for each level of accumulation, from a single sampling unit to the full reference sample (per climate and landscape) up to a sampling size of 300.
Prey species richness extrapolations
Based on species richness extrapolation from the reference pellets of each climate zone and landscape to a theoretical sample size of 300 pellets, rarefaction curves revealed that the analytical species richness S est kept increasing with the number of pellets in colonies located in urban and suburban landscapes and under sub-humid climates. S est was expected to reach 95.5, 71.8 and 49.7 prey species in sub-humid climates, urban and suburban landscapes, respectively (Fig. 3). The prey species expected in semi-arid and arid climates, rural landscapes as well as in the entire study area reached a plateau, with S est values expected for 300 pellets relatively close to those estimated for the reference sample of pellets. For 300 pellets, S est was expected to be 62.5 prey species for the entire study area, 59.6 and 35.3 prey species in semi-arid and arid climates, respectively, and 56.4 prey species in rural landscapes (Figs. 2 and 3). Table S5 (https://dx.doi.org/10.6084/m9.figshare.3463316) gives full diversity statistics including other species richness estimators and species diversity indices computed for climates, landscapes, and the total diet of White Storks in north-eastern Algeria based on the reference samples and extrapolated for a sample size of 300 regurgitated pellets.
Sample-based rarefaction and extrapolation curves of prey species richness estimated in the diets of White Storks along a gradient of climatic regions (sub-humid, semi-arid and arid) and urbanization landscapes (rural, suburban and urban) in North Africa. Reference pellet samples are indicated by black solid circles, rarefaction curves by solid lines, and extrapolation curves by dashed lines. Coloured areas represent lower and upper bounds of 95 % confidence intervals (CI) for the analytical estimated species richness S (est)
Spatial similarities of the prey species
Prey species compositions assessed between the climatic regions and landscapes of the studied White Stork colonies indicated high similarity based on qualitative (Classic Jaccard and Sørensen similarity indices) or abundance-based indices. Among the climatic regions, all indices revealed that dietary similarity was highest between sub-humid and semi-arid colonies (Table 5). Considering landscapes, all indices showed the highest similarity in diet between rural and urban colonies. Both Chao’s Jaccard and Chao’s Sørensen indices applied using raw and estimated data showed the highest similarity values (>0.83) among climates and landscapes.
Discussion and conclusion
The White Stork is distributed across multiple habitats in north-eastern Algeria, where a remarkably steep climatic gradient is prominent, but occurs mostly in humid, sub-humid and semi-arid areas. Some substantial colonies also exist under hot-arid climatic conditions (Si Bachir et al. 2013). My analysis of White Stork diet demonstrates that it feeds on larger and more diverse foods in sub-humid and semi-arid climatic conditions than in arid climates, as is also the case for urban landscapes compared to rural and suburban areas. This outcome could be explained by the greater availability of food resources in these areas. Arid regions are known as low productivity ecosystems due to their adverse ecological conditions that curtail the survival of many species (Noy-Meir 1973; Chenchouni 2012; Bradai et al. 2015).
Overall, dietary parameters were quite similar between sub-humid and semi-arid climates, as well as between colonies located in rural and urban landscapes. However, the White Stork seems to have a broader food niche under arid climates compared to sub-humid and semi-arid climates. This suggests that populations in arid zones expend more effort to attain their nutritional requirements (Ehrlich et al. 1993). Despite the diversity of prey species being low for arid climate colonies, the prey communities found in their diet showed higher values of evenness than those of the sub-humid and semi-arid areas. This reveals that the White Stork regularly consumes the same prey species in equivalent amounts in arid zones (and in rural landscapes), which is clearly shown in the tight confidence intervals for the extrapolation curves of prey species richness. In order to ensure a balanced diet under arid conditions, which is often synonymous with food shortages, birds need to increase foraging effort to match their nutritional needs, which can vary according to phenological breeding stages (Hilgartner et al. 2014; Chenchouni et al. 2015). This scenario is supported by FNB values, which are larger under arid conditions, representing clear evidence that generalist-foragers tend to expand their trophic niche under severe climatic conditions.
The observed differences in the frequencies of prey orders in diet across the studied climatic regions and landscapes may be explained by differences in foraging habitat productivities and food availability in these regions (Noy-Meir 1973). Certainly, arid areas are known to have lower primary productivity compared to mesic climatic areas (Bradai et al. 2015). So habitats under such conditions offer less abundant food resources, which forces birds to increase their feeding effort to attain the optimal diet.
Other studies investigating the dietary composition of the White Stork have indicated that it is an opportunistic forager (Peris 2003; Milchev et al. 2013). Two primary characteristics of its trophic niche are noteworthy: (1) in terms of consumed numbers of individuals, the diet is dominated by invertebrate prey (mainly coleopteran insects), with percentages exceeding 90 % of boli; and (2) in terms of biomass, these invertebrate prey represent only a small proportion of the total biomass so their energy contribution is marginal, with vertebrate prey contributing most energy (Barbraud and Barbraud 1998; Boukhemza 2001; Tsachalidis and Goutner 2002; Sbiki 2009; Vrezec 2009; Milchev et al. 2013). This opportunistic feeding behaviour explains the high values of dietary evenness (E), revealing that the White Stork is a dietary generalist par excellence.
This opportunism towards natural prey, as opposed to refuse scavenging, is more obvious for storks in arid climates that have a broader food niche breadth, indicating that they use the food resources of the foraging habitats in a complementary and compensatory manner to address their nutritional needs, impacting on fitness, reproductive performance and also migration outcomes (Drent et al. 2006; Byholm and Kekkonen 2008; van den Burg 2009; Hušek et al. 2013; Hilgartner et al. 2014; Djerdali et al. 2016). The GLMs revealed significant variation in the number of prey individuals, while other dietary parameters did not significantly differ, suggesting that the White Stork tends to balance its diet by ensuring a constant food supply whatever the climatic area or landscape type. The species achieves this balance by varying the numbers of consumed prey to match a constant biomass value, which results in a widening of its food niche breath by increasing its trophic niche in less productive areas/habitats or for periods of high food intake demand such as during pre- and post-migration and chick rearing.
Although insect prey represent only a small part of the dietary biomass, they are important for reproductive success, especially during the period of chick growth (Barbraud and Barbraud 1998). White Storks use insect prey when preferred prey (primarily vertebrates) are lacking in order to compensate and complement its food needs, particularly in hot-arid regions (Sekour et al. 2011). For quite similar reasons, chicken remains dominate the diet of the White Stork and constitute the major dietary biomass. White Storks consume chicken remains because they are constantly available, are abundant and are easily accessible in the uncontrolled trash dumps across North Africa. In addition, scavenging spares the White Stork as well as other bird species the effort of hunting down countless small prey of low calorific content. Therefore, the findings of this work indicate that urbanization level of colonies affects White Stork diet through intensive use of trash dumps as a food resource, which influences the productivity and numbers of breeding White Storks. This behaviour is not restricted to North African colonies (Chenchouni 2016), having been reported from European countries with mild climates such as Spain (Tortosa et al. 2002) and Poland (Kruszyk and Ciach 2010). In addition, the species can benefit from human socio-agricultural activities in North Africa, where agricultural development and rehabilitation of degraded arid lands is creating new favourable foraging habitats for White Storks (Johst et al. 2001; Latus and Kujawa 2005; Chenchouni 2016). However, agriculture intensification negatively affects bird productivity (Latus and Kujawa 2005; Tryjanowski et al. 2011; Janiszewski et al. 2014).
Scavenging from trash dumps is an increasingly common feeding strategy among several animal species, which usually select breeding and feeding habitats that are expected to provide perpetual abundant and available food of high quality and requiring low energy expenditure to acquire it (Alonso et al. 1991; Tortosa et al. 2002; Kosicki et al. 2006; Janiszewski et al. 2014). It is also possible that animal species frequently attend these man-made environments because natural environments do not always provide their food requirements, especially during periods of peak nutritional demand such as during brood rearing (Chenchouni et al. 2015; Djerdali et al. 2016), or during seasons of food scarcity occurring after severe climatic conditions or extreme weather events (Tryjanowski and Kuzniak 2002; Schaub et al. 2005; Tryjanowski et al. 2005a; Tryjanowski et al. 2009; Tobolka et al. 2015).
The White Stork also consumes other prey categories (such as Stylommatophora, Scorpionida, Blattodea, Heteroptera, Testudines) that are considered complementary to its diet, as they are found being consumed with low to moderate amounts on the one hand, and/or they are specific to a given climatic area on the other hand. In fact, the presence of some prey species in the diet and its composition in general depends on characteristics of the foraging habitats (Tryjanowski and Kuźniak 2002; Tsachalidis and Goutner 2002; Massemin-Challet et al. 2006).
As a synanthropic species, the diet of the White Stork is tightly linked to human activities. As a result, its bioecology and eco-ethology are significantly influenced and increasingly dependent on the resources and waste generated by humans and the habitat modifications due to diverse agricultural and urban activities (Chenchouni et al. 2016). Future studies of White Storks should investigate the effects of habitat modification and changes in land use (particularly urbanization and agricultural intensification) on food resources and breeding parameters (e.g. clutch size, chick survival and reproductive success), especially under arid conditions where habitats are less productive and the species is more reliant on anthropogenic food resources.
References
Alberti M (2005) The effects of urban patterns on ecosystem function. Int Reg Sci Rev 28:168–192. doi:10.1177/0160017605275160
Alonso JC, Alonso JA, Carrascal LM (1991) Habitat selection by foraging White Storks, Ciconia ciconia, during the breeding season. Can J Zool 69:1957–1962. doi:10.1139/z91-270
Barbraud C, Barbraud J-C (1998) Le régime alimentaire des poussins de Cigogne blanche Ciconia ciconia, en Charente-Maritime: importance des insectes. Alauda 65:259–262
Bensizerara D, Chenchouni H, Si Bachir A, Houhamdi M (2013) Ecological status interactions for assessing bird diversity in relation to a heterogeneous landscape structure. Avian Biology Research 6:67–77. doi:10.3184/175815513X13577344603957
Boukhemza M. (2001). Étude bio-écologique de la Cigogne blanche (Ciconia ciconia L, 1775) et du Héron garde-bœufs (Bubulcus ibis L, 1775) en Kabylie. Analyse démographique, éthologique et essai d’interprétation des stratégies trophiques. PhD Thesis, INA El Harrach, Algiers
Boukhemza M, Righi M, Doumandji S, Hamdine W (1995) Le régime alimentaire de la Cigogne blanche (Ciconia ciconia L, 1775) dans la vallée du Sébaou, région de Kabylie, Algérie. Alauda 63:199–207
Boukhemza M, Doumandji S, Voisin C, Voisin J-F (2004) Comparative utilization pattern of trophic resources by White Storks Ciconia ciconia and cattle egrets Bubulcus ibis in Kabylia (Algeria). Revue d’Ecologie-La Terre et la Vie 59:559–580
Boukhemza M, Boukhemza-Zemmouri N, Voisin J-F, Baziz B (2006) Écologie trophique de la Cigogne blanche (Ciconia ciconia) et du Héron garde-bœufs (Bubulcus ibis) en Kabylie (Algérie). Ecologia Mediterranea 32:15–28
Bradai L, Bissati S, Chenchouni H, Amrani K (2015) Effects of climate on the productivity of desert truffles beneath hyper-arid conditions. Int J Biometeorol 59:907–915. doi:10.1007/s00484-014-0891-8
Brasher MG, Steckel JD, Gates RJ (2007) Energetic carrying capacity of actively and passively managed wetlands for migrating ducks in Ohio. J Wildl Manag 71:2532–2541. doi:10.2193/2006-401
Brose U, Martinez ND (2004) Estimating the richness of species with variable mobility. Oikos 105:292–300. doi:10.1111/j.0030-1299.2004.12884.x
Brose U, Martinez ND, Williams RJ (2003) Estimating species richness: sensitivity to sample coverage and insensitivity to spatial patterns. Ecology 84:2364–2377. doi:10.1890/02-0558
Budyko MI (1974) Climate and life. Academic Press, New York
Byholm P, Kekkonen M (2008) Food regulates reproduction differently in different habitats: experimental evidence in the goshawk. Ecology 89:1696–1702. doi:10.1890/07-0675.1
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69. doi:10.1016/j.landurbplan.2004.08.007
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791. doi:10.2307/2531532
Chao A, Chazdon RL, Colwell RK, Shen T-J (2005) A new statistical approach for assessing compositional similarity based on incidence and abundance data. Ecol Lett 8:148–159. doi:10.1111/j.1461-0248.2004.00707.x
Chenchouni H (2012) Diversity assessment of vertebrate fauna in a wetland of hot hyperarid lands. Arid Ecosyst 2:253–263. doi:10.1134/s2079096113010022
Chenchouni H (2014) Diet of the little owl (Athene noctua) during the pre-reproductive period in a semi-arid Mediterranean region. Zool Ecol 24:314–323. doi:10.1080/21658005.2014.965919
Chenchouni H. 2016. Modelling the foraging habitats use of breeding white storks (Ciconia ciconia) across a mesic-xeric climatic gradient in North Africa. Under review
Chenchouni H, Zanati K, Rezougui A, Briki A, Arar A (2010) Population monitoring of pine processionary moth (Thaumetopoea pityocampa) by pheromone trapping at the southern limit of distribution of Pinus halepensis in Eastern Algeria. For Sci Technol 6:67–79. doi:10.1080/21580103.2010.9671974
Chenchouni H, Si Bachir A, AlRashidi M (2015) Trophic niche and feeding strategy of the White Stork (Ciconia ciconia) during different phases of the breeding season. Avian Biol Res 9:1–13. doi:10.3184/175815515x14232310459990
Chenchouni H, Smai I, Nezzar C, Si Bachir A, Tobolka M, Sparks TH, Tryjanowski P, Norris K. 2016. Breeding success of White Storks (Ciconia ciconia) nesting on phone masts and electricity pylons under North African conditions. Under review
Chetara S, Mechouma W. 2012. Contribution à la connaissance de la diversité des micromammifères du parc national de Belezma. Master dissertation, University of Batna, Algeria
Ciach M, Kruszyk R (2010) Foraging of white storks Ciconia ciconia on rubbish dumps on non-breeding grounds. Waterbirds 33:101–104. doi:10.1675/063.033.0112
Colwell RK. 2013. EstimateS: statistical estimation of species richness and shared species from samples. Version 9. Available at: http://purl.oclc.org/estimates
Colwell RK, Chao A, Gotelli NJ, Lin S-Y, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. J Plant Ecol 5:3–21. doi:10.1093/jpe/rtr044
R Core Team. 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at www.r-project.org.
Crampton LH, Longland WS, Murphy DD, Sedinger JS (2011) Food abundance determines distribution and density of a frugivorous bird across seasons. Oikos 120:65–76. doi:10.1111/j.1600-0706.2010.18624.x
De Martonne E. 1925. Traité de Géographie Physique: 3 tomes. Paris
Denac D (2006) Resource-dependent weather effect in the reproduction of the White Stork Ciconia ciconia. Ardea 94:233–240
Djerdali S, Tortosa FS, Hillstrom L, Doumandji S (2008) Food supply and external cues limit the clutch size and hatchability in the White Stork Ciconia ciconia. Acta Ornithologica 43:145–150. doi:10.3161/000164508x395252
Djerdali S, Guerrero-Casado J, Tortosa FS (2016) Food from dumps increases the reproductive value of last laid eggs in the White Stork Ciconia ciconia. Bird Study 63:107–114. doi:10.1080/00063657.2015.1135305
Drent RH, Fox AD, Stahl J (2006) Travelling to breed. J Ornithol 147:122–134. doi:10.1007/s10336-006-0066-4
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colonial Waterbirds 9:1–17. doi:10.2307/1521138
Durant JM, Hjermann DØ, Ottersen G, Stenseth NC (2007) Climate and the match or mismatch between predator requirements and resource availability. Clim Res 33:271. doi:10.3354/cr033271
Ehrlich PR, Ehrlich AH, Daily GC (1993) Food security, population and environment. Popul Dev Rev 19:1–32. doi:10.2307/2938383
Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ (2008) Garden bird feeding predicts the structure of urban avian assemblages. Divers Distrib 14:131–137. doi:10.1111/j.1472-4642.2007.00439.x
Fuller RA, Irvine KN, Davies ZG, Armsworth PR, Gaston KJ (2012) Interactions between people and birds in urban landscapes. In: Lepczyk CA, Warren PS (eds) Urban bird ecology and conservation. Studies in avian biology (no. 45). University of California Press, Berkeley, CA, pp. 249–266
Ghenam K, Si Bachir A. 2011. Approche biométrique et calorimétrique de quelques ordres d’insectes (Coleoptera, Orthoptera, Dermaptera et Hymenoptera) de la région de Batna (Algérie). Proceedings of SIBFA Conference, Univ. Ouargla, Algeria, pp. 150–156
Gregory RD, Strien AV (2010) Wild bird indicators: using composite population trends of birds as measures of environmental health. Ornithol Sci 9:3–22. doi:10.2326/osj.9.3
Heltshe J, Forrester NE (1983) Estimating species richness using the jackknife procedure. Biometrics 39:1–11. doi:10.2307/2530802
Hilgartner R, Stahl D, Zinner D (2014) Impact of supplementary feeding on reproductive success of white storks. PLoS One 9:e104276. doi:10.1371/journal.pone.0104276
Hušek J, Adamík P, Albrecht T, Cepák J, Kania W, Mikolášková E, Stenseth NC (2013) Cyclicity and variability in prey dynamics strengthens predator numerical response: the effects of vole fluctuations on White Stork productivity. Popul Ecol 55:363–375. doi:10.1007/s10144-013-0366-5
Janiszewski T, Minias P, Wojciechowski Z, Podlaszczuk P (2014) Habitat selection by White Storks breeding in a mosaic agricultural landscape of Central Poland. Wilson J Ornithol 126:591–599. doi:10.1676/13-219.1
Jin M, Dickinson RE, Zhang DA (2005) The footprint of urban areas on global climate as characterized by MODIS. J Clim 18:1551–1565. doi:10.1175/jcli3334.1
Johst K, Brandl R, Pfeifer R (2001) Foraging in a patchy and dynamic landscape: human land use and the white stork. Ecol Appl 11:60–69. doi:10.1890/1051-0761(2001)011[0060:FIAPAD]2.0.CO;2
Jovani R, Tella JL (2004) Age-related environmental sensitivity and weather mediated nestling mortality in white storks Ciconia ciconia. Ecography 27:611–618. doi:10.1111/j.0906-7590.2004.03925.x
Karasov WH. 1996. Digestive plasticity in avian energetics and feeding ecology. In Carey C. (ed) Avian energetics and nutritional ecology. Springer US, pp. 61–84. DOI 10.1007/978-1-4613-0425-8_3
Kosicki JZ (2010) Reproductive success of the White Stork Ciconia ciconia population in intensively cultivated farmlands in western Poland. Ardeola 57:243–255
Kosicki JZ (2012) Effect of weather conditions on nestling survival in the White Stork Ciconia ciconia population. Ethol Ecol Evol 24:140–148. doi:10.1080/03949370.2011.616228
Kosicki JZ, Profus P, Dolata PT, Tobółka M (2006) Food composition and energy demand of the White Stork Ciconia ciconia breeding population. Literature survey and preliminary results from Poland. In: Tryjanowski P, Sparks TH, Jerzak L (eds) The White Stork in Poland: studies in biology, ecology and conservation. Bogucki Wydawnictwo Naukowe., Poznań, pp. 169–183
Kruszyk R, Ciach M (2010) White Storks, Ciconia ciconia, forage on rubbish dumps in Poland—a novel behaviour in population. Eur J Wildl Res 56:83–87. doi:10.1007/s10344-009-0313-0
Latus C, Kujawa K (2005) The effect of land cover and fragmentation of agricultural landscape on the density of White Stork (Ciconia ciconia L.) in Brandenburg, Germany. Pol J Ecol 53:535–543
Levins R. 1968. Evolution in changing environments: some theoretical explorations. Princeton University Press
Magurran AE (2004) Measuring biological diversity. Wiley-Blackwell, New York
Mainwaring MC (2015) The use of man-made structures as nesting sites by birds: a review of the costs and benefits. J Nat Conserv 25:17–22. doi:10.1016/j.jnc.2015.02.007
Massemin-Challet S, Gendner JP, Samtmann S, Pichegru L, Wulgué A, Le Maho Y (2006) The effect of migration strategy and food availability on White Stork Ciconia ciconia breeding success. Ibis 148:503–508. doi:10.1111/j.1474-919x.2006.00550.x
Milchev B, Chobanov D, Simov N (2013) Diet and foraging habitats of non-breeding White Storks (Ciconia ciconia) in Bulgaria. Arch Biol Sci 65:1007–1013. doi:10.2298/abs1303007m
Mužinić J, Rašajski J (1992) On food and feeding habits of the White Stork, Ciconia c. ciconia, in the Central Balkans. Ökologie der Vögel 14:211–223
Myers RH, Montgomery DC, Vining GG, Robinson TJ (2012) Generalized linear models: with applications in engineering and the sciences, vol. 791. John Wiley & Sons, New York
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51. doi:10.1146/annurev.es.04.110173.000325
Peris SJ (2003) Feeding in urban refuse dumps: ingestion of plastic objects by the white stork (Ciconia ciconia). Ardeola 50:81–84
Ramo C, Aguilera E, Figuerola J, Máñez M, Green AJ (2013) Long-term population trends of colonial wading birds breeding in Doñana (SW Spain) in relation to environmental and anthropogenic factors. Ardeola 60:305–326. doi:10.13157/arla.60.2.2013.305
Rosenberg KV, Cooper RJ (1990) Approaches to avian diet analysis. Stud Avian Biol 13:80–90
Sbiki M. 2009. Contribution à l’étude comparative des niches trophiques de deux échassiers de la région de Tébessa: La Cigogne blanche (Ciconia ciconia) et le Héron garde-bœufs (Ardea ibis). Magister dissertation, University of Tebessa, Algeria
Schaub M, Kania W, Köppen U (2005) Variation of primary production during winter induces synchrony in survival rates in migratory white storks Ciconia ciconia. J Anim Ecol 74:656–666. doi:10.1111/j.1365-2656.2005.00961.x
Sekour M, Beddiaf R, Souttou K, Denys C, Doumandji S, Guezoul O (2011) Variation saisonnière du régime alimentaire de la chouette chevêche (Athene noctua)(Scopoli1769) dans l’extrême sud-est du Sahara algérien (Djanet, Algérie). Revue d’Ecologie-La Terre et la Vie 66:79–91
Si Bachir A, Chenchouni H, Djeddou N, Barbraud C, Céréghino R, Santoul F (2013) Using self-organizing maps to investigate environmental factors regulating colony size and breeding success of the White Stork (Ciconia ciconia). J Ornithol 154:481–489. doi:10.1007/s10336-012-0915-2
Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR (2001) Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291:2598–2600. doi:10.1126/science.1057487
Tobolka M, Zolnierowicz KM, Reeve NF (2015) The effect of extreme weather events on breeding parameters of the White Stork Ciconia ciconia. Bird Study 62:377–385. doi:10.1080/00063657.2015.1058745
Tortosa FS, Caballero JM, Reyes-López J (2002) Effect of rubbish dumps on breeding success in the White Stork in southern Spain. Waterbirds 25:39–43. doi:10.1675/1524-4695(2002)025[0039:EORDOB]2.0.CO;2
Tortosa FS, Pérez L, Hillström L (2003) Effect of food abundance on laying date and clutch size in the White Stork Ciconia ciconia. Bird Study 50:112–115. doi:10.1080/00063650309461302
Tryjanowski P, Kuzniak S (2002) Population size and productivity of the White Stork Ciconia ciconia in relation to common vole Microtus arvalis density. Ardea 90:213–217
Tryjanowski P, Jerzak L, Radkiewicz J (2005a) Effect of water level and livestock on the productivity and numbers of breeding white storks. Waterbirds 28:378–382. doi:10.1675/1524-4695(2005)028[0378:eowlal]2.0.co;2
Tryjanowski P, Sparks TH, Profus P (2005b) Uphill shifts in the distribution of the white stork Ciconia ciconia in southern Poland: the importance of nest quality. Divers Distrib 11:219–223. doi:10.1111/j.1366-9516.2005.00140.x
Tryjanowski P, Sparks TH, Profus P (2009) Severe flooding causes a crash in production of white stork (Ciconia ciconia) chicks across central and Eastern Europe. Basic Appl Ecol 10:387–392. doi:10.1016/j.baae.2008.08.002
Tryjanowski P, Hartel T, Báldi A, Szymański P, Tobolka M, Herzon I, Golawski A, Konvicka M, Hromada M, Jerzak L, Kujawa K, Lenda M, Orlowski G, Panek M, Skórka P, Sparks TH, Tworek S, Wuczynski A, Zmihorski M (2011) Conservation of farmland birds faces different challenges in western and Central-Eastern Europe. Acta Ornithologica 46:1–12. doi:10.3161/000164511X589857
Tsachalidis EP, Goutner V (2002) Diet of the White Stork in Greece in relation to habitat. Waterbirds 25:417–423. doi:10.1675/1524-4695(2002)025[0417:dotwsi]2.0.co;2
van den Burg AB (2009) Limitations of owl reproduction in the wild: is there a role for food quality besides quantity? Ardea 97:609–614
Van Der Jeugd HP, Eichhorn G, Litvin KE, Stahl J, Larsson K, Van Der Graaf AJ, Drent RH (2009) Keeping up with early springs: rapid range expansion in an avian herbivore incurs a mismatch between reproductive timing and food supply. Glob Chang Biol 15:1057–1071. doi:10.1111/j.1365-2486.2008.01804.x
Vivien ML (1973) Régime et comportement alimentaire de quelques poissons des récifs coralliens de Tuléar (Madagascar). Revue d’Ecologie-La Terre et la Vie 27:551–577
Vrezec A (2009) Insects in the White Stork Ciconia ciconia diet as indicators of its feeding conditions: the first diet study in Slovenia. Acrocephalus 30:25–29. doi:10.2478/v10100-009-0003-8
Zduniak P (2005) Forced regurgitation with tartar emetic as an effective and safe method to study diet composition in hooded crow nestlings. Eur J Wildl Res 51:122–125. doi:10.1007/s10344-005-0090-3
Acknowledgments
I warmly thank Prof. Abdelkrim Si Bachir (University of Batna 2, Algeria) for his help in prey identification and for the valuable conceptual advices. I am grateful to Dr. Omar Hamarsheh (University of Notre Dame, USA) and Dr. Craig Allen (USGS, New Mexico, USA) for their diligent copy editing of the manuscript. Many thanks extend to the field editor and the two reviewers for their constructive comments that greatly improved the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(ZIP 107 kb)
Appendix
Table 6 Location, human population size, climatic and agricultural characteristics of study colonies of the White Stork in northeastern Algeria (North Africa)
Rights and permissions
About this article
Cite this article
Chenchouni, H. Variation in White Stork (Ciconia ciconia) diet along a climatic gradient and across rural-to-urban landscapes in North Africa. Int J Biometeorol 61, 549–564 (2017). https://doi.org/10.1007/s00484-016-1232-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-016-1232-x