Abstract
The gypsy moth is a global pest that has not yet established in New Zealand despite individual moths having been discovered near ports. A climate-driven phenology model previously used in North America was applied to New Zealand. Weather and elevation data were used as inputs to predict where sustainable populations could potentially exist and predict the timing of hatch and oviposition in different regions. Results for New Zealand were compared with those in the Canadian Maritimes (New Brunswick, Nova Scotia, and Prince Edward Island) where the gypsy moth has long been established. Model results agree with the current distribution of the gypsy moth in the Canadian Maritimes and predict that the majority of New Zealand’s North Island and the northern coastal regions of the South Island have a suitable climate to allow stable seasonality of the gypsy moth. New Zealand’s climate appears more forgiving than that of the Canadian Maritimes, as the model predicts a wider range of oviposition dates leading to stable seasonality. Furthermore, we investigated the effect of climate change on the predicted potential distribution for New Zealand. Climate change scenarios show an increase in probability of establishment throughout New Zealand, most noticeably in the South Island.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gypsy moth, Lymantria dispar (L) (Lepidoptera: Lymantriidae), is a major pest in many parts of the Northern Hemisphere. Large numbers of larvae can strip trees of their foliage, damaging and exposing them to disease. The gypsy moth has been of particular concern to New Zealand’s Ministry of Agriculture and Fisheries (MAF) since egg masses were discovered in shipping containers at the beginning of the 1990s. A live adult moth was caught by an early warning trap in March 2003 near Hamilton, provoking a comprehensive eradication programme to prevent an outbreak of the pest (Ross 2004).
The gypsy moth has two strains, the European gypsy moth (EGM) and the Asian gypsy moth (AGM) (Ferguson 1978), and are both native in their namesake regions. The EGM was imprudently introduced to North America in 1869 (Dunlap 1980), resulting in considerable damage to important tree species (Liebhold et al. 1992), and millions of dollars were spent in attempts to control it (Wallner 1996). Within the United States, the EGM spread at an average rate of 20 km per year since its introduction (Liebhold et al. 1992). More recently, AGM incursions have also been discovered in North America, with their origins tracked back to multiple countries (Wallner 1996).
The AGM is considered the greater threat to New Zealand, as it feeds on a wider range of plants than does the EGM (Glare et al. 1997). In contrast to the flightless female EGM, AGM females can disperse up to 30 km (Wallner 1996). EGM and AGM are distinguishable by genetic markers although hybrids of the two can also form (Garner and Slavicek 1996). The gypsy moth is not host specific, and this has contributed to its extensive spread. It has been shown to complete development on 650+ species (53 families) of trees (Liebhold et al. 1995), and more specifically, the AGM completes development on 26 of 55 Australasian species tested by Matsuki et al. (2001), with no or low probability of development predicted on all but one of native New Zealand hosts, Nothofagus solandri. Despite this, the gypsy moth could still cause extensive economic damage to tree species introduced to New Zealand for forestry and agriculture, such as Pinus radiata plantations and apple orchards (Malus spp.). The gypsy moth can also survive on certain grasses, weeds, herbs, and garden crops (Cowley et al. 1993), which could assist population establishment.
We used a phenology model to indicate which areas of New Zealand could support a stable EGM seasonality. Since distribution and development data specific to AGM is sparse, we have assumed that there is sufficient similarity between the strains such that areas predicted to have a stable EGM seasonality may also be suitable for AGM. These results could help suggest appropriate port and region monitoring intensities based on the probabilities of establishment. Furthermore, the timing of life stages could assist in planning the optimal time to place traps as well as predicting when eradication spraying will be most effective. Additionally, we applied the phenology model to a region in Canada that already has an established population of EGM. This allowed us to judge the accuracy of establishment prediction where EGM is known to exist, as well as compare model behaviour between hemispheres.
Materials and methods
We compared the behaviour of a gypsy moth phenology model in New Zealand with its behaviour in another area with temperate–maritime climate. This area consists of three of Canada’s Maritime provinces (New Brunswick, Nova Scotia and Prince Edward Island) where the gypsy moth is marginally adapted and has been established since the 1960s (Benoit and Lachance 1990).
Model description
In comparison with the EGM, little is known about the quantitative development of each AGM life stage and their relative timing. We assumed that the similarities between strains were sufficient to use the EGM seasonality model described by Régnière and Nealis (2002), also by Gray (2004), called the gypsy moth life stage (GMLS) model. This model was used by Logan et al. (2006) to analyse the risk posed by EGM to forest resources in Utah, USA. The GMLS model is a distributed description of the developmental responses of the gypsy moth in all life stages—from the egg to the emergence of adult moths. The egg development component of the model was described by Gray et al. (1991, 1995, 2001). Larval and pupal development were described by Logan et al. (1991) and Sheehan (1992). The version of the GMLS model used in this study inputs daily minimum and maximum temperature either from historical records or from a stochastically generated weather trace (Régnière and Bolstad 1994; Régnière 2006) and uses sinusoidal interpolation between minimum and maximum temperature (Allen 1976) to create 4-h series. The GMLS model was modified to accommodate the particularities of seasonality in the Southern Hemisphere (change of calendar years in midsummer) and the warmer temperate climate of New Zealand. The modified model inputs 3 years of daily temperature data so that at least 2 years of weather data were available. Régnière and Nealis (2002) used an arbitrary limit date for peak oviposition as the single criterion for a viable seasonality. Under the substantially warmer climate of New Zealand, it was not clear that such a date exists. Instead, three criteria for life-cycle viability were used. Given that some adult emergence occurs over the period covered by the input temperature data:
-
1.
Egg hatch must start within 365 days (1 year) of oviposition (an egg viability criterion).
-
2.
All immature insects must be in the egg stage in midwinter (December 31 in the Northern Hemisphere; June 30 in the Southern Hemisphere). This ensures that all individuals in the population are overwintering in the diapause stage. This requirement is particularly important in areas where freezing temperatures occur.
-
3.
Once egg hatch has started, peak oviposition must be reached prior to the next midwinter. This ensures approximate synchrony of feeding stages with foliated deciduous host plants.
To determine if a given temperature time series leads to a stable seasonality, the model is run for several successive generations on the same input. An initial oviposition date is provided, and in each successive generation, the date of peak adult emergence is used to reset to peak oviposition date. If an uninterrupted series of successive viable seasonality cycles occurs, the input temperature trace is concluded to lead to a stable seasonality. The length of this series is arbitrary. Most weather regimes violate one of the viability criteria or reach a stable seasonality within two to four cycles. Logan et al. (2003) suggest that the quick attraction to a stable seasonality indicates that insect pest populations quickly track changes in climate. Gray’s (2004) version of the GMLS model simulates population variability in oviposition dates, whereas Régnière and Nealis’s (2002) version does not. Despite their differences, both models give similar results for GM establishment. Gray’s (2004) model may be more suitable for simulating population dynamics in greater detail, but that was not the purpose of this study.
Phenology prediction in urban areas
To examine detailed model behaviour under different temperature regimes, the GMLS seasonality model was run using weather data from eight cities in New Zealand: Auckland, Hamilton and Wellington in the North Island, and Nelson, Christchurch, Timaru, Dunedin and Queenstown in the South Island (Fig. 1a). The model was also run for three Canadian Maritime urban centres: Kentville, Nova Scotia (NS), Canterbury, New Brunswick (NB) and Bathurst (NB) (Fig. 1b). As the EGM has been established in parts of the Canadian Maritimes since the 1960s, the model’s results at can be compared with the insects’ actual distribution in that area.
The model was run on 30-year weather normals (1971–2000) for New Zealand and North America. The initial oviposition date (O 0) varied from ordinal date (OD) 10 to 360, in steps of 10. For each combination of location and O 0, 50 replicates were run to evaluate the average behaviour of the model across different temperature time series. Each replicate used a 3-year time series of daily temperature minima and maxima stochastically generated from the weather normals. Furthermore, each gypsy moth generation within a replicate reused the same weather trace, setting the oviposition date for each successive generation, O t , from the date of median female moth emergence in the previous generation, O t -1. The simulation stopped as soon as one of the above-mentioned criteria was not met (nonviable seasonality flag, F = 0) or after 15 generations (viable seasonality, F = 1). Average viability p was calculated per location and initial oviposition date, as follows:
We also compiled the final dates of first egg hatch (H 15) and median oviposition (O 15) in all cases of viable seasonality.
To further our understanding of specific model dynamics in both New Zealand and the Canadian Maritimes, we ran the model with 3 years of observed daily temperature data from 2001 to 2003 using four different initial oviposition dates: (OD) 40, 130, 220, and 310 (corresponding roughly to the middle of February, May, August and November). The simulation was continued for up to four generations, setting the oviposition date of each from the median adult female emergence date of the previous generation, with each generation receiving the same weather time series as the previous. The simulation stopped at four generations or as soon as one of the viability criteria was violated. The model output of interest in these simulations was the daily change in the proportion of eggs (distinguishing those in diapause) and of emerged female moths in the population.
Seasonality interpolated over entire region
A series of model runs was carried out for a large number (500) of randomly located points over New Zealand and over the Canadian Maritimes using normals from the two nearest stations to each simulation point (corrected for local thermal gradients; Régnière 1996). At each point, 50 replicate runs were carried out. Initial oviposition date was varied systematically between OD 10 and 360 in increments of 10 days. From the output of the model, we compiled the frequency with which a viable seasonality was obtained as a function of O 0. We also compiled the frequency distribution of final (viable) oviposition dates after 15 generations on the same weather time series (O 15).
Establishment probability maps and climate change
Establishment probability maps were generated as described by Régnière and Nealis (2002) by universal kriging (Cressie 1993), with elevation as an external drift variable, to interpolate between outputs from all of the simulation points. Probabilities were transformed to logits prior to kriging, and the resulting maps were retransformed to probability values. O 0 was selected to be optimal for stable seasonality. For New Zealand, this was O 0 = 40, and for the Canadian Maritimes, it was O 0 = 220. We also produced maps of establishment probability in New Zealand under climate change using the median of regional warming forecasts for 2030 and 2080 in New Zealand (Ministry for the Environment 2004) applied to 1971–2000 normals. These altered normals were then used to generate weather traces for a large number of simulation points, and maps were generated as described above.
To provide some assessment of the validity of our mapping approach, we compared the maps of EGM establishment probability under current climate (1971–2000 normals) with records of established populations (life stages other than male moths in traps) in the Canadian Maritimes between 1982 and 2005. These data were compiled by J. Edward Hurley (Canadian Forest Service, Fredericton, NB, Canada) from Canadian Forest Service annual insect survey reports for 1981–1995 (Magasi 1991–1993; Magasi and Hurley 1994; Hurley and Magasi 1995, 1996) and from the unpublished records of several forest pest survey agencies in New Brunswick, Nova Scotia and Prince Edward Island for 1996–2005.
Results
Influence of initial oviposition date (O 0)
The probability of establishment, p, was highly dependent on location and O 0. Oviposition dates outside of the warmer seasons, October–May in the Southern Hemisphere and May–September in the Northern Hemisphere, almost invariably led to nonviable seasonality (Figs. 2 and 3).
Influence of initial oviposition date (O 15) on the output of the phenology model at three locations in the Canadian Maritimes. Graphs are probability of establishment (top), peak oviposition date O 15 (middle) and date of first egg hatch H 15 (bottom), both after 15 generations simulated over the same weather regime
The influence of initial oviposition date (O 15) on the output of the phenology model at three locations in the North Island (a) and five locations in the South Island (b) of New Zealand. Graphs show probability of establishment (top); peak oviposition date O 15 (middle) and date of first egg hatch H 15 (bottom) after 15 generations simulated using the same weather regime
In Canada, summertime oviposition led to viable seasonality under nearly all temperature regimes generated from normals of Kentville (NS) but in only 10% of the cases in Canterbury and Bathurst (NB) (Fig. 2). In New Zealand, the North Island sites (Fig. 3a), the p value dropped sharply from 1.0 in the middle of May to 0.0 in the middle of June. They gradually returned to 1.0 by November for Auckland and Hamilton, and by December for Wellington. The South Island sites had a slightly narrower range of viable initial oviposition dates than did the North Island, as well as more variation in the date at which p decreased and again increased (Fig. 3b). At sites further south (Dunedin, Timaru, and Queenstown), the probability of establishment dropped at earlier O 0 values than at the others (Nelson and Christchurch). The southern cities also generally had lower probability of establishment than all other sites—except for Queenstown, where the probability of establishment increased at earlier O 0 than at other sites, from September onwards.
Assuming a suitable O 0, all sites with the exception of Dunedin had p approximately equal to 1. The probability of establishment at Dunedin varied from 0.2 to 0.4, indicating marginal climatic suitability for the gypsy moth. As these p values were greater than the values of Bathurst (NB) and Canterbury (NB), the model indicates that the New Zealand sites have a higher probability of establishment than those sites examined in the Canadian Maritimes.
Median oviposition after 15 generations with the same weather regime (O 15) stabilised to near-constant dates (about OD 240, late August) in Canada. In New Zealand, this date remained consistent irrespective of O 0, ranging from OD 19 to OD 59 (late January to end February) in the North Island and from OD 35 to OD 84 in the South Island. Similarly the date of first hatch after 15 generations (H 15) showed little dependence on O 0, but between New Zealand cities, H 15 varied between OD 229 and 266 in the North Island and OD 250 and 292 in the South Island. Canadian H 15 varied little between sites (OD 135–143).
Model behaviour on weather records from 2001 to 2003
In the three Canadian Maritime urban centres tested, it was only with summer oviposition (O 0 = 220) that eggs succeeded in going through the three phases of egg development (prediapause, diapause, postdiapause) in less than 365 days, with diapause occurring in winter as expected. However, these egg-development conditions were not sufficient to ensure viability. In Bathurst (Fig. 4a), oviposition in the first generation after initial oviposition on O 0 = 220 occurred too late, so that a large proportion of second-generation eggs failed to complete prediapause prior to the onset of winter. As a consequence, a large proportion of the second generation spent summer in the diapausing egg stage (Fig. 3c). So the main problem with the 2002–2003 weather regime in Bathurst was insufficient summer heat for timely development from hatchling to adult.
Detailed output for simulations at three Canadian Maritime locations, with initial oviposition dates O 0 = 40, 130, 220, 310: in New Brunswick (a, b), in Nova Scotia (c). The thin line represents the proportion in the egg stage; the shaded area is the proportion of eggs in diapause; the dashed curve is the proportion of female moths. Time scale is in years since initial oviposition. Simulation stopped whenever one (or more) of three viability criterion were not met. Daily weather records from 2001 to 2003 were used as input for each generation
Using weather records for 2001–2003 in the four New Zealand locations (Fig. 5a–d), a nonviable seasonality always resulted when initial oviposition was O 0 = 220. First egg hatch occurred just prior to mid inter (31June), violating our second viability criterion. In Queenstown, nonviable seasonality also resulted when initial oviposition was O 0 = 130, where eggs hatched in summer (January) ,but slow larval development continued into winter, with median adult emergence occurring the following spring, violating our second and third viability criteria (Fig. 5d).
Detailed model output for four New Zealand locations, with initial oviposition dates O 0 = 40, 130, 220, 310: North Island (a, b), and South Island (c, d). The thin line represents proportion in egg stage; the shaded area represents proportion of eggs in diapause; the dashed curve is the proportion female moths. Time scale is in years since initial oviposition. Simulation stopped whenever one (or more) of three viability criterion were not met. Daily weather records from 2001 to 2003 were used as input for each generation
Country-wide seasonality
In the Canadian Maritimes, viable seasonality was reached with initial oviposition dates between early June and the end of August (Fig. 6b), whereas in New Zealand, viability was achieved over a wider range of initial oviposition dates (early November to the start of April; Fig. 6a). Within these date ranges, there was little influence of initial oviposition date on the viability of the simulated seasonality. Outside of this period, viability became less likely as O 0 neared the winter months (June–August in New Zealand).
Final oviposition dates (O 15) converged to a narrow range between OD 225 and OD 65, with a mode at OD 245 in the Canadian Maritimes. In New Zealand, O 15 stabilised between OD 1 and 100, with a mode at OD 40. Thus, it is clear that in New Zealand, the gypsy moth phenology model is relatively insensitive to the date of initial oviposition provided that this date is outside of the winter months. Because O 15 converges between January and the start of April, it is sensible to assume that an established gypsy moth population would oviposit in this date range.
Establishment probability maps and climate change
The establishment probability map for the Canadian Maritimes, based on the proportion of weather regimes leading to viable seasonality, corresponds well to the area where the gypsy moth has already successfully established (Fig. 7; see also maps in Benoit and Lachance 1990 and Nealis and Erb 1993).
Probability maps of gypsy moth establishment in the Canadian Maritimes based on viability of seasonality over 50 stochastically different annual weather regimes. Interpolation between points was done by kriging with elevation as external drift. Normals for 1971–2000 were used to generate weather traces. Counties in the 2005 Canadian Food Inspection Agency regulated zone for Gypsy moth are outlined in white. White squares indicate new recoveries from 1981 to 2005
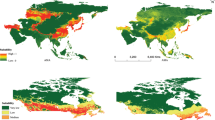
Using current temperature normals, the probability of EGM establishment is high over most of the North Island of New Zealand, except at higher elevations. In the South Island, establishment probability is low, except for the northern coastal areas and the northern part of the eastern and western coastal areas (Fig. 8a). Climate change is generally expected to alter the range of invasive species (Dukes and Mooney 1999). In New Zealand, the climate change expected by 2030 has only a slight influence on the area at risk of EGM establishment in the North Island, whereas suitable areas can be expected to expand into higher elevations, as well as into the southern parts of the South Island (Fig. 8b). By 2080, a large portion of the South Island, although still excluding some higher elevations, is predicted to have a climate suitable for establishment of the insect. Much of the high elevation terrain in the North Island may also become suitable (Fig. 8c).
New Zealand probability maps of gypsy moth establishment based on viability of seasonality using 50 stochastically different annual weather regimes for each of 500 simulation points. Interpolation between points was done by kriging, with elevation as external drift. Using current temperature normals (a), 2030 normals (b), and 2080 normals (c)
Date of peak oviposition
The date at which peak oviposition stabilised using current temperature normals was interpolated into a map through kriging. A mask was applied to this to exclude areas with less than a 50% probability of establishment (Fig. 9).
Maps for the date of peak oviposition (O 15) in the Canadian Maritime provinces and New Zealand. White areas have low (< 0.5) establishment probability and are masked out. The scale is the number of days since the earliest predicted peak oviposition for the region (OD 5 in New Zealand and OD 233 in Canadian Maritimes)
In the Canadian Maritimes, peak oviposition dates were centred around OD 245 (early September), and their range was very narrow (see Fig. 6b). In New Zealand, dates were more widely scattered [over a 100-day range (see Fig. 6a)] and centred around OD 40 (mid-February). This implies that pheromone monitoring operations in New Zealand would need to cover a much longer period of time and that timing of phenological events in the EGM life cycle would vary much more over the country than it would in eastern Canada. If a specific region within New Zealand should be monitored, such as near a port, then the range of oviposition dates to monitor can be significantly narrowed and tailored to local climatic conditions.
Discussion
The threat of gypsy moth establishment in New Zealand makes the prediction of its phenology and potential distribution important for risk assessment. If a reproducing population is detected, then this model would assist in the optimal timing of applying eradication treatments to the appropriate life stages. Validation of model output in the Canadian Maritime provinces of Nova Scotia, New Brunswick and Prince Edward Island further supports the Régnière and Nealis (2002) model and allows us to have some confidence in applying the model to regions in the Southern Hemisphere.
Model predictions can be used to assist timing of control and monitoring programs, specifically the definition of pheromone trapping periods as well as appropriate timing of pesticide applications targeting the most susceptible life stages. This is particularly critical with biological insecticides such as Bacillus thuringiensis (Sample et al. 1996). The GMLS model is being used in this fashion in the context of the Slow the Spread program aimed at reducing the rate of EGM spread across the continental United States (Sharov et al. 2002).
The GMLS model was designed and calibrated on the EGM, whereas New Zealand is currently threatened by AGM incursions. Several differences between these two strains could influence the accuracy of the model’s predicted risk of establishment. In particular, nondiapausing individuals are more likely to occur in AGM (Hoy 1977; Walsh 1993), and a shorter diapause chill period has been hypothesised that allows this species to become multivoltine under mild climates, such as that of New Zealand (Glare et al. 1997). Glare et al. (1997) stated that full eclosion in the AGM sometimes took several years, and additionally, this species has a higher rate of premature eclosion. Such attributes would invalidate the specifics of the viability criteria we used, and thus the establishment maps (Fig. 8) may underestimate the true extent of establishment risk.
Other climate-based models, such as CLIMEX (Sutherst et al. 1999), are often used to predict the potential distribution of invasive species. Our results generally agree with those of Matsuki et al. (2001), who used CLIMEX to predict the suitability of Australasia for gypsy moth survival. The GMLS model integrated with BioSIM gives a finer scale of establishment prediction that takes into account the initial date of oviposition and provides more detail about location-specific phenology. It is also a fundamentally different method of modelling distribution. The GMLS model mechanistically simulates the response of gypsy moth life stages to temperature, whereas CLIMEX compares the climate of a study area to the climates of regions where the gypsy moth is already found. Matsuki et al. (2001) use both EGM and AGM data to calibrate their model, since the strain the data pertained to was unable to be determined in many cases.
Our results indicate that New Zealand’s climate is suitable for the establishment of EGM and potentially AGM. The North Island is at greater risk, which may be one of the reasons for AGM individuals being detected around Hamilton along with more at-risk cargo arriving in North Island ports. The major limiting factor in the North Island will likely be land cover and the availability of suitable hosts rather than climate, whereas the South Island shows more variation in climatic suitability, and the gypsy moth will likely be limited by both vegetation and climate, although the two factors are correlated to some extent.
Further work could be done on developing a model that has more relaxed criteria allowing for nondiapausing individuals or even multivoltine seasonality. Given the variety of seasonal history pathways possible for AGM, an individual-based formulation of the GMLS would be most useful. Time series analysis could then be used to detect cycles, with a clear seasonal cycle indicating high risk of establishment. Such a model could more realistically simulate what is known about the differences between GM strains. Additionally, land cover and vegetation maps could be overlayed on establishment risk maps to create a composite map indicating risk based on suitable hosts as well as climate.
Despite the differences between gypsy moth strains, a phenology model such as this has the potential to contribute to more effective, and efficient, biosecurity and biocontrol measures against the gypsy moth in New Zealand.
References
Allen JC (1976) A modified sine wave method for calculating degree days. Environ Entomol 5:388–396
Benoit P, Lachance D (1990) Gypsy moth in Canada: behavior and control. Forestry Canada. Canadian Forest Service, 351 St. Joseph Blvd., Hull, Quebec. K1A 1G5. Information Report DPC-X-32
Cowley J, Bain J, Walsh P, Harte R, Baker R, Hill C, Whyte C, Barber C (1993) Pest Risk Assessment for Asian Gypsy Moth. Lymantria dispar L. (Lepidoptera:Lymantriidae). New Zealand Forest Research Institute
Cressie N (1993) Statistics for spatial data. Wiley, New York
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139
Dunlap T (1980) The gypsy moth. A study in science and public policy. J For Hist 24:116–126
Ferguson D (1978) The Moths of North America north of Mexico including Greenland. Fascicle. E.W. Classey Ltd and the Wedge Entomological Research Foundation, pp 90–95
Garner KJ, Slavicek JM (1996) Identification and characterisation of a RAPD-PCR marker for distinguishing Asian and North American gypsy moths. Insect Mol Biol 5:81–91
Glare TG, Walsh PJ, Barlow ND (1997) Strategies for the eradication or control of gypsy moth in New Zealand. AgResearch Internal Report
Gray DR (2004) The gypsy moth life stage model: landscape-wide estimates of gypsy moth establishment using a multi-generational phenology model. Ecol Model 176:155–171
Gray DR, Logan JA, Ravlin FW, Carlson JA (1991) Toward a model of gypsy moth phenology: using respiration rates of individual eggs to determine temperature-time requirements of prediapause development. Environ Ecol 20:1645–1652
Gray DR, Ravlin FW, Régnière J, Logan JA (1995) Further advances toward a model of gypsy moth (Lymantria dispar (L.)) egg phenology: Respiration rates and thermal responsiveness during diapause, and age-dependent developmental rates in postdiapause. J Insect Physiol 41:247–256
Gray DR, Ravlin FW, Braine JA (2001) Diapause in the gypsy moth: a model of inhibition and development. J Insect Physiol 47:173–184
Hoy MA (1977) Rapid response to selection for a nondiapausing gypsy moth. Science 196:1462–1463
Hurley JE, Magasi LP (1995) Forest pest conditions in the maritimes in 1994. Canadian Forest Service, Fredericton, NB, Information Report M-X-194E
Hurley JE, Magasi LP (1996) Forest pest conditions in the maritimes in 1995. Canadian Forest Service, Fredericton, NB, Information Report M-X-199E
Liebhold A, Gottschalk K, Muzika R, Montgomery M, Young R, O’Day K, Kelly B (1995) Suitability of north american tree species to the gypsy moth: a summary of field and laboratory tests. USDA General Technical Report NE-211. USDA, Forest Service, Delaware, OH
Liebhold A, Halverson J, Elmes G (1992) Gypsy moth invasion in North America: A quantitative analysis. J Biogeography 19:513–520
Logan JA, Casagrande RA, Liebhold AM (1991) A modeling environment for simulation of gypsy moth larval phenology. Environ Entomol 20:1516–1525
Logan JA, Régnière J, Powell JA (2003) Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ 1:130–137
Logan J, Régnière J, Gray D, Munson A (2006) Risk assessment in face of a changing environment: Gypsy moth and climate change in Utah. Ecol Appl (in press)
Magasi LP (1991) Forest pest conditions in the maritimes in 1990. Canadian Forest Service, Fredericton, NB, Information Report M-X-178
Magasi LP (1992) Forest pest conditions in the maritimes in 1991. Canadian Forest Service, Fredericton, NB, Information Report M-X-181E
Magasi LP (1993) Forest pest conditions in the maritimes in 1992. Canadian Forest Service, Fredericton, NB, Information Report M-X-183E
Magasi LP, Hurley JE (1994) Forest pest conditions in the maritimes in 1994. Canadian Forest Service, Fredericton, NB, Information Report M-X-188E
Matsuki M, Kay M, Serin J, Floyd R, Scott JK (2001) Potential risk of accidental introduction of Asian gypsy moth (Lymantria dispar) to Australasia: effects of climatic conditions and suitability of native plants. Agricult Forest Entomol 3:305–320
Ministry for the Environment (2004) Climate change effects and impacts assessment: A guidance manual for local government in New Zealand
Nealis VG, Erb S (1993) A sourcebook for management of the gypsy moth. Canadian Forestry Service, Sault Ste-Marie, ON. Catalogue No. Fo42-193/1993E
Régnière J (1996) A generalized approach to landscape-wide seasonal forecasting with temperature-driven simulation models. Environ Entomol 25:869–881
Régnière J (2006) Stochastic simulation of daily air temperature and precipitation from monthly normals in North America. Int J Biometeorology (in press)
Régnière J, Bolstad P (1994) Statistical simulation of daily air temperature patterns in eastern north america to forecast seasonal events in insect pest management. Environ Entomol 23:1368–1380
Régnière J, Nealis V (2002) Modelling seasonality of gypsy moth, Lymantria dispar, to evaluate probability of its persistance in novel environments. Can Entomol 134:805–824
Ross MG (2004) Response to a gypsy moth incursion within New Zealand. In: IUFRO conference, Hanmer, 2004. Biosecurity New Zealand, MAF
Sample BE, Butler L, Zivkovich C, Whitmore RC, Reardon R (1996) Effects of Bacillus thuringiensis berliner var. Kurstaki and defoliation by the gypsy moth [Lymantria dispar (L.) (Lepidoptera: Lymantriidae)] on native arthropods in West Virginia. Can Entomol 128:573–592
Sharov AA, Leonard D, Liebhold AM, Roberts EA, Dickerson W (2002) Slow the spread: a national program to contain the gypsy moth. J Forestry 100:30–35
Sheehan KA (1992) Users guide for GMPHEN: Gypsy moth phenology model. General Technical Report NE-158. USDA Forest Service
Sutherst R, Maywald G, Yonow T, Stevens P (1999) CLIMEX: predicting the effects of climate on plants and animals. CSIRO Publishing, Collingwood, Australia
Wallner WE (1996) Invasion of the tree snatchers. Am Nurseryman, March:41–43
Walsh P (1993) Asian gypsy moth: the risk to New Zealand. N Z Forestry 38:31–43
Acknowledgements
This research was funded by the National Center for Advanced Bio-protection Technologies, Lincoln University, New Zealand. Thanks to J.E. Hurley (Canadian Forestry Service, Atlantic Forestry Centre, Fredericton, NB, Canada) for the compilation of EGM records from the Maritime provinces. Thanks to Rémi St-Amant (Canadian Forest Service, Quebec City, QC, Canada) for help with model alterations. The National Institute of Water and Atmospheric Research (NIWA) of New Zealand allowed us use of historical New Zealand weather data from 1972 to 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pitt, J.P.W., Régnière, J. & Worner, S. Risk assessment of the gypsy moth, Lymantria dispar (L), in New Zealand based on phenology modelling. Int J Biometeorol 51, 295–305 (2007). https://doi.org/10.1007/s00484-006-0066-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-006-0066-3