Abstract
Flowering and fruiting as phenological events of 12 tree species in an evergreen tropical mountain rain forest in southern Ecuador were examined over a period of 3–4 years. Leaf shedding of two species was observed for 12 months. Parallel to the phenological recordings, meteorological parameters were monitored in detail and related to the flowering and fruiting activity of the trees. In spite of the perhumid climate of that area, a high degree of intra- and inter-specific synchronisation of phenological traits was apparent. With the exception of one species that flowered more or less continuously, two groups of trees could be observed, one of which flowered during the less humid months (September to October) while the second group started to initiate flowers towards the end of that phase and flowered during the heavy rains (April to July). As reflected by correlation coefficients, the all-time series of meteorological parameters showed a distinct seasonality of 8–12 months, apparently following the quasi-periodic oscillation of precipitation and related cloudiness. As revealed by power spectrum analysis and Markov persistence, rainfall and minimum temperature appear to be the only parameters with a periodicity free of long-term variations. The phenological events of most of the plant species showed a similar periodicity of 8–12 months, which followed the annual oscillation of relatively less and more humid periods and thus was in phase or in counter-phase with the oscillations of the meteorological parameters. Periods of unusual cold or dryness, presumably resulting from underlying longer-term trends or oscillations (such as ENSO), affected the homogeneity of quasi-12-month flowering events, fruit maturation and also the production of germinable seeds. Some species show underlying quasi-2-year-oscillations, for example that synchronise with the development of air temperature; others reveal an underlying decrease or increase in flowering activity over the observation period, influenced for instance by solar irradiance. As Ecuador suffers the highest rate of deforestation in South America, there is an urgent need for indigenous plant material for reforestation. A detailed knowledge of the biology of reproduction in relation to governing external factors (mainly climate) is thus required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To date, our understanding of the phenology, such as the periodicity of flowering and fruiting, or the shedding and flushing of leaves, of tropical rain forest trees is still limited. Aspects that have been addressed in this respect are the incidence of rhythmic cycles (Mikich and Silva 2001), the influence of climatic triggers and day-length as proximate factors, and the intra- and inter-specific synchronisation of phenological events (Borchert et al. 2005; Ims 1990; Poulin et al. 1999), these being ultimate factors that regulate plant life and plant-animal interactions (Frankie et al. 1974; Lobo et al. 2003; Opler et al. 1980; Stiles 1977; Wright et al. 1999). A study of the complex relationships between plant structures, vegetative development and the incidence of flowering of selected tropical trees has been presented by Borchert (1983).
In contrast to habitats subjected to a regular cycle of dry and wet seasons (Daubenmire 1972; Shukla and Ramakrishnan 1982), the synchronisation of phenological events may not a priori be expected in perhumid evergreen tropical rain forests lacking well-developed xeric periods, and, due to the high degree of species diversity, may also be difficult to examine. The proportion of trees and shrubs that show continuous flowering and fruiting appears to be small, as distinct peaks of flowering and fruiting are observed in the course of the year (Opler et al. 1980). A comprehensive 4-year study of the phenology of flowering and fruiting in a Philippine submontane rain forest found 34 tree species that reproduced once a year, 13 that reproduced continuously, 3 supra-annually and 7 irregularly (Hamann 2004). In a lowland Dipterocarp forest in Malaysia, Sakai et al. (1999) found that 35% of 257 species flowered only during mass flowering, 19% flowered supra-annually, 13% annually and only 5% more or less continuously.
With regard to climate as the triggering and synchronising master control factor of phenological cycles, a thorough physiological or ecological understanding is still lacking, although even in the perhumid regions changes between less and more humid seasons occur regularly. Species that flower during the wet season will fruit during the subsequent drier months, which will favour seed dispersal, while germination can take advantage of the rainy season following. On the other hand, species flowering during the drier months will find a broad spectrum of pollinators, including wind, and may still be able to ripen their seeds before the peak of the following wet season.
Various hypotheses have been presented over the last few decades addressing individual elements of the climate as proximate factors that trigger seasonality in the equatorial tropics. Cloudiness appears to play an important role because most of the meteorological cues that may have an impact on plant development, e.g. global radiation, UV-B intensity, rainfall and air temperature, are coupled with the occurrence and frequency of clouds. Higher doses of UV-B are known to affect flowering as well as the germination of pollen (Caldwell 1968; Tevini and Teramura 1989; Flint and Caldwell 1984; Caldwell et al. 1998). Wright et al (1999) attributed the seasonality of plant reproduction in the tropical forests of Panama to a shortage of assimilates during the rainy season when PAR is low, and Hamann (2004) reported that nearly all canopy tree species in a submontane rain forest (Philippines) flowered during the peak of solar irradiance. Numata et al. (2003) showed that the flower induction of tropical canopy trees was triggered by prolonged drought, high solar radiation and abnormally low temperatures. They presumed that a drop in nocturnal air temperatures due to cloudlessness, and thus enhanced radiation emission, is the most plausible cue for a supra-annual synchronisation of flowering. Considering changes in the light climate as the proximate factor that triggers periodicity in the aseasonal tropics, a differentiation must be made between direct and diffuse global radiation. The ratio of diffuse to direct global radiation increases under a cloudy sky (Graham et al. 2003). As a consequence, the light microclimate of sub-canopy trees improves, whereas that of canopy trees deteriorates as compared to clear sky conditions under which the proportion of direct radiation is higher (Endler 1993). Another potential factor explaining phenological phases could be the state of the saturation deficit of air (Wright and Cornejo 1990). However, many of these observations still require confirmation, as claimed by Sakai (2002), because the link between climate and reproductive cycles can be modified by biotic factors, such as low pest pressure combined with a maximum activity of pollinating insects during times of maximum solar irradiance (Hamann 2004), or competition for pollinators (Lobo et al. 2003).
To date, comprehensive studies of reproduction phenology in relation to climate have been performed only in tropical lowland rain forests (Frankie et al. 1974; Croat 1975; Opler et al. 1980; Newstrom et al. 1994; Hamann and Curio 1999; Sakai et al. 1999; Schöngart et al. 2002; Hamann 2004). Unfortunately, investigations of the phenological aspects of tropical mountain rain forests are scarce (e.g. Koptur et al. 1988). Hence, the current study aims to provide new insights into the phenological cycles and their relations to abiotic factors for a little-known ecosystem. In detail, the paper deals with the relationships of phenologically synchronised periods of selected tropical tree species to the relevant elements of weather cycles in a tropical mountain rain forest in southern Ecuador. Understanding these parameters is critical to understanding biodiversity accruement and to guarantee the biodiversity preservation of an evergreen tropical forest. It is of particular importance for Ecuador, the country which is at the present time suffering the highest annual rate (4%) of deforestation in the whole of South America (Miller 1998). Reforestation of abandoned agricultural areas with indigenous trees could help to rehabilitate biodiversity. Hence, the current investigation of periodicities in weather and tree phenology is indispensable for producing sufficient plant material in tree nurseries for reforestation purposes.
Materials and methods
Study area
The study area (location of the central research station: 3°58′18″S, 79°4′45″W) termed “Reserva Biológica San Francisco”, verges on the Podocarpus National Park in the Cordillera Real in the eastern part of the southern Ecuadorian Andes (Fig. 1). Geographical details of this region in the upper valley of the Rio San Francisco between the provincial capitals Loja and Zamora have been presented by Beck and Müller-Hohenstein (2001). The natural vegetation of the deeply incised valley is an evergreen forest covering the slopes from the valley bottom (in the research area 1,800 m a.s.l.) up to the tree line (∼2,700 m a.s.l.). This forest has been widely cleared for farming purposes on the south-exposed slopes, while it appears more or less intact on the slopes with a northern aspect. However, aerial photographs show that patches of the lowest part of this forest were also cleared during the course of the past century. Thus at least parts of the present forest must be considered as secondary forest. The area of the phenological studies reported here extends from 1,850 to 2,300 m a.s.l.
Botanical surveys and structural analyses of the forest have been presented by Bussmann (2001), Homeier et al. (2002), and Müller-Hohenstein et al. (2004). Based on tree species patterns, Homeier et al. (2002) described five major forest types for the Reserva Biológica San Francisco that relate to altitudinal ranges and differ in forest structure. Following current classification systems for Ecuador the investigated forest can be categorised as “bosque de neblina montano”, montane cloud forest (Valencia et al. 1999) or as “bosque siempreverde montano Iluvioso”, evergreen montane forest (Balslev and Øllgaard 2002). In total, more than 250 species of trees have been identified so far in this forest and plant families such as the Lauraceae, Melastomataceae and Rubiaceae reveal an above average representation in the species list (Bussmann 2001; Homeier 2004). Most of the tree species are evergreen; however, the highly diverse spectrum of trees also contains a few deciduous species, such as Tabebuia chrysantha, Cedrela montana and at least one Ficus species.
Botanical field survey
Table 1 shows the investigated tree species and respective numbers of regularly inspected individuals. For identification and nomenclature The Flora of Ecuador (Harling and Andersson 1973) and the collections of the National Herbarium of Ecuador (QCNE, Quito, Ecuador) were used. Images of the investigated species have been entered in the visual plants database (http://www.visualplants.de). Adult and to all appearances healthy individuals on different microsites (including gorges and ridges) between 1,850 and 2,300 m a.s.l. were selected for the study. To minimise scattering of the data, individuals that were overly attacked by pests or obviously suffering from a stressful microhabitat were not included. The trees were tagged and their phenological state was routinely examined every 2 weeks (every 4 weeks for Graffenrieda, Purdiaea and Viburnum) for 3.5 years and the percentage of flowering and fruiting individuals of each species was noted. Flowering comprises floral bud initiation, anthesis and floral persistence (Rathcke and Lacey 1985). However, when observing tropical trees, the crowns of which reach heights of 15 m and more, only the blossoming phase could be clearly distinguished. Since the transition from flowering to fruiting is gradual, fructification was recorded only when fruits became visible.
Meteorological equipment and data analysis
Meteorological data of the research area was collected by several automatic weather stations (ECSF-, TS1-, and Cerro del Consuelo-meteorological stations, see Fig. 1) and included the observation of wind, soil/air temperature, humidity, rainfall (tipping bucket rain gauge) and solar irradiance (Richter 2003). The longest available time series of the ECSF meteorological station was used for data analysis as detailed in the results. Additionally, several direct and indirect weather data collectors (including a scanning X-band weather radar on the top of Cerro del Consuelo and a vertically scanning K-band doppler rain radar profiler) were operated to investigate the spatial-temporal dynamics of fog, clouds and rain (Bendix et al. 2004a, 2006; Rollenbeck and Bendix 2006). Overlapping time series of meteorological and phenological data range from July 1999 to March 2003. The seasonality of weather and phenology is examined by standard time series analysis including numerical filters and power spectrum analysis (for methodological details, see Blackman and Tukey 1958; Andel 1984; Olberg and Rákóczi 1984; Bendix et al. 2000). Single Markov persistence is estimated as presented by Klaus (1977). A Markov persistence of unity means that the data sample is related to the markedness of the variable in the year before. It points out that the data series is embedded in a longer-term trend/oscillation and hence the significance of calculated periods must be tested against a red noise spectrum. Otherwise (zero Markov persistence), short-term (here seasonal) oscillations dominate and their significance has to be tested against a white noise spectrum. The possible relations between flowering, fruiting and leaf shedding within meteorological cycles were studied by moving correlations analysis. It should be stressed that interactions between climate, tree phenology and other external factors not considered in this study can be rather complex. Hence, the correlations presented just point to the most likely phenological triggers, bearing in mind that multiple factors could contribute to the observed phenological pattern. A stepwise multiple regression could be helpful to determine which factors explain the largest amount of variation; however, its application is complicated due to the different time lags between meteorological variables and phenology (Table 5). Hence, this procedure was not used in the current study.
Results
Climate of the study area
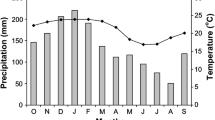
A synoptic overview of the climate of Ecuador has been presented by Bendix and Lauer (1992) and more specifically for the southern part of the country by Richter (2003) and Bendix et al. (2004b). The research area has a tropical humid climate with annual precipitation from around 2,000 mm at 1,850 m a.s.l. up to more than 6,000 mm at 3,100 m a.s.l. with an extremely wet season from April to July and a less humid period from September to December (Fig. 2).
Climate at three weather stations indicated in Fig. 1 for the time period 1998–2003. T Air temperatures (extremes, average amplitudes, monthly averages), P monthly and annual sums of precipitation (mm), E monthly and annual sums of potential evaporation (mm), W monthly and annual sums of cloud and horizontal rain water deposition (mm) (Rollenbeck et al. 2006), ΔTd average oscillation range of daily air temperature, ΔTa annual fluctuation range of monthly temperature averages, T med monthly average of air temperature, T min med monthly average of minimum temperature, T min abs Absolute monthly minimum temperature for the observational period, T max med monthly average of maximum temperature, T max abs absolute monthly maximum temperature for the observational period
Most of the precipitation results from the well-developed Atlantic trade winds by forced lifting and condensation in the eastern Andean ranges. Hence, cloudiness is high throughout the year especially in the elevated parts of the study area, with average cloud frequencies of ∼70% (Bendix et al. 2004a,b). While the advective easterly air stream dominates the climate between April and mid-October, westerly winds may cause short periods of sunny days from November to March with significantly enhanced solar irradiance especially in the lower regions (ECSF) of the study area (Fig. 3).
However, this period is also humid due to an intensification of local convective processes. With respect to the temperature regime, the investigated mountain forest belongs to the “Tierra templada” and the “Tierra fria”, with mean annual air temperatures of between 19° and 13°C (Richter 2003). During the relatively dry months the average temperature increased slightly, while an equally small decrease was observed during the main rainy season. The wind data show that the most elevated parts of the study area (Cerro del Consuelo) are affected by quasi-permanent easterlies with high wind speeds, whereas the lower regions of the San Francisco valley are influenced mainly by the diurnal regime of the mountain-valley breeze system that is characterised by relatively low wind speeds (Fig. 2).
Meteorological situation of the phenological observation period
The weather in the study area during the entire observation period is presented in Fig. 4.
All time-series show a distinct seasonality of meteorological parameters that apparently follows the quasi-periodic oscillation of precipitation (and related cloudiness), which peaks in May to August and shows significant minima from October to January in all years investigated. Solar irradiance is especially high during phases of lower precipitation and this consequently leads to higher average air and soil temperatures. This is also reflected by the correlation coefficients in Table 2. Only weak trends were observed for most of the meteorological parameters, with the exception of soil temperature (Ts) which is consistently negative. However, especially high soil temperatures and a longer period of increased solar irradiance were recorded between June 1998 and March 1999, which corresponds to the 1998/1999 La Niña event. It is well known that generally only weak correlations between ENSO (El Niño-Southern Oscillation) and the climate of the eastern Andean ranges exist in Ecuador [relative low correlation to southern oscillation index (SOI) values in Table 2; see also Bendix 2000, 2004; Vuille et al. 2000]. However, the time series points to an increase of irradiance and especially soil temperature as well as a slight reduction of rainfall during the transition from El Niño 1998 to La Niña 1999.
The periodicity of weather elements over the phenological observation period (July 1999 to January 2003) was tested by power spectrum analysis (Fig. 5, Table 3). The occurrence of simple Markov persistence (Briggs and Ruppert 2005) reflects the importance of longer periods in the variance spectrum of the time series and/or underlying trends. In this case, the significance of periods was tested against a red noise spectrum. Otherwise, a white noise spectrum was used. Figure 5 shows that spectral variance analysis is characterised by a reduced resolution for lower frequencies.
All meteorological parameters except soil temperatures revealed significant periods. This means that the climate of the study area exhibits a clear seasonality. Rainfall and minimum temperature appear to be the only factors that were free of long-term variations, whereas all the other time series showed additional periods of lower frequency or trends. A periodicity of 8–12 months is observed for most of the meteorological parameters and is especially well established for rainfall. Significant periods of greater frequency (5–8 months) were also recognised.
Flowering
The records of flowering of the examined 404 individuals of 12 tree species (see Table 1) show distinct periodicity on the one hand but also differences in the maximum extent of flowering between species (Fig. 6). High flowering percentages (>90%) were found for Piptocoma, Myrica, Isertia, Heliocarpus, Purdiaea and Viburnum, while Graffenrieda, Clethra, Tabebuia and Cedrela showed maximum rates lower than 80%.
Power spectrum analysis confirmed the occurrence of distinct flowering periods (Table 4, Fig. 7) for all species except Myrica, of which male flowers could be found all year round.
Most species show a significant periodicity of 8–12 months as was also observed for the meteorological time series. The flowering cycles of Heliocarpus were, in contrast to all other species, not modulated by underlying long term oscillations (Table 4, Fig. 7). High significance for an 8- to 12-month oscillation was also found for Vismia and Isertia. Due to the shorter time series, the 6-month periodicity (=harmonics of 12-month cycle) of Cedrela clearly indicates a yearly cycle of flowering (cf. Fig. 6). A long term oscillation (quasi 21 months) appears on top of the 8- to 12-month period of Isertia as indicated by a relatively high variance explanation. The time series of Vismia (Fig. 6) shows that the result of power spectrum analysis is probably related to a 24/25-month oscillation of increased flowering activity (2000, 2002). An 8- to 12-month periodicity was also found for Clethra and Purdiaea, but with decreasing flowering activity towards the end of the observation period. This refers also to an underlying long-term oscillation.
Viburnum also exhibited a significant 8- to 12-month oscillation. However, the periodicity is not as clear as for the above mentioned tree species. Viburnum and Purdiaea showed a trend of decreasing flowering activity from 2001 onwards. Some significant periods of Graffenrieda, Piptocoma and Tabebuia were shorter than the 8- to 12-month oscillation but power spectrum analysis indicated longer term trends of flowering activity. Figure 6 reveals that the flowering of Graffenrieda is modulated by the same supra-periodic tendency as Purdiaea. Tabebuia and Clethra showed an inverse supra-periodic behaviour with an increase of flowering activity at the end of the observation period (2002).
Weather cycles and flowering
If meteorological parameters are to trigger phenological cycles, the time series of flowering frequency and weather characteristics should be synchronisable, allowing for a species-specific time-lag between climate event and phenological response. A comparison of time series and power spectrum analysis suggests a synchronisation of flowering with the incidence of wetter and less wet periods, with an oscillation of 8–12 months. One group of trees (Piptocoma, Tabebuia, Cedrela, Myrica and Purdiaea) flowered during the less humid period of the year, while others (Clethra, Heliocarpus, Isertia, Viburnum and Vismia) started to flower during the more humid season (austral winter). Clethra and Heliocarpus reveal peaks of flowering in the months of highest rainfall at ECSF (May to July). Heliocarpus and Viburnum also started flowering activity in that month, but flowering lasted until September when the rainfall began to decline. Vismia showed less clear flowering intervals but maximum activity was generally increased in the main rainy season of the austral winter.
Moving correlation analysis (Δt=1 month) was used to uncover possible relations between single meteorological parameters and the flowering response of individual tree species, and the respective time-lag. The maximum correlation and the best correspondence for the time-lags are presented in Table 5 for every meteorological element in relation to the investigated tree species. An example of moving correlation analysis is given for two tree species with an inverse behaviour in comparison to wet-season and less wet-season flowering (Fig. 8). The flowering intensity of Clethra yields the highest positive correlation with a time lag of 11 months to precipitation (Table 5), indicating that the peaks of flowering are almost perfectly synchronised with periods of increased rainfall. In contrast Purdiaea flowered nearly in phase with air temperature and solar irradiance (high positive r at a time-lag of 6 months, Table 5) i.e. during the drier period.
Isertia also reveals a clear synchronisation with rainfall (Table 5). Although not entirely in phase, band pass filter analysis reveals that the flowering frequency of both tree species takes place in a quasi 12-month cycle, where maximum flowering intensity precedes maximum precipitation by 1–2 months (Fig. 9). This observation suggests that climatic factors other than precipitation could also trigger flowering.
Other conceivable meteorological parameters are solar irradiance and temperature, which are both inversely related to rainfall. This results from the fact that the second highest correlation between flowering and rainfall is solar irradiance (Clethra) or air temperature (Isertia, Table 5). Band pass filtered values in Fig. 10 show that the onset of flowering is nearly in phase with the maximum of solar irradiance for both tree species.
Graffenrieda, Heliocarpus and Viburnum revealed the highest positive correlation between flowering intensity and solar irradiance (Table 5). The time lag of 6 months for Heliocarpus means that flowering is nearly counter-phased to irradiance: flowering starts in the month in which irradiance begins to decrease (Fig. 11). The performance of Graffenrieda is less obvious, since all correlation coefficients are relatively low, and flowering intensity decreased considerably during the period of observation.
Another group of trees showed a maximum positive correlation of flowering with the average air temperature (Cedrela, Piptocoma, Purdiaea, Tabebuia, Vismia, Table 5). The flowering of Cedrela was clearly correlated with periods of higher air and soil temperatures during the period of reduced rainfall. Although the meteorological time series analysis (Fig. 4, Table 2) reveals that there is no perfect correspondence of air temperature and precipitation with the dominant 8- to 12-month oscillation, Purdiaea showed the highest positive correlations to this oscillation of precipitation and irradiance. From Fig. 12, it is obvious that the initiation of flowering is generally correlated with lower temperatures. The decrease in amplitude of the filtered temperature series towards the end of the observation period (2002/2003) is also displayed for the filtered flowering series. The prolonged flowering period in 2001/2002 (October to March), which may be related to the lowest average temperature (August 2001) of the entire observation period, is particularly remarkable. The flowering of Tabebuia and Piptocoma seems also to be initiated by decreasing temperatures, although flowering activity was less well in phase (Fig. 12).
Three types of underlying long-term oscillations or trends, as indicated by the Markov persistence, can be distinguished upon low pass filtering of the time series: (1) Heliocarpus, which revealed no long-term oscillations as also observed for the climate element precipitation. The regular annual oscillation with maximum flowering during the very wet season (March to September) also applies for Isertia and Tabebuia. (2) A nearly 2-year oscillation is discernible for Vismia and Piptocoma, which is nearly in phase with, or completely opposite to the oscillation of air temperature (Fig. 13). High flowering percentages of Vismia corresponded to increased air temperatures which peaked in November 1999 and 2001, but not in 2000 and 2002. The counter-current 2-year oscillation of Piptocoma suggests that its flowering activity responds positively to lower spring temperatures. The warmer years are apparently related to the westerly mode of the Quasi-Biennial Oscillation (QBO) and the colder years to the easterly QBO mode. Warming in late 2001 was concomitant with the weakening of the extended La Niña cold phase (1999–mid 2001) and the onset of a central Pacific El Niño event (2002/2003, McPhaden 2004). Graffenrieda, like Piptocoma, belongs to the quasi 2-year oscillation type (Fig. 6), but the highest percentages of flowering also correlated with significant cold events such as in JJA 1999 and 2001. (3) Purdiaea exhibited a decline in flowering activity after the austral spring of 2000 and was slightly out of line with the 12-month oscillation; Viburnum performed similarly. Such a decline could be associated with a general decrease in the solar irradiance peaks after the year 2000.
Seasonality of fructification
Due to the differing species-specific time-spans required for seed-set and fruit ripening, the annual phases of fruiting were wider and in most cases not as clearly defined as for flowering (Fig. 14).
Periods of fruiting of 12 tree species of the evergreen mountain forest in the Rio San Francisco valley. Fructification was quantified as percent of fruiting trees of the respective sample (see Table 1)
Nevertheless, a differentiation between groups of trees can again be made with respect to fructification (Fig. 14). Piptocoma and Tabebuia were found fruiting during the less humid season, i.e. in NDJ. Another group of trees consisting of Cedrela, Clethra, Heliocarpus, and Vismia exhibited maximum fruiting predominantly during the wet season. Fruiting trees of Isertia were present all year round; however, with a pronounced minimum in February/March (in February 2001, no fruiting trees were found).
Power spectrum analysis shows that the clarity of periods of fruiting is less pronounced in comparison to flowering activity but generally shows the same tendencies. A short period of fructification does not, however, necessarily mean a high percentage of simultaneously fruiting trees. Tabebuia, for example, shows a narrow window (about 3 months) of fructification, and simultaneously only a low percentage of fruiting trees (usually less than 50%).
Moving correlation analysis reveals that flowering and fruiting activity is well correlated for most tree species, an exception possibly being Purdiaea (Table 6).
Table 6 also shows the time required for seed set and fruit production with an average of 3–4 months for most of the tree species. Vismia, on the other hand shows a long fruiting period of 8 months (in 2002), but no flowers or fruits in 2001. Moving cross-correlation analysis of meteorological parameters and fruiting data revealed the same tendency as shown for flowering in Table 5, but with generally lower correlation coefficients.
Seasonality of leaf shedding
A correlation of phenological activity with seasonal events is best exemplified by patterns in leaf fall and leaf flushing (Frankie et al. 1974). In an evergreen forest such phenomena are difficult to quantify. Two of the deciduous tree species presented here, Tabebuia chrysantha and Cedrela montana shed their leaves predominantly during the heavy rains and presented as more or less leafless during the less humid months (September to December). Tabebuia produced new foliage in November after flowering, while Cedrela developed flowers after the flushing of new leaves (Fig. 15).
Moving correlation analysis (Table 7) confirms that leaf shedding of the deciduous species relates best with the rainfall cycles and, in the case of Tabebuia, to solar irradiance.
Tabebuia starts leaf shedding immediately after the major rain period in July, and Cedrela follows slightly later. The shedding frequency of Tabebuia culminated approximately 2 months before the incidence of the annual maximum of solar irradiance. The maximum leaf shedding of Cedrela coincided with the maximum period of solar irradiance (October to November).
Discussion
The data presented in this paper show a high degree of species-specific synchronisation of phenological events of the investigated tree species in a tropical mountain rain forest. These events follow quasi-annual oscillations, which in general coincide with an 8–12 month oscillation of most meteorological parameters. To date, this has also been reported for several more or less perhumid lowland forests of the paleo- and the neo-tropics (Daubenmire 1972; Frankie et al. 1974; Shukla and Ramakrishnan 1982; Mikich and Silva 2001; Sakai 2002; Hamann 2004). The question is which meteorological parameters or combination of elements as proximal factors induce flowering or leaf shedding and thereby cause synchronisation and proper placement in the changing weather conditions during the course of a year. In many tree species, to switch from vegetative growth to flower production requires a signal in which drought or shortage of soil moisture is involved (Borchert 1983; Reich and Borchert 1982). Nevertheless, peaks in humid periods and, in a few cases, also continuous reproduction have been observed (refer to Frankie et al. 1974; Hamann 2004).
In the tropical mountain rain forest of southern Ecuador, the synchronisation of reproduction generally follows two different response types, each represented by several species: one group starts reproduction with the onset of the less humid period of the year (e.g. Tabebuia, Piptocoma, Purdiaea), while the other commences flowering shortly before (Isertia, Clethra), or with, the onset of the rainy season (Vismia, Heliocarpus, Graffenrieda).
The question as to whether rainfall as such can be a meteorological trigger for the phenological cycle of these species, as induced by correlation analysis e.g. for Clethra and Isertia, and as partly stressed for tropical lowland forests (Frankie et al. 1974; Opler et al. 1980) must be discussed carefully: the climate in the study area is more or less perhumid, and thick humus layers as well as an effective interflow from the wetter upper parts of the mountains may provide extensive soil-water storage for the short rainless phases. A seasonal deficiency of moisture is therefore less probable as a proximate synchronising factor. However, rainfall is an indicator for clouds, which reduce solar irradiance, alter the spectral composition of radiation, affect air and soil temperature, and may also have an impact on the activity of insects (Wright and van Schaik 1994). Floral control by the daily photoperiod and light quality and annual oscillation of PAR is well known (e.g. Simpson and Dean 2002; Boss et al. 2004) also for tropical trees (Zipparro 2004), and therefore the shading effect of clouds (Graham et al. 2003) combined with a change in the spectral composition due to an increase in the proportion of diffuse radiation (Nann and Riordan 1991; Nunez et al 1994) is suggested as a conceivable hypothesis to explain the synchronisation of flowering. The activity of insects, pollination, fruit ripening, and fruit or seed dispersion as ultimate factors (Ims 1990) also greatly depends on meteorological conditions. Most of the investigated species produce conspicuous flowers that are pollinated by specialised animals (bats, insects, birds; Dziedzioch et al. 2003; Wolff et al. 2003), which find intervals of drier conditions for their interaction with the plants even during the months of maximal rainfall. Flowering of (female) Myrica, as the only obligatory wind-pollinated species, is well positioned in the less humid period of the year. Clethra and Isertia start flowering 1–2 months in advance of the heavy rains. In this case factors other than cloudiness must be considered as triggers of flower production even if maximum flowering extends into the peak of precipitation. Correlation analysis for Clethra and Isertia showed the second highest correlation coefficients with solar irradiance and temperature, respectively. Hence, it is not surprising that the onset of the flowering of both species is clearly related to the maximum of solar irradiance.
Oscillations of the flowering of Graffenrieda and Heliocarpus were contrary to solar irradiance: flowering activity peaked when irradiance was below ∼13 MJ m−2 day−1, which is >5 MJ m−2 day−1 less than the radiation peak in the drier season (November). A relationship between flowering and low average temperatures during the cloudy and humid periods is observed especially for Heliocarpus. Low temperatures as the trigger of the flowering and fruiting of tropical trees have also been reported by Numata et al. (2003).
Some trees apparently respond to thermal oscillations of the air temperature, which exhibit an increase of the daily temperature range (i.e. higher day and lower night temperatures) during the less humid and a decrease during the overly wet months. Although a thermal response is typical also for dry forests, a recent review paper shows that it is frequently linked with the periodicity of rainfall (Singh and Kushwaha 2005).
Piptocoma belongs to the group of trees that regularly flower during the less humid months. It requires 3–4 months from flowering to maturation of the achenes, which are dispersed predominantly by wind but also by pigeons. Thus flowering, as well as seed dispersal, both dependent on dry weather, are well positioned in the less humid period of the year. From laboratory studies with the model plant Arabidopsis, a modifying effect of temperature on the control of flowering by the quality of light has been suggested (Blázquez et al. 2003). Appreciating the entirely different situation of a laboratory model plant and the trees in a tropical forest, lowering of the minimum temperatures in the course of the less wet months in addition to changes in the light climate could also contribute to the synchronisation of flowering. Likewise, the flowering of Tabebuia and Purdiaea is generally initiated by low air temperatures but with different intensity thresholds.
Although reasonably good correlation coefficients were obtained with precipitation, proximate factors for leaf shedding are less obvious than for flowering. Nevertheless, an influence of the climate is likely, as the example of Tabebuia chrysantha shows: whilst this species drops all of its leaves during, or at the end of, the heavy rains, when there is still ample moisture in the soil, and appears completely leafless during the early less wet phase (September to November), the same species in Costa Rica was reported to shed its leaves early in the year and to enter the leafless phase at the beginning of the wet season (Frankie et al. 1974; see also Reich and Borchert 1982). With respect to the ultimate factors, the stagnation of stem growth due to a complete replacement of the foliage was observed only with the deciduous species Tabebuia and Cedrela. In both species, annual growth rings could also be demonstrated in the wood (Homeier 2004). Of the evergreens, a rhythmicity of stem growth concomitant with a maximum of leaf shedding could not be observed.
For most tree species, the entire phenological periodicity could not be completely established over the observation period of 3 – 4 years. The extent to which individual species participated in a phenological event seems to be triggered by irregular meteorological events which are, however, most likely a result of underlying longer term cycles or trends that could not yet be fully resolved by the relatively short phenological and meteorological time series. A significant decrease of flowering was, for example, observed for Tabebuia in 2001, which can be explained by specific low temperatures. An absence of flowering and/or the production of germinable seeds in one or two of the years studied was observed for Vismia and Piptocoma. Vismia did not flower in 2001 and Piptocoma flowered only negligibly (Fig. 6). Consequently, there were no fruits of Vismia in 2001 and only a few fruits of Piptocoma (Fig. 7), which, however, did not germinate. Interestingly, the ample seed production of Piptocoma late in the year 2000 also did not result in germinable seeds. An unusually long period of cold occurred between 31 October and 13 November 2000, following a short breakdown of the easterly air flow between 24 and 26 October. An air stream from the north-west, overflowing the easterlies, even temporarily interrupted the diurnal mountain-valley breeze system. During that 2-week episode, nocturnal temperatures repeatedly dropped to about 6°C. Cold as a factor responsible for the failure to produce germinable seeds is in line with an appreciation of the altitudinal range of Piptocoma and Vismia. Both are typical representatives of the lower, i.e. warmer montane forest, which does not occur above 2,100 m (Piptocoma) and 2,300 m a.s.l. (Vismia), and thus may indeed be sensitive to unusually low temperatures.
Isertia also showed a reduced flowering frequency in the colder period of 2001, whereas flowering intensity was higher during the preceding and following years. This underlying quasi 24-month oscillation is also present in air and soil temperature. Flowering of Purdiaea, Viburnum and Piptocoma declined consistently from the year 2000 onwards, which is in general coincidence with a decrease of peak phases in solar irradiance after 2001. The flowering of Vismia is related to soil temperature, and the dominant flowering peak in early 2000 occurs after a phase of significant increased soil temperature during the La Niña event in 1999.
To summarise, it can be concluded that phenological activity of the investigated trees generally follows an annual oscillation connected with relatively less and more humid periods. However, the extent to which most of the investigated species produce flowers and fruits is clearly modified by apparently irregular events, which most likely result from underlying oscillations of lower frequency (as e.g. ENSO) or trends. Hence, longer time series are required to examine the influence of these events in future studies.
References
Andĕl J (1984) Statistische Analyse von Zeitreihen. Akademie-Verlag, Berlin
Balslev H, Øllgaard B (2002) Mapa de vegetación del sur de Ecuador. In: Aguirre Z, Madsen JE, Cotton E, Balslev H (eds) Botánica austroecuatoriana. Estudios sobre los recursos vegetales en las provincias de El Oro, Loja y Zamora-Chinchipe. Ediciones Abya-Yala, Quito Ecuador, pp 51–64
Beck E, Müller-Hohenstein K (2001) Analysis of undisturbed and disturbed tropical mountain forest ecosystems in Southern Ecuador. Die Erde 132:1–8
Bendix J (2000) A comparative analysis of the major El Niño events in Ecuador and Peru over the last two decades. Zbl Geol Paläontol Teil I 1999, H. 7/8:1119–1131
Bendix J (2004) Climate variability and extreme events in the Andes of Ecuador and Peru (in German). Geogr Rdsch 56:10–16
Bendix J, Lauer W (1992) Die Niederschlagsjahreszeiten in Ecuador und ihre klimadynamische Interpretation. Erdkunde 46:118–134
Bendix J, Bendix A, Richter M (2000) El Niño 1997/98 in Nordperu: Anzeichen eines Ökosystem-Wandels? Petermanns Geogr Mitt 2000/4:20–31
Bendix J, Fabian P, Rollenbeck R (2004a) Gradients of fog and rain in a tropical montane cloud forest of southern Ecuador and its chemical composition. In: Proceedings of the 3rd International Conference on Fog, Fog Collection and Dew, 11–15 October 2004, Cape Town, South Africa, pp H7-1–H7-4
Bendix J, Rollenbeck R, Palacios WE (2004b) Cloud detection in the Tropics—a suitable tool for climate-ecological studies in the high mountains of Ecuador. Int J Remote Sensing 25:4521–4540
Bendix J, Rollenbeck R, Reudenbach C (2006) Diurnal patterns of rainfall in a tropical Andean valley of southern Ecuador as seen by a vertically pointing K-band Doppler radar. Int J Climatol 26 DOI:10.1002/joc.1267
Blackman RB, Tukey JW (1958) The measurement of power spectra. Dover, New York
Blázquez M, Ahn JH, Weigel D (2003) A thermosensory pathway controlling flowering time in Arabidopsis. Nat Genet 33:168–171
Borchert R (1983) Phenology and control of flowering in tropical trees. Biotropica 15:81–89
Borchert R, Renner SS, Calle Z, Navarrete D, Tye A, Gautier L, Spichiger R, von Hildebrand P (2005) Photoperiodic induction of synchronous flowering near the Equator. Nature 3295:1–3
Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell 16:S18–S31
Briggs W, Ruppert D (2005) Assessing the skill of yes/no forecasts for Markov observations. http://www.orie.cornell.edu/~davidr/papers/weathermarkov03.pdf
Bussmann RW (2001) The montane forest of Reserva Biológica San Francisco (Zamora-Chinchipe, Ecuador). Die Erde 132:9–25
Caldwell MM (1968) Solar ultraviolet radiation as an ecological factor for Alpine plants. Ecol Monogr 38:243–268
Caldwell MM, Björn LO, Bornman JF, Flint SD, Kulandaivelu G, Teramura AH, Tevini M (1998) Effects of increased solar ultraviolet radiation on terrestrial ecosystem. J Photochem Photobiol B46:40–52
Croat T (1975) Phenological behavior of habitat and habitat classes on Barro Colorado Island (Panama Canal Zone). Biotropica 7:270–277
Daubenmire R (1972) Phenology and other characteristics of tropical semideciduous forest in north-western Costa Rica. J Ecol 60:147–170
Dziedzioch C, Stevens A-D, Gottsberger G (2003) The hummingbird plant community of a tropical montane rain forest in Southern Ecuador. Plant Biol 5:331–337
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63:1–27
Flint SD, Caldwell MM (1984) Partial inhibition of in vitro pollen germination by simulated solar ultraviolet-B radiation. Ecology 65:792–795
Frankie GW, Baker HG, Opler PA (1974) Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 62:881–913
Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ (2003) Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc Natl Acad Sci USA 100:572–576
Hamann A (2004) Flowering and fruiting phenology of a Philippine submontane rain forest: climatic factors as proximate and ultimate causes. J Ecol 92:24–31
Hamann A, Curio E (1999) Interactions among frugivores and fleshy fruit trees in a Philippine submontane rain forest. Conserv Biol 13:766–773
Harling G, Andersson L (eds) (1973–2003) Flora of Ecuador, vol 1–68. University of Göteborg, Göteborg
Homeier J (2004) Baumdiversität, Waldstruktur und Wachstumsdynamik zweier tropischer Bergregenwälder in Ecuador und Costa Rica. PhD Thesis, University of Bielefeld
Homeier J, Dalitz H, Breckle S-W (2002) Waldstruktur und Baumartendiversität im montanen Regenwald der Estación Científica San Francisco in Südecuador. Ber Reinh-Tüxen Ges 14:109–118
Ims RA (1990) The ecology and evolution of reproductive synchrony. Trends Ecol Evol 5:135–140
Klaus D (1977) Klimafluktuationen in Mexiko seit Beginn der meteorologischen Beobachtungsperiode. Abh Akad Wiss Lit Mainz Jhrg 1977 Nr 1. Franz Steiner Verlag, Wiesbaden
Koptur S, Haber WA, Frankie GW, Baker HG (1988) Phenological studies of shrubs and treelet species in tropical cloud forests of Costa Rica. J Trop Ecol 4:323–346
Lobo JA, Quesada M, Stoner KE, Fuchs EJ, Herrerías-Diego Y, Rojas J, Saborio G (2003) Factors affecting phenological patterns of bombacaceous trees in seasonal forests in Costa Rica and Mexico. Am J Bot 90:1054–1063
McPhaden MJ (2004) Evolution of the 2002/03 El Niño. Bul Am Meteor Soc 85:677–695
Mikich SB, Silva SM (2001) Floristic and phenological study of zoochoric species of semideciduous seasonal forest remnants in the mid-west region of Parana State, Brazil. Acta Bot Bras 15:89–113
Miller C (1998) Rates of rainforest destruction and species loss. http://www.ecuadorexplorer.com/html/body_rf_destuction.html
Müller-Hohenstein K, Paulsch A, Paulsch D, Schneider R (2004) Vegetations- und Agrarlandschaftsstrukturen in den Bergwäldern Südecuadors. Geogr Rdsch 56:48–55
Nann S, Riordan C (1991) Solar spectral irradiance under clear and cloudy skies: measurements and a semiempirical model. J Appl Meteorol 30:447–462
Newstrom LE, Frankie GW, Baker HG (1994) A new classification for plant phenology based on flowering patterns in lowland tropical forest trees at La Selva, Costa Rica. Biotropica 26:141–159
Numata S, Yasuda M, Okuda T, Kachi N, Noor NSM (2003) Temporal and spatial patterns of mass flowerings on the Malay peninsula. Am J Bot 90:1025–1031
Nunez M, Forgan B, Roy C (1994) Estimating ultraviolet radiation at the Earth’s surface. Int J Biometeorol 38:5–17
Olberg M, Rákóczi F (1984) Informationstheorie in Meteorologie und Geophysik. Akademie-Verlag, Berlin
Opler PA, Frankie GW, Baker HG (1980) Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 68:167–188
Poulin BS, Wright SJ, Lefebvre G, Calderon O (1999) Interspecific synchrony and asynchrony in the fruiting phenologies of congeneric bird-dispersed plants in Panama. J Tropical Ecol 15:213–227
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16:179–214
Reich PB, Borchert R (1982) Phenology and ecophysiology of the tropical tree, Tabebuia neochrysantha (Bignoniaceae). Ecology 63:294–299
Richter M (2003) Using epiphytes and soil temperatures for eco-climatic interpretations in Southern Ecuador. Erdkunde 57:161–181
Rollenbeck R, Bendix J (2006) Experimental calibration of a cost-effective X-band radar for climate-ecological use in southern Ecuador. Atmos Res 79:296–316
Rollenbeck R, Fabian P, Bendix J (2006) Precipitation dynamics and chemical properties in tropical mountain forests of Ecuador. Adv Geosci 6:73–76
Sakai S (2002) General flowering in lowland mixed dipterocarp forest of South Asia. Biol J Linn Soc 75:233–247
Sakai S, Momose K, Yumoto T, Nagamitsu T, Nagamasu H, Hamid AA, Nakashizuka T (1999) Plant reproductive phenology over four years including an episode of general flowering in a lowland Dipterocarp forest, Sarawak, Malaysia. Am J Bot 86:1414–1436
Schöngart J, Piedade MTF, Ludwigshausen F, Horna V, Worbes M (2002) Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. J Trop Ecol 18:581–597
Shukla RP, Ramakrishnan PS (1982) Phenology of trees in a sub-tropical humid forest in north-eastern India. Vegetatio 49:103–109
Simpson GG, Dean C (2002) Arabidopsis, the Rosetta stone of flowering time? Science 296:285–289
Singh KP, Kushwaha CP (2005) Emerging paradigms of tree phenology in dry tropics. Curr Sci 89:964–975
Stiles FG (1977) Coadapted competitors: the flowering seasons of hummingbird-pollinated plants in a tropical forest. Science 198:1170–1178
Tevini MM, Teramura AH (1989) UV-B effects on terrestrial plants. Photochem Photobiol 50:479–487
Valencia R, Cerón C, Palacios W, Sierra R (1999) Las formaciones naturales de la sierra del Ecuador. In: Sierra R (ed) Propuesta preliminar de un sistema de clasificación de vegetación para el Ecuador continental. Proyecto INEFAN/GEF-BIRF y EcoCiencia, Quito, pp 79–108
Vuille M, Bradley RS, Keimig F (2000) Climate variability in the Andes of Ecuador and its relation to tropical Pacific and Atlantic sea surface temperature anomalies. J Clim 13:2520–2535
Wolff D, Braun M, Liede S (2003) Nocturnal versus diurnal pollination success in Isertia laevis (Rubiaceae): a sphingophilous plant visited by hummingbirds. Plant Biol 5:71–78
Wright SJ, Cornejo FH (1990) Seasonal drought and leaf fall in a tropical forest. Ecology 71:1165–1175
Wright SJ, van Schaik CP (1994) Light and the phenology of tropical trees. Am Nat 143:192–199
Wright SG, Carrasco C, Calderón O, Paton S (1999) The El Niño southern oscillation, variable fruit production, and famine in a tropical forest. Ecology 80:1632–1647
Zipparro VB (2004) Climate as a proximate factor affecting phenology of Atlantic rain forest trees. http://ams.confex.com/ams(AFAPUBBIO/techprogram/paper 78878.htm
Acknowledgements
This work was performed as part of the research programme FOR 402 “Functionality in a Tropical Mountain Rainforest: Diversity, Dynamic Processes and Utilisation Potentials under Ecosystem Perspectives”, funded by the Deutsche Forschungsgemeinschaft (DFG) and also supported by the foundation Nature and Culture International (San Diego and Loja). The authors gratefully acknowledge the skillful assistance of Dr. Rütger Rollenbeck with the meteorological recordings and the correction of the raw data sets.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendix, J., Homeier, J., Cueva Ortiz, E. et al. Seasonality of weather and tree phenology in a tropical evergreen mountain rain forest. Int J Biometeorol 50, 370–384 (2006). https://doi.org/10.1007/s00484-006-0029-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-006-0029-8