Abstract
Campylobacter is among the most important agents of enteritis in developed countries. We have described the potential environmental determinants of the seasonal pattern of infection with campylobacter in Europe, Canada, Australia and New Zealand. Specifically, we investigated the role of climate variability on laboratory-confirmed cases of campylobacter infection from 15 populations. Regression analysis was used to quantify the associations between timing of seasonal peaks in infection in space and time. The short-term association between weekly weather and cases was also investigated using Poisson regression adapted for time series data. All countries in our study showed a distinct seasonality in campylobacter transmission, with many, but not all, populations showing a peak in spring. Countries with milder winters have peaks of infection earlier in the year. The timing of the peak of infection is weakly associated with high temperatures 3 months previously. Weekly variation in campylobacter infection in one region of the UK appeared to be little affected by short-term changes in weather patterns. The geographical variation in the timing of the seasonal peak suggests that climate may be a contributing factor to campylobacter transmission. The main driver of seasonality of campylobacter remains elusive and underscores the need to identify the major serotypes and routes of transmission for this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Campylobacter is an important agent of enteritis. In England and Wales, Canada, Australia and New Zealand, campylobacter infection now accounts for more cases per year than salmonella (Schmidt and Tirado 2001; Blummer et al. 2003; Health Canada 2003). Species type is not routinely ascertained in surveillance but Campylobacter jejuni is thought to be the most commonly isolated species, accounting for around 80–90% of infections in England (IID Study Team 2002).
Transmission occurs through consumption of contaminated food, water and milk products, as well as directly from animals and the environment. Risk factors identified for campylobacter infection include recent travel abroad (Schorr et al. 1994), occupational exposure in abattoirs and the consumption of chicken in restaurants (Eberhart-Phillips et al. 1997; Rodrigues et al. 2000). Water is thought to be an important route of transmission in New Zealand (Duncanson et al. 2000; Skelly and Weinstein 2003) and Canada (Clark et al. 2003). Treated drinking water can contain campylobacter but usually at levels considered too low to cause disease (Savill et al. 2001). In Norway, campylobacter levels in surface waters are generally higher in the colder months (Brennhovd et al. 1992; Obiri-Danso et al. 2001; Kapperud and Aasen 1992).
Reservoirs of campylobacter include a wide range of animals and birds. The spring peak of campylobacter may be related to birds and mammals foraging for food as activity in wild species generally increases after winter (Lacey 1993; Southern et al. 1990). The serotype distribution of Campylobacter in wildlife in Denmark was significantly different from known serotype distributions in broilers and humans, suggesting that the importance of wildlife as a reservoir of infection is limited (Petersen et al. 2001).
Poultry are considered to be the most important source of campylobacter in food, although any raw meat may be contaminated with the bacteria. The consumption of chicken is estimated to account for between 40% and 70% of the human infections. Case control studies, however, have not consistently implicated particular food vehicles (de Wit et al. 2000; IID Study Team 2002). Campylobacter does not multiply in food and is seldom identified as a cause of common-source outbreaks of food poisoning.
Despite the importance of poultry as a reservoir of infection, it does not explain the seasonal pattern in human infection. No seasonal variation was observed either in the prevalence of positive flocks, or in the percentage of birds infected in one area of the UK (Humphrey et al. 1993). However, one study found a summer peak in the prevalence of positive flocks and in the number of isolates in Lancashire, UK (Wallace et al. 1997). In Denmark, seasonal peaks in the prevalence of campylobacters in broiler chickens coincided with the seasonal pattern of human campylobacteriosis (Wedderkopp et al. 2000, 2001). The major peak in isolates in broiler chickens corresponds to a lesser second peak in humans seen in some countries, although this latter peak has also been associated with travel-related infections. Seasonal peaks in isolates in lambs, cattle and sewage generally appear 1–2 months prior to the peak in human infections indicating that this may be an important route of transmission (Stanley et al. 1998a, b; Wallace et al. 1997, 1998). However, it is possible that observed rates in animal reservoirs are biased by differences in surveillance intensity throughout the year. The seasonality of infection in pets does not correspond to the observed seasonality in humans (Lopez et al. 2002).
In this paper, we investigate the seasonal patterns of campylobacter infection using surveillance data for 15 populations in Europe, Australia, Canada and New Zealand. We also investigate any relationship of cases with meteorological factors in England and Wales, and the role of temperature in the timing of seasonal peaks of infection.
Materials and methods
Surveillance data
Surveillance data on laboratory-confirmed cases of campylobacteriosis were obtained from national surveillance centres in the following countries: the Czech Republic, Denmark, England and Wales, Estonia, Greece, Ireland, Malta, the Netherlands, Scotland, Spain, Switzerland (Schmidt and Tirado 2001), New Zealand, and Australia. Health Canada kindly supplied figures and information for comparison for Alberta. Data by species type or source/vehicle were unavailable.
There is an unknown and variable delay between the onset of illness and the date of report of confirmed infections to national surveillance systems. The average time from infection to the appearance of symptoms is a matter of days. The average time from becoming ill to a stool sample being sent to the laboratory varies between countries and depends on the health system and surveillance structures. In England and Wales, the mean delay between onset date and the date a specimen was recorded was 4.72 days, with over 90% of the specimen dates within 15 days of the onset date. We assume that the reported date corresponds to the onset date with an average delay, as reported by our partners in Table 1.
Meteorological data
For populations in Europe and New Zealand, a representative temperature series was constructed using daily temperature data from three to four weather climate stations in each country obtained from the archives at the German meteorological office. The national series were validated against an independent national (monthly) data series. We used the Central England temperature series for England and Wales (Parker et al. 1992). Temperature data for Canada were supplied by the Meteorological Service of Canada (Environment Canada) and for Australia by the Queensland Department of Natural Resources and Mines. We used 92 stations in Alberta to ensure geographical representativeness for the region. For all countries, we obtained baseline national monthly and seasonal temperatures (averaged for years 1961–1990) from the Tyndall Centre for Climate Change Research (dataset TYN_CY_1.1, Mitchell et al. 2002). For the analysis of the effects of weather in the North East region of England we used the following daily variables from the UK Meteorological Office for the Durham weather station: temperature, relative humidity, and sunshine hours. Area-averaged daily rainfall values for North East England coherent precipitation region were obtained from the Climatic Research Unit, University of East Anglia (Jones and Conway 1997). The daily series were collapsed to weekly average values.
Study design
The surveillance data were converted to weekly series (i.e. total number of cases per week). Each series was averaged by week for all years of data available and the seasonal patterns were plotted. At the first stage of the analysis, the relationship of campylobacter with short-term weather effects was investigated for the North East region of England. This region was chosen because rainfall patterns were relatively homogenous and the public water supplies were primarily from surface water sources within the region, thereby increasing the likelihood of detecting a potential water-borne transmission route. Weekly numbers of campylobacter cases were plotted against various weather parameters in this region to determine any crude associations. The relationships were summarized by smoothing the data using natural cubic splines of each weather variable as part of the Poisson generalised linear models (Kovats et al. 2004).
The next stage of the analysis concerned the more specific question of whether the timing of the peak of cases is affected by seasonal temperature variability. The “peak” week for each year was defined as the maximum total weekly count of cases each year. Conditional logistic regression models were used for this purpose with time to occurrence of the peak week each year as the dependent variable. To control for confounding from seasonal factors other than temperature, the analysis was matched on week. To explore the possibility of long time delays between temperature exposure and peak infection, the temperature series was lagged and then averaged across various weeks before being simultaneously entered into the model. Results are presented for the Czech Republic, Denmark, England and Wales, Scotland, Spain and Switzerland combined because the data were of suitable length and quality, although no allowance was made for possible clustering by year or country.
To investigate the role of climate variability between countries in determining the timing of infection, peak week was correlated with average winter (December–February) and spring (March–May) temperatures. For southern hemisphere populations, peak weeks were estimated as number of weeks from 1 July (week 28). Additional countries were included from a previous study that reported the peak week for Austria, Germany, Sweden, France, Finland and Norway (Nylen et al. 2002).
All analyses were undertaken in STATA version 8 (STATA 2003) or SPSS version 12 (SPSS 2003).
Results
Many countries showed an early spring peak (typically in April or May); however, not all countries followed this pattern (Fig. 1). The Czech Republic appeared to have two peaks of infection in summer. Denmark, Switzerland and the Netherlands had late summer peaks with the peak of cases occurring after the peak of temperature, and these may be associated with foreign travel. The seasonality was less pronounced in Australian cities than in New Zealand. There was also a difference in seasonality between North and South Island. In Alberta, Canada, infections peaked in late June–early July, and were lowest in February–March. The timing of the peak showed least variation from year to year in England and Wales, Greece, Denmark, and the Netherlands (SD <2 weeks). The timing of the peak was most unstable in Spain (SD 8 weeks) where the seasonal peak in spring was not clearly defined. Most countries with >8 years of data showed a small decline in seasonality over the last decade, but in no country was this trend statistically significant (results not shown).
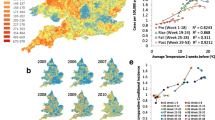
Figure 2 shows the simple relationships between weather parameters and numbers of cases of campylobacter infection in the North East region of England. The correlations are crude (i.e. not adjusted for potential confounders) and have splines overlaid to help summarize the basic relationship. The relationships shown are with weather in the previous week (lag 1), although results were also similar at longer lag periods. Similar patterns were found with temperature in other regions in UK and in other countries (not shown). Rainfall (averaged for North East England) and humidity in the previous month had little effect on the transmission of cases. However, the effect of temperature (and the closely linked parameter of sunshine hours) is more complex, and may have some influence on transmission during the beginning of the campylobacter “season”.
Crude relationship of campylobacter cases with weather variables in the North East region of England. The relationships are summarised using natural cubic splines of each weather parameter. The thick centre line is the estimate of the relationship, and the upper and lower lines represent the 95% confidence intervals. The dashed line represents the average number of cases in the dataset
Table 2 shows results of the analysis between temperature and the timing of the peak in candidate countries. Odds ratios are given for a 1°C increase in the temperature measure. The only association that reached statistical significance at the 5% level was an increase in cases associated with exposure to temperature measured 10–14 weeks prior to infection. Temperature accounted for only 4% of the inter-annual variability of the timing of the peaks. The estimates were similar when recalculated without the Spanish data series, as this country showed a different seasonal pattern.
Figure 3 illustrates the variation in climate and timing of peak infection between countries. In Australia/New Zealand, spring was defined as September–November and winter as June–August. Countries with warmer climates were shown to have earlier peaks. Table 3 shows that there was a fairly strong degree of correlation between both mean winter (r=−0.62) and spring temperature (r=−0.67) and the timing of peak of reported cases.
Discussion
There is a wide and varying literature in relation to campylobacter transmission mechanisms and seasonality, both within humans and animal species. The transmission of campylobacteriosis in humans is a complex ecological process with multiple hosts and routes (Skelly and Weinstein 2003). The literature does not imply a single main transmission route that would explain the seasonal pattern in human cases. We found that climate factors explain some differences in the seasonal variation between countries. Milder winter temperatures may favour some transmission routes, as well as enhance the survival and multiplication of the bacteria in the environment.
We were unable to find a strong effect of temperature variability on campylobacter transmission at either the short time scales (weather) or at longer (seasonal) time scales. We found no other published studies that had addressed similar questions, although some time series analyses of temperature effects have been investigated (Christensen et al. 2003; Tam et al. 2004). Our results are also consistent with the laboratory studies of the effects of temperature on the biology of campylobacters which only replicate in microaeorophilic environments (Altekruse et al. 1999; Kapperud and Aasen 1992). We observed no effect of rainfall. Although there is some evidence that outbreaks of infection with pathogens such as cryptosporidium are linked to extreme rainfall in conjunction with water treatment failures, the evidence for sporadic cases is lacking. In part, this is due to the difficulty in characterising the “extreme weather” over a large area and due to the complexity of the transmission pathway.
We have made several assumptions about the timing of seasonal peaks. Only a proportion of cases in the community are reported in national surveillance data, and those reported cases are not necessarily representative of all cases (Tam et al. 2003). Given the lack of routine direct surveillance of infectious disease morbidity, however, surveillance data are the best source of information available. Where possible, we have validated our assumptions by comparing reporting date with information on the onset of illness. We excluded data series from the regression analyses where the number of years was small, or where the number of cases per year was small (Malta). The data from most countries were case-based (i.e. repeat isolates from the same patient were excluded from the data series). Cases associated with travel were excluded from data for England/Wales, Scotland, Denmark and the Netherlands. Our analysis was limited by the lack of data from countries in southern Europe and by the lack of long time series (>10 years). Aggregation of national data will obscure important local or sub-national effects. The relative important of transmission routes may vary within countries, as has been shown in New Zealand (Hearnden et al. 2003). Further studies are needed when data become available that can identify the effect of climate on the different campylobacter species and animal reservoirs.
The role of weather in triggering short-term increases in infection has yet to be resolved. There are various potential transmissions routes (water supplies, bird activity, food borne and recreational contact) that could be affected by weather. However, the effect of short-term increases in temperature on campylobacter transmission is, at most, weak, in contrast to that consistently observed with salmonella transmission (Kovats et al. 2004; D’Souza et al. 2004). There is limited person to person transmission in infection with campylobacter and changes in human host susceptibility are unlikely to be important determinants of the seasonal pattern (Dowell 2001). However, intra-annual changes in the animal host reservoirs may be an important explanation for the seasonal patterns.
The timing of seasons is now shifting in the mid to high latitudes due to climate change. The onset of spring events moved forward by, on average, 6.3 days from 1959 to 1996 in Europe (Menzel 2000) and associated changes have been observed in both animal and plant species (Root et al. 2003). The lack of long-term health data from routine sources, in addition to the imprecision of disease onset or infection date, means that it is not possible to detect such small effects in seasonality in infectious disease data at present (Wilkinson et al. 2003). If the climate warms and the onset of spring becomes earlier, our study indicates that climate change is unlikely to significantly affect the seasonal pattern of campylobacteriosis in humans.
More specific questions relating to the role of climate variability can be addressed once more detailed information on vehicles or serotyping become available. Campylobacters are so widely distributed in nature that there is no prospect of reducing the reservoir of bacteria. At present there is no routine surveillance of campylobacter in animals, either food animals or wild species, but selective monitoring of sentinel species may provide useful information on the main transmission pathways. We recommend that countries develop integrated surveillance systems to monitor pathogens in animals as well as humans (WHO 2004). Currently, there are insufficient data to determine the seasonality or temperature-sensitivity of pathogens in animals and other environmental reservoirs. Complex systems analysis should be used to describe the relationship between the many meteorological, behavioural and other factors affecting the transmission of food-borne disease.
References
Altekruse SF, Stern NJ, Fields PI, Swerdlow DL (1999) Campylobacter jejuni—an emerging foodborne pathogen. Emerg Infect Dis 5:28–35
Blummer C, Roche P, Spence J et al (2003) Australia’s notifiable diseases status 2001: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell 27
Brennhovd O, Kapperud G, Langeland G (1992) Survey of thermotolerant Campylobacter spp. and Yersinia spp. in three surface water sources in Norway. Int J Food Microbiol 15:327–338
Christensen LE, Evans MC, Waino M, Ethelberg S, Madsen H, Wegener HC (2003) Climate as a predictor of prevalence of Campylobacter spp. in humans and broilers in Denmark. In: The 12th International Workshop on Campylobacter, Helicobacter and Related Organisms, Aarhus, Denmark, September 2003
Clark CG, Price L, Ahmed R, Woodward DL, Melito PL, Rogers FG, Jamieson D, Ciebin B, Li A, Ellis A (2003) Characterization of water borne disease outbreak associated Campylobacter jejuni, Walkerton, Ontario. Emerg Infect Dis 9:1232–1241
de Wit MA, Hoogenboom-Verdegaal AM, Goosen ES, Sprenger MJ, Borgdorff MW (2000) A population-based longitudinal study on the incidence and disease burden of gastroenteritis and campylobacter and salmonella infection in four regions of the Netherlands. Eur J Epidemiol 16:713–718
Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7:369–374
D’Souza RM, Becker N, Hall G, Moodie KB (2004) Does ambient temperature affect foodborne disease? Epidemiology 15:86–92
Duncanson M, Russell N, Weinstein P, Baker M, Skelly C, Hearnden M, Woodward A (2000) Rates of notified cryptosporidiosis and quality of drinking water supplies in Aotearoa, New Zealand. Water Res 34:3804–3812
Eberhart-Phillips EJ, Walker N, Garrett N, Bell D, Sinclair D, Rainger W, Bates M (1997) Campylobacteriosis in New Zealand: results of a case-control study. J Epidemiol Community Health 51:686–691
Health Canada (2003) Canadian integrated surveillance report: salmonella, campylobacter, pathogenic E. coli and shigella, from 1996 to 1999. Can Commun Dis Rep 29
Hearnden M, Skelly C, Eyles R, Weinstein P (2003) The regionality of campylabacteriosis seasonality in New Zealand. Int J Environ Health Res 13:337–348
Humphrey T, Henley A, Lanning DG (1993) The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol Infect 110:601–607
IID Study Team (2002) A report of the study of infectious intestinal disease in England. Stationery Office, London
Jones PD, Conway D (1997) Precipitation in the British Isles: an analysis of area-averaged data updated to 1995. Int J Climatol 17(4):427–438
Kapperud G, Aasen S (1992) Descriptive epidemiology of infections due to thermotolerant Campylobacter spp. in Norway, 1979–1988. Acta Pathol Microbiol Immunol Scand 100:883–890
Kovats RS, Edwards S, Hajat S, Armstrong B, Ebi KL, Menne B and Collaborating Group (2004) The effect of temperature on food poisoning: time series analysis in 10 European countries. Epidemiol Infect 132:443–453
Lacey R (1993) Food-borne bacterial infections. Parasitology 107:S75–S93
Lopez CM, Giacoboni G, Agostini A, Cornero FJ, Tellechea DM, Trinidad JJ (2002) Thermotolerant Campylobacters in domestic animals in a defined population in Buenos Aires, Argentina. Prev Vet Med 55:193–200
Menzel A (2000) Trends in phenological phases in Europe between 1951 and 1996. Int J Biometeorol 44:76–81
Mitchell TD, Hulme M, New M (2002) Climate data for political areas. Area 34:109–112
Nylen G, Dunstan F, Palmer SR, Andersson Y, Bader F, Cowden JM, Feierl G, Galloway Y, Kapperud S, Megraud K, Molbak K, Petersen LR, Ruutu P (2002) The seasonal distribution of campylobacter infection in nine European countries and New Zealand. Epidemiol Infect 128:383–390
Obiri-Danso K, Paul N, Jones K (2001) The effects of UVB and temperature on the survival of natural populations and pure cultures of Campylobacter jejuni, Camp. coli, Camp. lari and urease-positive thermophilic campylobacters (UPTC) in surface waters. J Appl Microbiol 90:256–267
Parker DE, Legg TP, Folland CK (1992) A new daily Central England temperature series, 1772–1992. Int J Climatol 12:317–342
Petersen LR, Nielsen EM, Engberg J, Dietz HH (2001) Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl Environ Microbiol 67:3115–3121
Rodrigues LC, Cowden JM, Wheeler JG, Sethi D, Wall PG, Cumberland P, Tompkins DS, Hudson MJ, Roberts JA, Roderick PJ (2000) The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol Infect 127:185–193
Root T, Price J, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Savill MG, Hudson JA, Ball A, Klena JD, Scholes P, Whyte RJ, McCormick RE, Jankovic D (2001) Enumeration of Campylobacter in New Zealand recreational and drinking waters. J Appl Microbiol 91:38–46
Schmidt K, Tirado C (2001) Seventh Report on Surveillance of Foodborne Diseases in Europe 1993–1998. Federal Institute for Health Protection of Consumers and Veterinary Medicine, European Centre on Environment and Health, Berlin, Rome
Schorr H, Schmid H, Rieder HL, Baumgartner A, Vorkauf H, Burnens AP (1994) Risk factors for Campylobacter enteritis in Switzerland. Zentralbl Hyg Umweltmed 169:327–337
Skelly C, Weinstein P (2003) Pathogen survival trajectories: an eco-environmental approach to the modelling of human campylobacteriosis ecology. Environ Health Perspect 111:19–28
Southern JP, Smith RM, Palmer SR (1990) Bird attack on milk bottles: possible mode of transmission of Campylobacter jejuni to man. Lancet 336:1425–1427
SPSS (2003) SPSS version 12. SPSS, Chicago, Ill.
Stanley KN, Wallace JS, Currie JE, Diggle PJ, Jones K (1998a) The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J Appl Microbiol 85:472–480
Stanley KN, Wallace JS, Currie JE, Diggle PJ, Jones K (1998b) Seasonal variation of thermophilic campylobacters in lambs at slaughter. J Appl Microbiol 84:1111–1116
STATA (2003) STATA 8.1. Stata, Tex.
Tam CC, Rodrigues LC, O’Brien S (2003) The study of infectious intestinal disease in England: What risk factors for presentation to general practice tell us about potential for selection bias in case-control studies of reported cases of diarrhoea. Int J Epidemiol 32:99–105
Tam CC, Rodrigues LC, O’Brien SJ (2004) Temperature dependence of campylobacter enteritis in England. International Conference on Emerging Infectious Diseases, Atlanta, Georgia, USA, February–March 2004
Wallace JS, Stanley KN, Currie JE, Diggle PJ, Jones K (1997) Seasonality of thermophilic Campylobacter populations in chickens. J Appl Microbiol 82:219–224
Wallace JS, Stanley KN, Jones K (1998) The colonization of turkeys by thermophilic Campylobacters. J Appl Microbiol 85:224–230
Wedderkopp A, Rattenborg E, Madsen M (2000) National surveillance of broilers at slaughter in Denmark in 1998. Avian Dis 44:993–999
Wedderkopp A, Gradel KO, Jorgensen JC, Madsen M (2001) Pre-harvest surveillance of campylobacter and salmonella in Danish broiler flocks: a 2-year study. Int J Food Microbiol 68:53–59
WHO (2004) Report of WHO Meeting on Climate and Foodborne Disease. WHO, Rome (in preparation)
Wilkinson P, Campbell-Lendrum D, Bartlett C (2003) Monitoring the health effects of climate change. In: McMichael AJ, et al. (eds) Climate change and health: risks and responses. WHO, Geneva
Acknowledgements
S. H., S. E., K. L. E. and S. K. were funded by the European Commission as part of the project Climate Change and Adaptation Strategies for Human Health (cCASHh) (EVK2-2000-00070). The authors would also like to thank: C. Tirado at the WHO Regional Office for Europe for help with obtaining the surveillance data; C. Koppe at DWD, Germany, for supplying meteorological data; M. Fleury at Health Canada for analysis of the Canadian health data; A. Maarouf at Environment Canada for Canadian meteorological data; Communicable Diseases Network Australia, National Notifiable Diseases Surveillance System for providing the data for notifications of campylobacterosis for the years 1991 to 2001 and the Queensland Department of Natural Resources and Mines for providing interpolated temperature data for the same years; At Health Protection Agency, I Gillespie for extracting and cleaning the England and Wales campylobacter surveillance data, and G. K. Adak for advice; C. McGuigan at the Scottish Centre for Infection and Environmental Health for the Scottish surveillance data; M. Baker at Environmental Sciences Research, for advice and provision of New Zealand surveillance data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sari Kovats, R., Edwards, S.J., Charron, D. et al. Climate variability and campylobacter infection: an international study. Int J Biometeorol 49, 207–214 (2005). https://doi.org/10.1007/s00484-004-0241-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-004-0241-3