Abstract
To assess differences in the lag-effect pattern in the relationship between particulate matter less than 10 μm in aerodynamic diameter (PM10) and cause-specific mortality in Seoul, Korea, from January 1995 to December 1999, we performed a time-series analysis. We used a generalized additive Poisson regression model to control for time trends, temperature, humidity, air pressure, and the day of the week. The PM10 effect was estimated on the basis of the time-series models using the 24-h means and the quadratic distributed-lag models using a cumulative 6-day effect. One interquartile range increase in the 6-day cumulative mean of PM10 (43.12 µg/m3) was associated with an increase in non-accidental deaths [3.7%, 95% confidence interval (CI): 2.1, 5.4], respiratory disease (13.9%, 95% CI: 6.8, 21.5), cardiovascular disease (4.4%, 95% CI: –1.0, 9.0), and cerebrovascular disease (6.3%, 95% CI: 2.3, 10.5). We found the following patterns in the disease-specific lag-effect window: respiratory mortality was more affected by air pollution level on the day of death, whereas cardiovascular deaths were more affected by the previous day's air pollution level. Cerebrovascular deaths were simultaneously associated with the air pollution levels of the same day and the previous day. The patterns in the lag effect from the distributed-lag models were similar to those of a series of time-series models with 24-h means. These results contribute to our understanding of how exposure to air pollution causes adverse health effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of studies have shown that daily air pollution concentrations are associated with daily mortality in several areas (Katsouyanni et al. 1997; Hong et al. 1999; Lee et al. 1999, 2000; Schwartz and Dockery 1992). These associations have been evaluated using pollution levels on the same day or those within a few previous days. In recent years, several studies examining the effect of air pollution on cause-specific deaths rather than on deaths from all causes have been reported (Hong et al. 2002; Kwon et al. 2001; Rossi et al. 1999). There are several reasons to study cause-specific mortality risks, one being that different causes of death can be affected by air pollution with different latent periods or through different mechanisms. If so, then using data for deaths from all causes will tend to confuse the lag structure of the association and underestimate the overall effect of air pollution.
The distribution of the effect over time (or the lag-effect window) may shed some light on the nature of the link between air pollution and cause-specific mortality (Braga et al. 2001). We considered differences in the lag structure pattern in the relationship between particulate matter less than 10 μm in aerodynamic diameter (PM10) and cause-specific mortality in Seoul, Korea, from 1995 to 1999. To assess the pattern, we used a distributed-lag model (Schwartz 2000), taking account of PM10 on the same day and for up to the previous 5 days. We also applied a series of time-series Poisson regression models with 24-h mean PM10 values and various starting times.
Materials and methods
We obtained hourly PM10 measurements for the years between 1995 and 1999 from the Ministry of Environment, Korea. We calculated the hourly mean levels for 27 monitoring stations in Seoul, and averaged these to calculate the daily mean PM10 level in Seoul. To reduce the impact of outliers in the pollution variables, the days when PM10 levels were above 123 µg/m3 (95th percentile) were excluded from the analysis. The daily numbers of deaths from non-accidental causes [International Classification of Diseases, 10th Revision (ICD-10): all causes except S01–S99, T01–T98], all respiratory diseases (ICD-10: J00–J98), specifically pneumonia (ICD-10: J10–J18), chronic obstructive pulmonary diseases (COPD) (ICD-10: J40–J47), all cardiovascular diseases (ICD-10: I00–I52), specifically myocardial infarction (ICD-10: I21), cerebrovascular diseases (ICD-10: I60–I69), and specifically ischemic stroke (ICD10: I63) were calculated from the mortality data supplied by the National Statistics Office of Korea. Meteorological data on 24-h mean temperatures, relative humidity, and sea-level air pressure were obtained from the Korean Meteorological Administration.
We utilized a distributed-lag model for each cause of death to verify and compare the lag-effect window pattern. Distributed-lag models have been used recently as an analytical approach in the study of epidemiology associated with air pollution (Schwartz 2000; Braga et al. 2001). The unconstrained distributed-lag model, which assumes that the number of deaths on any one day depends on the pollution level on that day and those on several previous days, can be written as:
where Y t is the number of deaths on day t, and X t–q is the PM10 concentration q days before the deaths and the overall effect can be expressed as β0 + ··· + β q . This model, however, produces unstable estimates because of autocorrelation between air pollution concentrations. The common approach to solving this problem is to constrain the shape of the coefficients with a lag number. This can be written as
If we combine Eq. 2 with Eq. 1, we obtain
This is called a dth-degree polynomial distributed-lag model. We have chosen a second-degree polynomial distributed-lag model with a maximum lag of 5 days before the death (q = 5), estimating η0, η1, η2 and the 6-day cumulative effect. It is generally accepted that a second-degree or cubic polynomial distributed-lag model is suitable.
We also used generalized additive Poisson regression models (Hastie and Tibshirani 1990), which include nonparametric smooth functions to control the potential nonlinear dependence of daily time-trends and weather variables on the logarithm of the mortality. We used the following basic model:
where Y is the daily count of deaths, the X is the PM10 level, Z i are the time and meteorological variables and S i are the Loess smooth functions (Pope and Schwartz 1996). Z i values cover temperature, relative humidity, sea-level pressure on the day on which deaths occurred, the previous day's temperature, time trends and the day of the week.
To evaluate the lag effect in detail, we computed 24-h means starting from various times: the 0-h lag starts from 00:00 hours on the corresponding day, the 6-h lag starts from 18:00 hours on the previous day, and the 12-h lag starts from 12:00 hours on the previous day, … etc., and they last for 24 h. The 6-, 12-, 18-, …, 72-h lags were calculated and used to get the estimated effects. The 0-h lag is the pollutant value on the same day, the 24-h lag is the 1-day lag value, the 48-h lag is the 2-day lag value, etc. In order to allow for different lag structures for different causes of death, the smoothing parameters were optimized separately for each disease. Estimated relative risks and Akaike's information criteria (AIC; Akaike 1973) were supplied for model comparison. The smaller the AIC, the better the fit. Distributed-lag models and Poisson regression models generally give similar lag-effect windows. The relative risks estimated from the distributed-lag models are generally bigger than those derived from time-series models. Detailed comparisons and explanations are also given in Results and Discussion.
Results
Table 1 shows the distributions of the PM10 levels, the daily cause-specific deaths and meteorological variables in Seoul from 1995 to 1999. The average PM10 level in Seoul satisfied the Korean standard of air pollution, but was higher than that for most cities of North America and some cities of Europe (Braga et al. 2001; Daniels et al. 2000; Goldberg et al. 2001a, b; Ostro et al. 1999). Table 2 shows correlations between PM10, the weather and various mortalities. Temperature and humidity have negative relationships with mortality from all the causes listed.
Table 3 shows the estimated percentage increase in daily mortality associated with one interquartile range (43.12 µg/m3) increase of PM10 for various causes of death. The percentage increases in daily mortality for all deaths and those from, respiratory, cardiovascular, and cerebrovascular causes are 3.7%, 13.9%, 4.4%, 6.3% respectively. The estimated effects on deaths from respiratory causes are considerably greater than those for other causes of death. The distributed-lag models always have smaller AIC values and bigger estimated relative risks than time-series models. This is not surprising because the distributed-lag model utilizes all the data for 6 days whereas a time-series model uses 24-h mean values of PM10. The estimated effects on deaths from COPD and ischemic stroke were not significant in any of the time-series models but were significant in the distributed-lag models. We compared the three time-series models for lags of 0, 1, and 2 days. For respiratory deaths, the estimated effect of lag 0 is the largest and the AIC value is the smallest, so we concluded that respiratory deaths are more affected by the air pollution levels on the same day. Similarly, we concluded that cardiovascular deaths are more affected by the air pollution levels on the previous days. For cerebrovascular deaths, effects of conditions on the day of death and on the previous days were found to be similar.
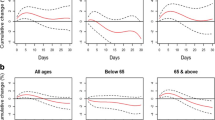
Similar patterns are observable in Fig. 1. Figure 1a shows estimated PM10 effects associated with one interquartile range from time-series models with different lags (6 h apart). Figure 1b shows lag effects from the distributed-lag models. We again observe that the lag effects for respiratory deaths are shorter that those for cardiovascular deaths. Generally there are few differences between the two patterns except for myocardial infarction (not shown): the effect estimated by the distributed-lag model decreases steadily whereas it is flat in the time-series models.
Estimated daily cause-specific relative risks of death and 95% confidence intervals associated with one interquartile range (IQR) increase of PM10 (43.12 µg/m3) from Poisson regression models and distributed-lag models. Akaike's information criteria are also plotted for the time-series models. a Time-series models. b Quadratic-distributed-lag model
Discussion
We examined the pattern of lag in the relationship between PM10 and different causes of death, using two methods. One fitted a time-series Poisson regression model using 24-hour mean PM10 values with different starting times and the other fitted distributed-lag model. Generally, the estimated effects were large in the lags when AIC values were small and the effect patterns resulting from two methods showed little difference. For respiratory deaths, including pneumonia, adverse effects after exposure to particulate matter were immediate. On the other hand, for cardiovascular deaths, the effects of pollution had a lag period of 1 or 2 days. For cerebrovascular deaths, effects were constant for up to 2 days and subsequently decreased. Interestingly, the effect of temperature on respiratory and non-accidental mortality was explained largely by the temperature on the previous day rather than that on the same day. Increased mortality was highest for respiratory deaths, then cardiovascular deaths; the effects on cerebrovascular and non-accidental deaths were lowest.
These dissimilarities of effect magnitude and lag structure suggest that there are different mechanisms for different causes of death. Lag structures in the relationship between air pollution and respiratory and cardiovascular deaths have been reported in several studies. Some studies reported a shorter lag period for respiratory deaths and some reported the reverse. Peters et al. (2000) reported that defibrillator discharges in patients with implanted cardioverter defibrillators did not follow exposure to particulate matter immediately but required an induction time of 1 or 2 days. In the study of Goldberg et al. (2001b) in Montreal, the effect on respiratory deaths (specifically of persons more than 65 years old) was larger following same-day exposure. Cardiovascular disease was more affected by exposure on the previous day. Our study shows similar results. However, Braga et al. (2001) reported that cardiovascular deaths represented an acute response to exposure to air pollution, but respiratory deaths were more affected by exposure on the 1 or 2 previous days.
These different results can be explained by the following. First of all, the mixture of particles is likely to vary with the study areas in the distribution of size, number, and chemical composition. The toxicity of particulate matter depends on its chemical composition and size distribution. Recently, fine particles (PM2.5) have been found to have bigger effects on health than PM10 (Bremner et al. 1999; Lee et al. 1999). The PM2.5 fraction in PM10 is likely to be different in different geographical regions. A further reason is that different populations have different structures. Some studies reported different susceptibilities for different groups (especially infants and the elderly) (Ha et al. 2001; Bremner et al. 1999). It is quite reasonable to assume that the actual effects are different for different study populations.
In summary, we present a statistically significant and positive association between PM10 and cause-specific mortality in Seoul, Korea. Respiratory deaths presented a more acute response to exposure to air pollution, with deaths occurring on the same day. Cardiovascular deaths were more affected by the previous day's air pollution level. Cerebrovascular deaths were simultaneously associated with the air pollution levels of the same day and the previous days. These results contribute to our understanding of how exposure to air pollution causes adverse health effects.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Caski F (eds) Second International Symposium on Information Theory, Budapest, 1973. Akademiai Kiado, Budapest, pp 267–281
Braga A, Zanobetti A, Schwartz J (2001) Structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J Occup Environ Med 43:927–933
Bremner SA, Anderson HR, Atkinson RW, McMichael AJ, Strachan DP, Bland JM, et al (1999) Short term associations between outdoor air pollution and mortality in London 1992–4. Occup Environ Med 56:237–244
Daniels MJ, Dominici F, Samet JM, Zeger SL (2000) Estimating particulate matter – mortality dose-response curves and threshold levels. An analysis of daily time-series for the 20 largest US cities. Am J Epidemiol 152:397–406
Goldberg MS, Burnett RT, Bailar JC III, Brook J, Bonvalot Y, Tamblyn R, et al (2001a) The association between daily mortality and ambient air particle pollution in Montreal, Quebec. 1. Nonaccidental mortality. Environ Res [A] 86:12–25
Goldberg MS, Burnett RT, Bailar JC III, Brook J, Bonvalot Y, Tamblyn R, et al (2001b) The association between daily mortality and ambient air particle pollution in Montreal, Quebec. 2. Cause-specific mortality. Environ Res [A] 86:26–36
Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC (2001) Is air pollution a risk factor for low birth weight in Seoul? Epidemiology 12:643–648
Hastie T, Tibshirani R (1990) Generalized additive models. Chapman and Hall, London
Hong YC, Leem JH, Ha EH, Christiani CD (1999) PM10 exposure, gaseous pollutants, and daily mortality in Incheon, south Korea. Environ Health Perspect 107:873–878
Hong YC, Lee JT, Kim H, Ha EH, Schwartz J, Christiani CD (2002) Effects of air pollutants on acute stroke mortality. Environ Health Perspect 110:187–191
Lee JT, Shin D, Chung Y (1999) Air pollution and daily mortality in Seoul and Ulsan, Korea. Environ Health Perspct 107:149–154
Lee JT, Kim H, Hong YC, Kwon HJ, Schwartz J, Christiani DC (2000) Air pollution and daily mortality in seven major cities of Korea, 1991–1997. Environ Res [A] 84:247–254
Katsouyanni K, Touloumi G, Spix C, et al (1997) Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from the APHEA projects. Br Med J 314:1658–1663
Kwon HJ, Cho SH, Nyberg F, Pershagen G (2001) Effects of ambient air pollution on daily mortality in a cohort of patients with congestive heart failure. Epidemiology 12:413–419
Ostro BD, Hurley S, Lipsett MJ (1999) Air pollution and daily mortality in the Coachella Valley, California. A study of PM10 dominated by coarse particles. Environ Res [A] 81:231–238
Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, et al (2000) Air pollution and incidence of cardiac arrhythmia. Epidemiology 11:11–17
Pope CA III, Schwartz J (1996) Time series for the analysis of pulmonary health data. Am J Respir Crit Care Med 154:S229–S233
Rossi G, Vigott, MA, Zanobetti A, Repetto F, Gianelle V, Schwartz J (1999) Air pollution and cause-specific mortality in Milan, Italy, 1980–1989. Arch Environ Health 54:158–164
Schwartz J (2000) The distributed lag between air pollution and daily deaths. Epidemiology 11:320–326
Schwartz J, Dockery DW (1992) Increased mortality in Philadelphia associated with daily air pollution concentrations. Am Rev Respir Dis 145:600–604
Acknowledgements.
This study was supported by a grant for reform of university education under the BK project of S.N.U. and was also partly supported by the Ministry of Health and Welfare, Republic of Korea (HMP-99-M-09 0007). The authors declare that the whole process of this study complies with the current law of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H., Kim, Y. & Hong, YC. The lag-effect pattern in the relationship of particulate air pollution to daily mortality in Seoul, Korea. Int J Biometeorol 48, 25–30 (2003). https://doi.org/10.1007/s00484-003-0176-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-003-0176-0