Abstract
Monte Carlo simulation (MCS) methodology has been applied to explain the variability of parameters for pollutant transport and fate modeling. In this study, the MCS method was used to evaluate the transport and fate of copper in the sediment of the Tibagi River sub-basin tributaries, Southern Brazil. The statistical distribution of the variables was described by a dataset obtained for copper concentration using sequential extraction, organic matter (OM) amount, and pH. The proposed stochastic spatial model for the copper transport in the river sediment was discussed and implemented by the MCS technique using the MatLab 7.3™ mathematical software tool. In order to test some hypotheses, the sediment and the water column in the river ecosystem were considered as compartments. The proposed stochastic spatial model makes it possible to predict copper mobility and associated risks as a function of the organic matter input into aquatic systems. The metal mobility can increase with the OM posing a rising environmental risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal pollution has drawn increasing attention worldwide due to a dramatic increase of anthropogenic contaminant metal to the ecosystems since that may pose a hazard to human livelihoods and sustainable development (Liu et al. 2007). As a result, significant footprints in sediments has stimulated studies that directly relate dispersal, storage and remobilization of sediment-associated metals in the fluvial system to sediment transport processes (Massoudieh et al. 2010).
River sediments play an important role for pollutants and reflect the river pollution history (Singh et al. 2005). The sediments act as a sink and carrier of contaminants in aquatic systems, and are the final fate of trace metals as a result of sorption process, diffusion, chemical reactions and biological activities. Natural and anthropogenic activities may remobilize the contaminated sediments through changes in sediment behavior and be a potential source of metals to the water column. The metal leaching process can take place shortly after the releases in the sediment and its extension depends on the metal interactions with different mineralogical fractions of the sediment, named solid speciation (Ramirez et al. 2005). The exposition to a different chemical environment could result in desorption and transformation of contaminants to bioavailable and toxic forms. In this way, bioavailability and bioaccumulation of contaminants in aquatic systems depend principally on the distribution coefficient or the contaminant binding strength in the sediment (Eggleton 2004).
According to Singh et al. (2005), metals may be present in sediments in several chemical forms and generally have different physicochemical behavior regarding chemical interactions, mobility, bioavailability, and potential toxicity. Hence, to identify and qualify the forms in which the metal occurs in the sediment is required to know the actual metal impacts and evaluate the transport processes in a river, through deposition and leaching under changing environmental conditions.
The evaluation of impacts on ecosystems and the proposition of environmental mathematical models are considered to be multidisciplinary scientific problems representing a principal challenge to the process understanding. The transport and the metal transformation in rivers have been subject of many studies concerning the application of mathematical models (He et al. 2001; Liu et al. 2007; Lindenschmidt et al. 2007), whose main goal is related to the low cost and transferability.
Studies on the transport of metal contaminants is incipient in Brazil (Silva et al. 2002; Allard et al. 2004), and due to its great territorial extension, metal-related impacts associated to the accelerated economic development even have been adequately evaluated. Although models have been developed for metal transport in river systems, speciation from sequential extraction applying Monte Carlo simulations have not been considered. Different forms of heavy metals with different mobilities and therefore different bioavailabilities must be considered.
In environmental systems, the mathematic models can be described from deterministic and stochastic approach, in order to predict the chemical species concentration in the water column, sediment, and biota (Zhang et al. 2011; Paleologos 2011; Sahoo et al. 2011; Jang et al. 2009). Whereas the deterministic approach describes the models from a single fixed value for each one of the input parameters, resulting in a single value for each one of the predicted results, in the stochastic approach, a wider range prediction is obtained, taking a distribution frequency for each input parameter in order to produce a distribution for the output parameters (Bansidhar et al. 2001).
In this study, a model of metal transport was developed to simulate the distribution of total copper concentration, which is partitioned into dissolved and solid phases, in a catchment that drains a medium, but in accelerated development, metropolitan area of south Brazil, and to build on what is presently known about metal transport in fluvial systems. The model predictions and measured data were compared to evaluate the model reliability.
Speciation is related to the distribution of an element among its different chemical species in water, sediment and soil. It is of increasing interest the use of sequential extraction techniques to relate mobility degree with risk assessment—the larger is the metal mobility, the larger is the associated risk. One of the methodology applied more thoroughly for the sequential extraction was proposed by Tessier et al. (1979), with the partition in to five geochemical fractions, defined operationally as: exchangeable, carbonate (acid-soluble), reducible (Fe/Mn oxides), oxidizable (organic matter), and residual. However, a three-stage sequential extraction procedure considering exchangeable, reducible, and oxidizable fractions (Thomas et al. 1994) can be applied to the sequential extraction when mobility and availability is concern in freshwater sediments.
The rest of the paper has the following organization: material and methods are expeditiously treated in Sect. 2, while the aquatic system, as well as the proposed metal transportation stochastic model are described in details in Sect. 3. The experimental aspects and adopted procedures in order to obtain and adjust the principal parameters values used in the model are discussed in the Sect. 4. Prediction and risk impact results applying the proposed model are extensively discussed in Sect. 5. The main conclusions are summarized in Sect. 6.
2 Methods and materials
2.1 Area of study
The area of study consist of a sub-basin River that drains to a major basin of the Tibagi River. This basin has a great importance to the city where, along with the stream, are installed industries, serve clubs, commercials and residence areas implying in big paving areas intercalated by some little rural areas. The sub-basin receives a significant amount of sewer and industrial discharges and have already had changes in their course as barrier, canalization and sanding. There are contribution of effluent rich in nutrients as phosphate, nitrate, and nitrite in addition to metals, besides commitment of the fish health due to histologic and physiologic alterations.
2.2 Sampling sites
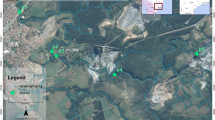
Four sampling stations were defined at the Londrina city hydrographic network (south Brazil): BT, which is characterized by industrial activities, JL is located downstream a water treatment station, LG with neighborhood anthropogenic activities profile, and PL, where the river come through a dense forest area. In Fig. 1 it can be observed that the sampling stations are approximately equidistant (at each 5 km). The sediment samples (short testimony) were collected with an acrylic tubular sampler (9.0 cm of diameter and 50 cm of height) and sliced to each 2 cm from top to bottom. The slices were oven-dried at 40°C, milled and sieved through 63 μm mesh before analysis. The water samples were collected in polyethylene bottles, acidified and reserved at 4°C until analysis. The analyses were made in triplicate.
2.3 Copper
The copper concentration was determined by taking into account the anthropogenic activity and its xenobiotic character. Due to the copper properties and characteristics, as well as the availability and influence on the biota, the studies were carried out for the metal mobility through the sediment by using sequential extraction.
The copper speciation in the sediment was determined by a three-stage sequential extraction procedure (Thomas et al. 1994) to obtain three copper fractions: (i) limited to exchangeable and carbonate, (ii) limited to Fe/Mn oxides, and (iii) limited to organic matter.
A Spectroflame Modula ICP-atomic emission spectrometer was used to determine the concentration of major and trace elements in the water and the extracts from sequential extraction. All reagents were analytical grade or extra-pure quality. All standard reagent solutions were stored in polyethylene bottles.
2.4 Organic matter
The dissolved organic matter (DOM) of the sediment samples was oxidized with a solution of \((\hbox{K}_{2}\hbox{Cr}_{2}\hbox{O}_{7}\)–\(\hbox {H}_{2}\hbox{SO}_{4})\) in a digester (150°C, 30 min), and determined by titration with ammonium iron(II) sulfate. The organic matter amount was calculated from stoichiometry of the reactions involved.
2.5 Monte Carlo simulation method
Monte Carlo simulation (MCS) is a stochastic method that can be applied to explain the behavior of variables for pollutants modeling, sediment transport and nutrient loading analysis (Giri et al. 2001; Osidele et al. 2003). Hence, MCS was the adopted simulation method to implement and evaluate the proposed pollutant transport and fate model. In this work, a two-dimensional spatial-stochastic model able to describe the iterations and relations with the water column and the sediment in a tropical river is proposed. Adopting MCS method, and after validation, the stochastic outputs of the MCS algorithm, which is fed with the input and internal variables values, are able to provide a sufficient quantity of information to predict the behavior and risk assessment of the metal in the river sediment.
Specifically, in this work, the MCS was applied to evaluate the fate and transport of copper in the sediment of the Tibagi River sub-basin tributaries—Paraná State, south Brazil. The statistical distribution of the variables were described based on the copper concentration dataset using sequential extraction, organic matter amount and pH. Monte Carlo computational realizations (trials) were extensively performed in order to evaluate the average response behavior for the cooper transport in the river sediment, under a specific scenario, mathematically described by the proposed metal transportation model, and by the numerical values of the internal and the input variables in the model as well. Depending on the analysis scenario, MCS realizations for our model ranges from 150 to 2,500 trials.
3 Proposed metal transportation model
In environmental systems, the mathematical models can be described in deterministic and stochastic forms under constant or variable conditions, in order to predict the chemical species concentration in water column, sediment, and biota. The deterministic way describes the models at a single fixed value for each input parameter, resulting in a single value for each predicted result. Average values may be used for each input parameter, resulting in output average values. The average and percentage values are based on the assumption the occurrence of a frequency distribution of the input parameters as a result of temporal variation. In the stochastic way, the distribution frequency for each input parameter is used to produce a distribution for the output parameters (Bansidhar et al. 2001).
Typically, there is an uncertainty degree for both the input variable values and the model due to variability and complexity of the environmental conditions. It is important to quantify the effects of each uncertainty regarding the model quality predictions (Wallach 1998). However, if the designed variables can be expressed as analytical equations or functions of the input parameters, and the probability distributions of the parameters with the uncertainty based on the observed values, the probability distributions for any dependent variable will be generated in terms of corresponding equations (Wang et al. 2004).
The increase in the number of designed variables in the model, enlarges the number of interacting processes between variables, being necessary additional parameters to control these processes. However, despite of the dynamic nature of those processes, i.e., the interacting among the species along the time and space, it is important to hold the model as simple as possible. According to Lindenschmidt (2006), if a model will be used under non-stationary conditions, both the parameters and the process descriptions should be transferable. In that way, the transferability is required. The transferability concept can be defined as the capacity to adapt the hydrological regime in different areas to the relevant space-temporal scales without requesting changes in the model structure. Therefore, the parameters must be identified in order to give good results, not only for the situation in which they have been calibrated, but for other situations as well.
3.1 Modeling approach
3.1.1 General aspects and hypothesis
A dynamic model offers a more flexible representation of possible fluctuations of pollutant sources, recognizing chemical substances that do not reach the standard state instantaneously. Temporal characteristics must be considered for any system that has steady input variables, finite decay velocity, and non-instantaneous transition velocities among the averages, within specific time intervals.
Some assumptions were made to take into account only the impacts of random parameters in the model construction: (i) copper concentration is an input variable, and the effluent flow velocity is negligible in relation to the river flow velocity, (ii) the metal transport is bidimensional, (iii) the river flow velocity and the sediment settling velocity are constants, and the dispersion effects are spatially constants, (iv) temperature fluctuations and biochemical variables are negligible. Both the sediment and the water column were considered as compartments studied. In Fig. 2 a schematic overview of the mechanisms and interactions considered in the model is sketched.
A concentration range was considered in the proposed model for punctually added copper \((\hbox{Cu}_{\rm add})\) in the water sampling sites, depending on the human activity (low, medium, high, and saturated) in relation to population (inhabitants number) and industrial activities. The adopted copper concentration ranges regarding the local characteristics (BT, LG, PL and JL sampling locals) were taken as random variables, as described in Table 1.
3.1.2 pH-dependent Cu–DOM interaction
In freshwater, the complexation by the dissolved organic matter (DOM) may dominate the trace metal speciation and control its toxicity and bioavailability (Lu and Allen 2002). It has been demonstrated that the Cu complexation is strongly pH-dependent and increases with pH. In order to describe more accurately the speciation and the Cu partition, Lu and Allen (2006) pointed out that the Cu–DOM complexation increases with pH firstly due to the Cu ions and protons competition for reactive functional groups of organic matter. For Lu and Allen (2002), the complexation by DOM is based on the following assumptions: the Cu–DOM complexation is only by \(\hbox{Cu}^{2+}, \) and hydrolyzed Cu is not complexed by ligand sites; \(\hbox{Cu}^{2+}\) and \(\hbox{H}^+\) ions compete for the ligand sites, being the monoprotic sites normally used, although polyprotic sites can be used in some cases; only the CuL 1:1 stoichiometry is considered.
Based on the interactions considered by Lu and Allen (2006), the Cu speciation might be expressed as the sum of free \(\hbox{Cu}^{2+},\) Cu-inorganic \((\hbox{Cu}(\hbox{L}_{\rm inorg})),\) C-organic \((\hbox{CuL}_{\rm org}\)), and the solid phase (CuS) as follows:
where
with \(\hbox{L}_{\rm inorg}\) being the inorganic ligand, \(\hbox{L}_{t,\rm org}\) the free DOM binding site, and S t the total surface site.
Taking into account the relations in the Eqs. 1–4, a general model for the distribution coefficient K d , expressed as a ratio of the copper species sorbed in the solid phase (mol kg−1) and the total concentration of the dissolved species (mol L−1), can be obtained:
The dissolved organic matter ligands are involved in many metal sorption processes with consequences for the metal mobility in the aquatic environment. Simulations of the copper speciation in aqueous phase, under typical river water conditions and based on the equilibrium constants (Radovanovic 1998), have indicated that the Cu–DOM complexation is the prevailing process, and the two most important inorganic ligands for Cu2+, depending on pH and/or alkalinity, are OH− and CO3 2−, Cu(OH)2, Cu(OH)+, and CuCO3 are the most important inorganic complexes, but they only have a significant effect in the Cu speciation at pH > 7. Under typical freshwater conditions, other inorganic ligands such as Cl− and \({\hbox{SO}_{4}}^{2-}\) normally have negligible effects on the K d , and therefore, can be disregarded (Lu 2006).
3.1.3 Water column
The copper transport in the water column is modeled by:
where the first term describes the longitudinal flux of the Cu transport at each sample station, and the second term, the metal dispersion process exactly at the sampling point. x is the distance between the sampling stations, \(\frac{\Updelta C_{t,\rm w}(x)}{\Updelta t}\) is a space-time function of the total copper concentration variation in the water (subscript \(\rm w), V_x\) is the river flux velocity, \(\frac{\Updelta C_{t}}{\Updelta{x}}\) is the copper concentration variation as a function of space, and D d is the dispersion coefficient. A decreasing exponential or still a third-degree polynomial behavior regarding the longitudinal river distance can be assumed for the two terms in Eq. 6. The river flux velocity and the dispersion coefficient are considered constant in the distance of the river.
3.1.4 Sediment
In the sediment, the copper concentration averages for each extractor, at each sampling station (i.e., determined for each distance x) were used as input data for the MCS trials. The settling of the dissolved and particulate copper was considered. For copper bound to organic matter, the relationship with organic ligands and stability constants, as well as the metal distribution in the solid–liquid phases and the pH influence were set up in C 3 term of the Eq. 7. For this equation, a decreasing exponential fitting and, alternatively, a third-order polynomial fitting were taken into account to model the term K d /H.
where
where C t,sed(x) is the total sediment copper concentration, H is the sediment depth, V d is the settling velocity, [Cu2+] is the free copper concentration, K d is the distribution coefficient, B 3 is the dissolved copper in the water column. The distribution between these two phases can be described by a partition coefficient and defined as \(K_d =\frac{C_{3}}{B_3}\cdot k_1, k_2, k_3\) are proportionality constants, \(K_{\hbox{Cu}\hbox {L}_{\hbox {org}}}\) is the stability constant, and e 1, e 2, e 3 are Cu-exchangeable, Cu–Fe/Mn oxides (reducible), and Cu–OM fractions, respectively. The proportionality constants k 1, k 2, and k 3 represent the contribution of Cu bound to the exchangeable, the oxides, and the OM fractions, respectively, in relation to the Cu-total fraction. The ρ represents the amount of exchangeable (ex), oxidable (ox), and organic matter (OM) particulate fractions.
4 Evaluation of the proposed model using MCS method
From a stochastic perspective, MCS method was applied, using the MatLab 7.3™ mathematical software tool, to quantify the input variables changes and added effects, as well as to carry out a sensibility analysis for several parameters, aiming at studying the prior environmental impacts and risks. The copper concentration in each extractor fraction, as well as the organic matter amount at each site, and the pH variation, were considered as stochastic parameters, and the other parameters as deterministic ones. The Uniform statistical distribution \(\mathcal{U}\) was assumed for several inputs and internal variables in the model, taking into account a large confidence interval within a certain range of values around the average.
Indeed, in order to evaluate the effect of uncertainty in the random parameters of the model, Monte Carlo simulations have been performed. We have assumed probability distributions for these parameters since it is very complicated to describe the true nature of randomness in these parameters. Hence, let Z denote a random variable (r.v.). The range [Z L , Z U ] describes the lower and upper bound of values. Assuming r.v. Z to be uniformly distributed, i.e., taking values in the range \(Z\,\sim \, \mathcal{U}[Z_L,Z_U],\) or equivalently \(Z\,\sim \, \mathcal{U}\left[\frac{Z_L+Z_U}{2},\pm \frac{Z_U-Z_L}{2}\right],\) then
Analogously, if we assume Z to be either a random variable with normal distribution, then
or other probability value near to one. As a result, once a probability distribution is assumed for the parameter Z, first (mean) and second (variance) statistical moments can be calculated directly from lower and upper bounds Z L and Y U by using the above equations.
Table 2 shows the principal variables of the model, their meanings, units and the type of the distribution, if deterministic or stochastic, with the respective values of the statistical moments deployed in the Monte Carlo simulations (MCS). Of course, for the adopted stochastic parameters in Table 2, alternatively, it is reasonable to choose Gaussian distributions (or Log-Normal distributions or even a combination of them) with suitable first and second statistic moments if an extensive parameter measurements could be available to guide this choice.
However, for the general purpose of our model validation, herein, uniform statistical distributions have been assumed for several parameters based on the literature discussion (Radovanovic 1998; Bansidhar et al. 2001) and references inside. For instance, in Bansidhar et al. (2001) the dispersion coefficient is considered a deterministic parameter in a wide range \(D_d\in [20;198]\, \hbox{m}^2\) s−1 (river scenarios) or in the range \(D_d \in [150; 300]\, \hbox{m}^{2}\) s−1 (lake scenarios). Furthermore, for the flux velocity V x and the amount of oxidable particulate fraction ρox, generally in the literature, they have been adopted as a deterministic parameters, with some discrepancy of value; for instance, in (Bansidhar et al. 2001) it has been considered different but deterministic values for flux velocity in the range \(V_x\in[0.1; 1].\) On the other hand, the mean value of 0.30 for ρox was obtained from the Fe and Mn determinations by atomic emission (ICP-AES), using the extractor for oxides of iron and manganese.
For the second set of parameters \(\rho_{\hbox{OM}},\) pH and Cu added, it was admitted a uniform distribution with low and upper bound values (Z L , Z U ) based on measurements of the anthropogenic activities in the sampling locals referred to Table 1. For instance, the added Copper values in the ranges namely low, medium, high and very high, Cul, Cum, Cuh and Cuvh, respectively, are given in Table 2. A relative narrow range is adopted for the \(\rho_{\hbox{OM}}\) and pH (20%), since measurements for these parameters indicated small deviations. It is worthing to note that in literature a deterministic approach have been adopted for both parameters (Radovanovic 1998; Bansidhar et al. 2001).
The model implemented in Eq. 10 takes into account the copper transport in the water column, as described in Eq. 6, and the sediment settling term as well, Eq. 7. It is worth to note that the assumed values (as a function of longitudinal distance, x) of Terms 1 and 2 in (10) were obtained by exponential and polynomial data fitting using the site sampling data.
where in this case the metal [Mn+] stands for [Cu2+].
The model equation is constituted of three terms. The Terms 1 and 2 describe the metal longitudinal flux in the water column and the metal dispersion at the sampling point, respectively, according to Eq. 6, and the Term 3 describes the sediment copper concentration related to the speciation: Cu-exchangeable, Cu-oxides, and Cu–OM fractions, as well as to the copper settling rate in the water, according to Eq. 7. The metal settling velocity was taken into account as a deterministic depth variable, with an average value of 2.89 × 10−4 m s−1 for the sediment, and related to the copper speciation, according to Vuksanovic et al. (1996). The settling velocity depends on the particle diameter and density, presenting a range of 9.26 × 10−6 to 9.78 × 10−4 m s−1 for natural particles.
The incremental differential behavior of the first two terms from Eq. 10 is obtained in the model by the first and the second derivatives, respectively, of the adopted exponential and the third order polynomial approximations:
Figure 3 shows the results for the curve adjustments taking into account the average copper concentration in the water column, obtained from each sampling site dataset. The adopted parameter values, i.e., A 1, t 1 and y 0 parameters for the exponential and p 0, p 1, p 2, p 3 coefficients for the third-order polynomial fitting curves from Eqs. 11 and 12, as well as the fitting goodness for both approximations are presented in Table 3.
Because the distribution coefficient is an important parameter for metal transport modeling or risk assessment due to describing the speciation in sorbed and dissolved states, the Term 3 from Eq. 10 refers to the sediment, where the metal bound to the OM (C3) is related to the distribution coefficient (K d ):
where C s is the solid-phase metal concentration (particulate, mol kg−1 or mg kg−1), and C d is the dissolved-phase metal concentration (mol L−1 or mg L−1). The K d values depend on both the solid-surface properties and the chemical properties of the water. The K d presents different values for different geographical areas, as well as for the same area with different conditions.
The K d has been widely used to describe the solid–liquid metal distribution in transport models, and several problems can be identified in current models in aquatic environments. Firstly, the K d values are found out to be specific constants of the metals or are empirically estimated using, among others, solid and solution characteristics as pH, salinity, CEC, and total dissolved solids. Parameters such as CEC and salinity, represent interactions that are not taken into account individually. However, the parameters used for empirical models are conditional and depend on the model (Radovanovic 1998).
5 Discussion
Taking into account the environmental characteristics, in the first instance, was reasonable to consider the flux velocity (V x ) as a deterministic variable, with an average value of 0.35 m s−1. In the second instance, it was assumed a stochastic behavior for this variable with Uniform distribution, an average value of 0.35 m s−1, and a percentage interval of ±5%. With this value of flux velocity, the Term 1 in the Eq. 10, which represents the metal longitudinal point-to-point flux down the river (Fig. 4a), can be associated to the transport of approximately 30−40% of the initial copper concentration.
Term 2 in the Eq. 10 is represented by the metal radial dispersion at the sampling point. The dispersion coefficient is a river hydraulic parameter. For rivers, this parameter is represented by a range of 19.78 to 197.84 m2s−1. In this study, the correlation given by Mccutcheon (1989) was assumed as follows:
where D d is the dispersion coefficient, n is the Manning rugosity coefficient, V x is the flux velocity, and H depth (m). Considering a stochastic variable behavior and Uniform distribution for D d , in the range of 150 to 300 m2 s −1, it can be observed from Fig. 4b that the Term 2 of Eq. 10 suggests a metal concentration contribution close to the initial copper concentration in the water, with acceptable variation in each sampling point. Besides, Term 3 of Eq. 10 is related to the sediment, and is able to discriminate the copper concentration in the fractions, as well as its relations with the pH variation (6.0–8.0), and the metal complexation with the organic matter at each sampling point.
Furthermore, in order to describe the copper distribution observed in the sediment, the Term 3 was decomposed into 3-1 to 3-4, which are the contributions of Cu-settled, Cu-exchangeable, Cu–Fe/Mn oxides, and Cu–OM fractions, respectively, in relation to the K d and pH, as shown in Fig. 5.
The basin headwater at the BT point (Fig. 5a) shows the larger deposition rate due to the higher copper amount in the dissolved and particulate phases, probably due to the type of particulate derived from local industry. In Fig. 5b, the Cu-exchangeable fraction with the depth is highlighted at the LG point. This fraction can easily equilibrate with the aqueous phase and become bioavailable. At the LG point evidences of environmental overload conditions could be found. Great amounts of micropollutant organic compounds and clays in the sediment may act as sorbents, keeping metals by ionic exchange, resulting in higher Cu-exchangeable fraction amounts. Still at the LG point, the Cu–OM trend highlights again the local environmental overload characteristics (Fig. 5d), as the OM from human activity. Otherwise, Fig. 5c shows the predominance of the Cu–Fe/Mn oxides fraction at the PL site. Therefore, for this dataset conditions and implemented MCS, Fe/Mn oxides and OM have worked as a sink for copper, and their matrix leaching can be affected by redox and pH conditions. Figure 6 shows the total copper behavior, from the three Terms of Eq. 10, 15 km across this hydrographic basin segment, where the effect of a more impacted site can be observed down the basin.
Copper behavior taking into account the three Terms of the model, Eq. 10, and exponential approximation: a a hundred MCS trials curves; b average values curve
5.1 Model validation
In order to validate the proposed model, from the initial results obtained by the simulations, and based on goodness-of-fit shown in Fig. 3, it was assumed that the exponential approximation has higher representativity regarding the third order polynomial equation for the copper transport when the pH and OM variables are modified. Although both approximations present similar fitting goodness values (RMSE and R2 values in Table 3), the exponential fitting presents a suitable monotonic behavior in contrast with the oscillating dependence found in the third order polynomial fitting. The model consistence depends on the behavior described by these modified variables. The distribution coefficient is directly proportional to the cited parameters, since an increase of the H+ concentration and the OM amount could cause the raise of the K d and the Cu–DOM complexation, respectively, decreasing the Cu availability in the aquatic environment.
5.1.1 Distribution coefficient (K d )
In order to evaluate the K d evolution, the H+ concentration was fixed at 10−6 mol L−1, whereas for only one site the variation was within an interval of {10−8; 10−4} mol L−1. Figure 7 points out the K d behavior with pH variation in Eq. 7 for the four sampling sites. Figure 7 shows the increasing monotonic behavior of K d dependence with pH. Different ranges of K d are obtained in each sampling site (BR, LG, PL and JL). All the other values for the model parameters, described in Table 2, were kept constant or near-constant in the case of random variables, with the variance being close to zero. Besides, according to Tessier et al. (1989), the pH increase promotes a K d increase within a range of 4.5–6.5. Depending on the metal, the K d may vary up to three orders of magnitude. Moreover, models for chemical species in soils and groundwater have been frequently applied by using distribution coefficients, because these species are easily incorporated in advection-dispersion processes.
5.1.2 K d versus organic matter variation
Studies of Lu and Allen (2002) demonstrated that the copper complexation appears to be strongly pH-dependent, and it increases with the pH. The Cu–OM complexation takes place mainly through the Cu–H change over a wide pH range. However, in our study, the Cu–DOM interaction was described according to the partition model for Cu sorption processes by taking into account the pH and the organic matter influence in both the water column and the sediment. For the Terms 3–4 of Eq. 10, it was considered the partition model described by Lu and Allen (2006).
Based on Eq. 7, Fig. 8 indicates the K d behavior taking into account the organic matter present in the sediment within a range of 1 to 1,000 mg kg−1 and an average value of OM (33.9 mg kg−1). The K d is monotonically dependent on the OM increase. At the PL site, a higher value for the K d could be observed when the solid-phase (C 3) had a larger influence than the aqueous phase (B 3).
From Fig. 8, one can see K d behavior quite similar on the BT and PL sites, but distinct. LG and JL sampling locals present K d similarity as well. This can be explained as although there are different origins for anthropogenic activity in BT and PL locals, both are into dense forest areas, whose similar characteristics related to the OM might be influencing the interactions with Cu ions, stated through C 3 term. In this way, the simulation involving variation of organic matter leads to similar K d ranges. Likewise, LG and JL locals hold similar relations as anthropogenic activity, keeping similar K d values. Furthermore, it is worth to note that even small differences in the K d regarding the differences in the anthropogenic activities are able to be captured by the proposed stochastic transport model.
5.1.3 Total copper versus organic matter variation
Figure 9 shows the Cu-concentration variation trends in the aquatic system after the assumed OM input ranging from 1 to 500 mg kg−1. This adopted range follows the same assumptions used in the ranges of Cu-addition, as described in Table 1. Hence, in the Monte Carlo simulations, the adopted OM addition values associated to low, medium, high and saturated human activity were 1, 10, 200 and 500 mg kg−1, respectively.
As expected, with the OM increase, the contribution of the Term 3 (sediment, Eq. 10) is larger in relation to that of the first and the second ones (water). This contribution is due to the direct proportionality between K d and OM. For the largest OM range (200–500 mg kg−1), there was a turnover of the Term 3 contribution. The larger the OM amount, the larger its contribution to the copper distribution, regarding the metal transport downstream.
The river flow is changed by a small impoundment that decrease the residence time of the water at LG local that incorporates OM from diverse human activity. As the residence time is greater, the OM takes part of transformation processes different of those that occur at PL local, whose OM incorporation is mainly associated to the dense vegetation characteristic. Various types of organic ligands will produce compounds with stability constants and solubility with different background for sorption capacity in the aquatic system. In this way, as can be seen from Fig. 9, the proposed model offers different answers to these two local when OM quantity was added. It can also be observed that the incorporation of a large quantity of OM (200 and 500 mg kg−1) increases the differences in the copper mobilization for LG and JL sampling sites. LG tends to reserve copper in the river bed, as long as PL, with higher water velocity, promotes higher mobilization of the metal–OM association.
5.1.4 Total copper versus pH variation
Within a pH range of 4.0–8.0, found in aquatic systems, a low change of the total Cu contribution was observed in the studied system. The total Cu contribution increases with pH 6.0 and 8.0, in relation to the results for pH 4.0, as displayed in details in Fig. 10.
5.1.5 Scenarios of organic matter influence on aquatic system
After the proposed model has been validated, assumptions related to pH and organic matter variables were considered to analyze different scenarios of the aforementioned variables, as a consequence of environmental contamination caused by human activity. The scenarios can be observed in Figs. 11 and 12.
In Fig. 11, the additions of 10, 50, 100, 200, and 500 mg kg−1 of organic matter were assumed for the reference site (BT) with the pH being constant (6.0). This OM increase produced a decrease in the Cu-transport contribution to the aquatic system. The BT and PL sites showed the higher variation than the other ones, due to the high K d values.
As it is seen from Fig. 12, the scenario is set up from both the organic matter amounts and the pH values determined in the sediment. Separate additions of 100 mg kg−1 of OM at each site were simulated and after that the copper contribution trend was evaluated at the subsequent sites. In this way, a great increase of the Cu contribution at the BT site could be observed. At the PL site, the Cu contribution increases significantly, providing larger representativity of the solid phase in the metal dispersion. On the other hand, the JL site shows a low variation in relation to the initial condition. The reason for this low influence of the added OM is due to this sampling local presents the higher anthropogenic activity. So, probably, the added OM at previous sampling local (PL) may not been enough to show great differences in the copper mobilization.
From Figs. 11 and 12, clearly one can see an increasing in the concentration of mobilized Cu along the river sub-basin, as a function of OM addition, approaching to concentration range of [1.0; 9.0], [2.3; 3.8], [1.6, 14.0] and [1.0, 3.5] mg kg−1, on the BT, LG, PL, and JL sites, respectively. It is worthing to note the higher variance for the PL local in Fig. 11, and low for JL and LG (in both figures), due to the environmental characteristics, such as the quality of the OM introduced in the environment, as well as the residence time of the water, which would involve in the variations on the mobility, by imposing greater risk of mobilization in the BT and PL locals, which does not mean that there is no risk associated with the LG site. Environmental events, such as large volumes of rain in the sampling sites or above these locals can promote metal remobilization, increasing substantially the possibility of environmental risk associated.
Finally, in accordance with the Central Limit Theorem (Dudle 2002) it is possible to infer from Fig. 13 that the copper concentration variation in the proposed model shows a Normal (or Gaussian) statistical distribution at all sampling sites. Specifically, under the analyzed conditions by assuming the input variables pH and OM with Uniform distribution and the OM addition at the PL sampling site, the simulated results show that along the Tibagi River sub-basin tributaries the \(\Updelta\)[Cu] (output variable in the proposed model) could be described by the Gaussian probability density functions (Gaussian PDFs), with mean and variance values strongly dependent on the input variable values at each sampling site.
6 Conclusions
The proposed mathematical model have described properly the copper transport and behavior along the analyzed aquatic system. In the proposed stochastic-spatial model, four complementary mechanisms that describe the copper transport were considered.
The stochastic character attribution from the uniform probability density functions assumption was appropriated for description of essential variables for the metal transport process (copper concentration, pH, and organic matter amount), and can be transferred to other metals and river systems. Hence, the proposed model made it possible to determine the essential variables for the three Terms of the general equation for the aquatic system, regarding the sediment depth and the spatial distribution. Therefore, the contribution of copper related to the three Terms in the suggested model could be highlighted from the solid–liquid copper distribution.
For each sampling site, the Term 3 contribution to the equation model showed a direct relation with the solid–liquid distribution coefficient, whereas the Term 1 and Term 2 demonstrated a contribution more possibly marked by the variation of other factors altering the aquatic system.
An increase of the organic matter load into the aquatic system would increase the copper mobility, and consequently, enlarge the associated risks. Therefore, recovery/remediation environmental actions, such as for copper contamination, could be evaluated by taking into account the regulation of organic matter amount input into the aquatic system.
References
Allard T, Menguy N, Salomon J, Calligaro T, Weber T, Calas G, Benedetti M (2004) Revealing forms of iron in river-borne material from major tropical rivers of the Amazon Basin (Brazil). Geochim Cosmochim Acta 68(14):3079–3094
Bansidhar SG, Karimi IA, Ray MB (2001) Modeling and Monte Carlo simulation of TCDD transport in a river. Water Res 35(5):1263–1279
Dudley RM (2002) Real analysis and probability, 2nd edn. Cambridge University Press, Cambridge
Eggleton J, Thomas KV (2004) A review of factors affecting the release and biovailability of contaminants during sediment disturbance events. Environ Int 30:973–980
Giri BS, Karimi IA, Ray MB (2001) Modeling and monte carlo simulation of tcdd transport in a river. Water Res 35(5):1263–1279
He M, Wang Z, Tang H (2001) Modeling the ecological impact of heavy metals on aquatic ecosystems: a framework for the development of an ecological model. Sci Total Environ 266:291–298
Jang C, Lin K, Liu C, Lin M (2009) Risk-based assessment of arsenic-affected aquacultural water in blackfoot disease hyperendemic areas. Stoch Environ Res Risk Assess 23:603–612
Lindenschmidt KE (2006) Testing for the transferability of a water quality model to areas of similar spatial and temporal scale based on an uncertainty vs. complexity hypothesis. Ecol Complex 3:241–252
Lindenschmidt K, Fleischbein K, Baborowskib M (2007) Structural uncertainty in a river water quality modelling system. Ecol Model 204:298–300
Liu WC, Chang SW, Jiann KT, Wen LS, Liu KK (2007) Modelling diagnosis of heavy metal (copper) transport in an estuary. Sci Total Environ 388:234–249
Lu Y, Allen HE (2002) Characterization of copper complexation with natural dissolved organic matter (DOM)—link to acidic moieties of DOM and competition by Ca and Mg. Water Res 36:5083–5101
Lu Y, Allen HE (2006) A predictive model for copper partitioning to suspended particulate matter in river waters. Environ Pollut 143:60–72
Massoudieh A, Bombardelli F, Ginn T (2010) A biogeochemical model of contaminant fate and transport in river waters and sediments. J Contam Hyd 112:103–117
Mccutcheon C (1989) Water quality modeling—transport and surface exchange in rivers, vol 1. CRC Press, Boca Raton
Osidele OO, Zeng W, Beck MB (2003) Coping with uncertainty: a case study in sediment transport and nutrient load analysis. J Water Resour Plan Manage 129(4):345–355
Paleologos E, Sarris T (2011) Stochastic analysis of flux and head moments in a heterogeneous aquifer system. Stoch Environ Res Risk Assess 25:747–759
Radovanovic H, Koelmans AA (1998) Prediction of in situ trace metal distribution coefficients for suspended solids in natural waters. Environ Sci Technol 32:753–759
Ramirez M, Massolo S, Frache R, Correa JA (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar Pollut Bull 50:62–72
Sahoo G, Schladow S, Reuter J, Coats R (2011) Effects of climate change on thermal properties of lakes and reservoirs, and possible implications. Stoch Environ Res Risk Assess 25:445–456
Silva I, Abate G, Lichtig J, Masini J (2002) Heavy metal distribution in recent sediments of the Tietê–Pinheiros river system in São Paulo state, Brazil. Appl Geochem 17:105–116
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. J Hydrol 312:14–27
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Tessier A, Carignan R, Dubreur B, Rafin B (1989) Partitioning of zinc between the water column and the oxic sediments in lakes. Geochim Cosmochim Acta 53(7):1511–1522
Thomas R, Ure A, Davidson C, Littlejohn D, Rauret G, Rubio R, López-Sánchez J (1994) Three-stage sequential extraction procedure for the determination of metals in river sediments. Anal Chim Acta 286:423–429
Vuksanovic V, Smedt FD, Meerbeeck SV (1996) Transport of polychlorinated biphenyls (PCB) in the Scheldt Estuary simulated with the water quality model WASP. J Hydrol 174:1–18
Wallach D, Genard M (1998) Effect of uncertainty in input and parameter values on model prediction error. Ecol Model 105:337–345
Wang XL, Tao S, Dawson RW, Wang XJ (2004) Uncertainty analysis of parameters for modeling the transfer and fate of benzo(a)pyrene in Tianjin wastewater irrigated areas. Chemosphere 55:525–531
Zhang Y, Xia J, Shao Q, Zhai X (2011) Water quantity and quality simulation by improved SWAT in highly regulated Huai River Basin of China. Stoch Environ Res Risk Assess (12). doi:10.1007/s00477-011-0546-9
Acknowledgments
This work was partially funded by the Brazilian agency The National Council for Scientific Technological Development (CNPq) under Research Grant Contract #303426/2009-8 and scholarship. The authors would like to express gratitude to the Associate Editor and anonymous reviewers for their invaluably constructive suggestions and helpful comments, which have enhanced the readability and quality of the manuscript. Finally, authors would like to thank Evgeny Galunin for providing language help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corazza, M.Z., Abrão, T., Lepri, F.G. et al. Monte Carlo method applied to modeling copper transport in river sediments. Stoch Environ Res Risk Assess 26, 1063–1079 (2012). https://doi.org/10.1007/s00477-012-0564-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00477-012-0564-2