Abstract
Key message
Climate responses of radial growth of major hardwood species growing in a cool temperate forest in Japan were clarified by dendrochronological analysis combining the phenology observation of radial growth.
Abstract
To better understand which climate factors limit the radial growth of major hardwood species growing in a cool temperate forest in Japan, we clarified the phenology of radial growth and developed ring width residual chronologies for Betula ermanii, Fagus crenata and Quercus crispula at Takayama, an LTER research site located in central Japan. We inspected stem tissue for division of cambial cells in the early stage of wood formation and examined the cumulative radial growth by the wounding method. The onset of cambial cell division in Q. crispula was observed to be about 2 weeks earlier than in the other species. Wood formation in the final part of the tree ring occurred approximately 1 month earlier in B. ermanii than in the other species. The correlation analysis was performed between chronologies and moving averages of 31-day climate data with a 1-day lag. The periods which revealed significant correlations with climate factors were categorized into the previous growing season, previous autumn, just before the current growing season and current growing season according to their characteristic responses and the phenology of wood formation and leaves. The negative correlations between ring width of all the species and mean and minimum temperatures of previous year’s autumn, when the growth ending of ring width and leaf coloring occurred, suggested that temperature may have affected the respiration rate and/or prolonged the start of leaf fall, resulting in variations of radial growth through the consumption of photosynthetic products. On the other hand, different responses to climate were observed among species during the other phenological periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change scenarios have predicted a rise in temperature and more uneven distribution of precipitation, including more frequent droughts and extreme rain fall events (Bergant and Kajfež-Bogataj 2005; IPCC 2014), and these changes will inevitably affect tree growth in the world’s forests. This is especially important because forests are a key component of the carbon cycle; trees sequester large amounts of carbon (Roy et al. 2001), making their radial growth one of the major sources of long-term carbon fixation. Thus, it is important to understand the impact of climate change on the radial growth of trees. In Japan, forest covers 67% of the total land area; about half of the forests are natural, and 82% of the natural forests are broadleaf forests (Japan Forestry Agency 2012). Among Japanese forests, the cool temperate region is widely distributed from the northern to eastern part of the Japanese islands and in the subalpine zone of the main island (Numata et al. 1972). In these forests, Betula ermanii, Betula platyphylla, Quercus crispula and Fagus crenata are the dominant broadleaf trees (Horikawa 1972; Miyawaki 1985). Thus, increased knowledge of any changes in the radial growth of these trees in response to climate would be of great importance in forecasting the carbon sequestration.
The Dendrochronological technique is useful for evaluating the effects of climate on tree growth and for reconstructing climates of the past (e.g., Fritts 1976; Schweingruberm 1996; García-Suárez et al. 2009). Various studies have reported on the climate responses of ring width of deciduous hardwoods, such as Fagus, Quercus, and Betula species in Europe, North America and Central Asia (e.g., García-Suárez et al. 2009; Jansons et al. 2016). In Japan, several dendrochronological studies have been conducted on hardwood species. Two studies in the 1990s revealed that Fraxinus mandshurica var. japonica (Yasue et al. 1996), and Quercus dentata (D’Arrigo et al. 1997) were indicators of climate reconstruction. Hoshino et al. (2008) tested the synchronization of ring width variations and climate responses of F. crenata at various sites in Tohoku to confirm the applicability for cross-dating in archeological analyses. In other studies, climate responses of the ring widths of B. ermanii (Takahashi et al. 2003, 2005), F. crenata (Shen et al. 2018), Fagus japonica and Magnolia hypoleuca (Takahashi and Okuhara 2012) were analyzed to evaluate the growth-limiting factors of these species. In addition, the dendrochronological approach has been used to analyze climate responses of one species at a single site (e.g., Yasue et al. 1996; D’Arrigo et al. 1997), or one species growing at various sites (e.g., Hoshino et al. 2008; Shen et al. 2018). Takahashi et al. (2003, 2005) showed that the growth of B. ermanii responded differently to climatic factors at its upper and lower distribution limits in a subalpine forest on Mt. Norikura, central Japan. Our previous study investigated the ring width responses to climatic conditions at 13 sites of F. crenata in Japan, and revealed that some of the sites in Tohoku and Hokkaido exhibited common climatic responses, but not all the sites showed common responses (Shen et al. 2018). These reports highlight that the climate responses differ depending on the growing environment. Finally, there have also been studies on multiple species at a single site (e.g., Takahashi et al. 2003; Takahashi and Okuhara 2012). By comparing the climate responses of different tree species at the same site, it becomes possible to elucidate differential responses within the same growing environment, and thereby isolate the unique properties of individual species. Thus, the dendrochronological technique can provide important detailed information on the differential growth changes among tree species in the cool temperate forest.
The dendrochronological technique is also a useful tool for detecting growth-limiting environmental factors when analyzing relationships between tree-ring width indices and meteorological data. However, it is difficult to understand the physiological process by itself. To understand the factors affecting the tree-ring development process, it is necessary to identify both the wood formation phenology and the specific climatic signals that affect particular features in the wood (e.g., Fonti et al. 2007). Yasue et al. (1994) reported that growth-limiting climate factors differ among ring variables (ring width, earlywood width, latewood width ring density and maximum density) for Picea glehnii, and the difference can be attributed to the tree-ring development processes at different xylem phenology stages. Recently, Peng et al. (2019) found a significant correlation between canopy phenology observed using satellite images and the start of the stem growth season as estimated by a Vaganov–Shashkin (V–S) model adjusted by 1-year dendrometer observation in spring in Qinghai spruce (Picea crassifoloa). On the other hand, Čufar et al. (2015) found that there was no close relationship between the length of the vegetation growing season and tree-ring width in the European beech (Fagus sylvatica). Furthermore, a positive correlation between tree-ring width and moisture conditions (soil moisture content and precipitation) was observed in European beech during its leaf developing period (Kolář et al. 2016). Thus, the leaf phenology should have significant effects on radial growth. Despite the likelihood of correlation, however, there have been few studies on the relationship between leaf phenology and radial growth. Therefore, integrated analysis of the climate responses of tree-ring width, xylem and leaf phenology would improve our understanding of the mechanisms of radial growth response to changing climate (e.g., Čufar et al. 2008).
In statistical analysis of dendrochronology, the monthly climate data are usually used to evaluate the effects of climate on radial growth (e.g., Fritts 1976; Schweingruber 1988, 1996). However, the tree’s responses to climate factors do not always follow our monthly calendar. Vaganov et al. (1999), Kirdyanov et al. (2003), and Kujansuu et al. (2007a, b) calculated the correlations between ring widths and 5-day or 10-day average meteorological data and found significant responses to temperature on a short time scale for Larix gmelinii. Variable temporal width and moving correlations on daily climate data (e.g., Nechita and Chiriloaei 2018; Arvai et al. 2018) were employed and these analyses revealed that climate influences radial growth (Beck et al. 2013). Thus, moving correlations on daily climate data are effective for revealing the climate response at various periods, and this information would be helpful to understand the mechanism by which climate factors affect tree growth.
In the present study, we focused on diffuse-porous B. ermanii, F. crenata and ring-porous Q. crispula, which are major deciduous hardwood species in the cool temperate zone and subalpine zone in central Japan. Field observations and tree-ring analysis were performed for adult trees with canopy dominance. The objectives of the study were: (1) to clarify the phenology of radial growth; and (2) to identify the relationship between climate factors and radial growth by the dendrochronological technique using a moving average of metrological data. We also used local data on leaf phenology and soil water content to clarify how climate affects the growth of tree-ring width. Finally, we discussed the expected radial growth changes associated with the predicted climate changes.
Materials and methods
Study site
The study site is located in a cool-temperate deciduous broadleaf forest (36°08 N′, 137°25′ E, 1420 m a.s.l.) on the northwestern slope of Mt. Norikura of central Japan (Fig. 1). The sampling stands were adjacent to the TKY site, which is one of the core research sites of AsiaFlux under FLUXNET and is a site of JaLTER under ILTER. Long-term research into carbon cycling has been performed at the TKY site for more than a decade, using micrometeorological measurements, ecological process research and remote sensing (e.g., Saigusa et al. 2008; Muraoka and Koizumi 2009; Ohtsuka et al. 2009; Saitoh et al. 2012). The forest canopy is predominated by B. ermanii, Q. crispula, Betula platyphylla and F. crenata. The understory is dominated by an evergreen dwarf bamboo, Sasa senanensis. The site is covered by snow from December until April, and its peak depth ranges from 100 to 180 cm (Noda et al. 2015). The warmth index [WI, Kira (1949)] of the site is 54.9. WI was calculated by the difference in altitude from Takayama Meteorological Station with a temperature lapse rate of 0.568 °C/100 m (Ueno et al. 2013). The WI is in the middle of the distribution for F. crenata (45–85) according to Kira (1949), and for Q. crispula (40–80) according to Hoshino (1998). The altitude of the site is near the lower distribution limit for B. ermanii, which dominates between 1600 and 2500 m a.s.l. on the east slope of Mt. Norikura (Takahashi et al. 2003).
Tree-ring sampling and measurement
We investigated B. ermanii, F. crenata and Q. crispula. Twenty to thirty trees were chosen for each species. The tree-ring records of F. crenata have been reported by Shen et al. (2018). We extracted two increment cores of 5 mm in diameter from each sample tree at breast height. Increment cores were cut transversely into strips that were 1.6-mm thick with a twin-bladed saw. The strips were air-dried and subjected to X-ray analysis (Softex Co., Tokyo). The resultant radiographs were scanned at 2400 dpi on an EPSON GT-X970 (Epson, Nagano, Japan). Ring widths were obtained using the program WinDENDRO 2009b (Regent Instruments Inc., Quebec). The raw ring width series were cross-dated visually (Stokes and Smiley 1996) and confirmed statistically using the program COFECHA (Holmes 1983, 1994). The series were standardized by a cubic smoothing spline (Cook and Peters 1981) with a cutoff of 32 years. To remove the effects of autocorrelation, we transformed each of the standardized series to a residual series through pooled autoregressive (AR) modeling (Cook 1985). Residual chronologies for the species were developed by averaging the individual series by using a biweight robust mean (Mosteller and Tukey 1977). The chronology quality was estimated by means of the Expressed Population Signal (EPS; Briffa and Jones 1990), by calculating a 31-year moving window with a 1-year lag. EPS values > 0.85 were used as threshold representing the reliable part of the chronologies (Wigley et al. 1984). For development of chronologies, all statistical analyses were done using the dlpR program (Andrew 2008) in the R environment (version 3.3.3; R Development Core Team 2017).
Climate data and climate growth response analysis
To analyze the response of tree-ring width to climate, daily temperatures (mean, maximum, minimum temperature), precipitation and sunshine duration data from Takayama Meteorological Station over the 53 years from 1961 to 2013 were employed (Japan Meteorological Agency; https://www.jma.go.jp/jma/index.html) (36°09 N′, 137°15′ E, 560 m a.s.l.); the station was located 15 km from the study site. The daily variation in meteorological data between the Meteorological Station and the study site were nearly same (Nagai et al. 2013). To determine the correct period of climate response to tree-ring width, correlations were calculated between the residual chronologies and moving average of 31 days of meteorological data with a 1-day lag using the R software environment (version 3.3.3; R Development Core Team 2017). The analysis period was from 16 May of the previous year until 15 November of the current year.
Wood formation phenology
To investigate the seasonal changes in radial growth, we collected the intact tissue samples in the early stage of wood formation and conducted the wounding method in the subsequent stage. For each species, three dominant trees were chosen for the observation of stem tissue. The phenology was examined from 10 June 2014 to 1 October 2015 except in the winter months.
We collected tissue samples from three trees for each species at the early stage of wood formation. Block samples (15 × 15 mm) containing the phloem, cambium and xylem were taken from the breast height (1.3 m) with a chisel and knife. The samples were fixed in 3% glutaraldehyde solution. The fixed blocks were cut into small blocks (approx. 3 × 3 × 3 mm), dehydrated in a graded ethanol series and embedded in epoxy resin (Kudo et al. 2015). Transverse sections were cut at thickness of 2–3 µm on a rotary microtome with tungsten steel knives (RM2265; Leica, Germany). Sections were stained with 0.5% toluidine blue solution and examined by light microscopy (BX53; Olympus, Japan). A new cell wall that was thinner than the other walls of cambial cells was used to examine whether the beginning of cell division (i.e., growth beginning) occurred in spring (Fig. 2a, b).
The transverse images show stages of wood formation phenology. a No new thin cell walls (NCW) are visible, indicating that the cambium is in a dormant stage (B. ermanii on April 27). b NCW formed in cambial cells (arrow), indicating the beginning of cambial cell division (B. ermanii on May 11). The proportional radial cumulative growth for each wounding date was calculated by the wounding position (Rwi) on complete one ring width (RW). c A wound tissue formed in B. ermanii wounded on July 9, 2014, and collected on November 17, 2014. The broken line shows the assumed site of cambium at the date of wounding. The thick arrow indicates the position of the cutter-knife wound. c1 Magnified image of the rectangle in (c). Increase of radial cell files (triangles) is used as the mark of cambium at wounding (Kuroda 1986). d A Q. crispula sample wounded on September 10, 2015, and collected on October 15, 2015. Additional xylem (AX) (Seo et al. 2007) was laid down onto the completed tree ring due to the wound. e A B. ermanii sample wounded on August 27, 2015, and collected on October 15, 2015. There is only a crack at the wound site, indicating the cessation of cambium division
The wounding method (Wolter 1968; Yoshimura et al. 1981; Kuroda 1986; Kuroda and Kiyono 1997) was performed with a 2-week interval using an NT cutter knife, which enables wounding on hard bark. The incisions were spaced at 3-cm intervals laterally. After forming an annual ring, the wounded areas were harvested with a chisel for 1 tree for each species in October of 2014 and 2015, respectively. Thin radial sections of 15–25-µm thickness were cut by a sliding microtome. The sections were stained with 1% safranin solution and 0.5% fast green solution, and observed under an ordinary light microscope with a polarizing attachment. The position of the cambium at the time of wounding was determined by a sudden increase in the number of radial cell files (Kuroda 1986; Fig. 2c1). The proportional radial cumulative growth was calculated by the wounding position on a completed tree ring (Fig. 2c). The termination of cell division was classified into two cases. In the first case, additional xylem (AX) was laid down onto the completed tree ring due to the wound (Seo et al. 2007; Fig. 2d). The AX indicates termination of cell division; whereas cambial cells have the ability to react to injury by marking. In the second case, no AX occurred, indicating complete dormancy (Fig. 2e). We divided wood formation into four stages as follows: (1) before division of cambial cells; (2) division of cambial cells; (3) cessation of cambial division with AX due to wounding; and (4) cessation of cambium division. Leaf phenological stages were identified using the previously established categories (e.g., Fujimoto 2007; González-González et al. 2013; Kudo et al. 2015): (1) winter buds, (2) bud breaking, opening of buds with partly green color, (3) leaf expansion and appearance of small leaves. These phenological images were clarified by the camera images (FinePix S100FS; FUJIFILM, Tokyo) taken at the same timing of tissue sample collection.
We will discuss the cause of the climate responses, by combining our wood formation observations with the leaf phenology and moisture conditions data obtained at the field station located close to the study site. The images of leaf phenology of the dominant canopy species, which were Q. crispula, B. ermanii, and B. platyphylla, were obtained from the Phenological Eyes Network at the TKY site (http://www.pheno-eye.org, Nagai et al. 2018). Soil water content and precipitation data were from the Takayama field station of the River Basin Research Center, Gifu University (http://www.green.gifu-u.ac.jp/takayama), which is located about 300 m away from the study site.
Results and discussion
Phenology of wood formation
During initial phase, we confirm the division of cambial cells by the stem tissues of three trees for each species. We got at least one series of wood formation phenology for each species for both years (Fig. 3). We collected samples of the wounding method from two trees for each species, although some of the wounds were too large to identify the exact positions of the cambium and some blocks did not contain complete annual rings. We excluded such samples from the subsequent analysis.
The wood formation phenology. Cumulative proportional growth of xylem is measured by the wounding method. Beginning of cell division (white arrow) was confirmed in the intact tissue samples from three trees in the early stage of xylem formation. The end of radial growth (black arrow) was confirmed at the particular trees by the wounding method (for details see Fig. 2). Leaf expansion (black triangle) was visually observed in all the trees. B. e. indicates B. ermanii, F. c. indicates F. crenata, and Q. c. indicates Q. crispula
On 27 April 2015, divisions of cambial cells were observed in the stems of all three Q. crispula trees as shown by the white arrow in Fig. 3; whereas, no new cell wall was observed in any of the stem samples of either B. ermanii or F. crenata. No bud breaks were observed in any of the species at that time. On 11 May, many dividing cambial cells and enlarging vessel elements were observed on Q. crispula, and some new cell walls were observed on all of the B. ermanii and F. crenata trees. Leaf expansion of these three species was observed at the same time, as shown by the black triangles in Fig. 3. The onset of the cambial cell division of Q. crispula was about 2 weeks earlier than the others even though there was little difference in the bud breaks or leaf expansion among the species.
The end of radial growth was confirmed by AX laid down onto the completed tree ring (Fig. 2d) or cessation of cambium division (Fig. 2e) as shown by the black arrows in Fig. 3. The end of radial growth of B. ermanii was observed on 6 August 2014 having AX, and was observed on 27 August 2015 with cessation of cambium. The end of radial growth of F. crenata was observed on 8 September 2014 having AX, and was observed on 10 September 2015 with cessation of cambium division. The end of radial growth of Q. crispula was observed on 8 September 2014, and 10 September 2015 having AX. Over 2 years, the ends of radial growth were observed at the same month for the same species. The end of radial growth of B. ermanii occurred 2–4 weeks earlier than that of the other two species. We noted that cessations of cambium division (Fig. 2e) were observed within 2 weeks after observation of AX (Fig. 2d) or absence of AX for all species. Thus, the transition from cessation of the ability to respond to wounding to complete cessation occurred within 2 weeks.
The divisions of cambial cells for 3 trees were observed at the same time for each species. The previous observations on cambial onset of hardwood species based on a number of trees reported that differences among individuals were 15 days for Q. robur, 19 days for Q. pyrenaica with a 6–8-day sampling interval (Pérez-de-Lis et al. 2017); 0 day for Q. robur and Q. pyrenaica with a 2-week sampling interval (González-González et al. 2013); 0–10 days for F. orientalis with a 10-day sampling interval (Oladi et al. 2011). Our results with the 2-week sampling interval are consistent with previous reports and can be regarded as representative for the species at the site.
At the early stage of xylem and leaf phenology, the onset of cambial cell division for ring-porous Q. crispula was about 2 weeks earlier than those of diffuse-porous B. ermanii and F. crenata, and was even before its bud break (Fig. 3). Then, leaf expansion for all species occurred simultaneously. The onset of cambial cell division for B. ermanii and F. crenata was observed at the same timing of their leaf expansion. These results indicate that the timing of the beginning of cambial cell division in ring-porous Q. crispula was different from those in diffuse-porous B. ermanii and F. crenata, even in the same growth environment. Similar observations to our study were reported for the ring-porous species Q. serrata, in which cambial cell division was observed before bud break, and the deposition of secondary walls in earlywood vessels was observed at the onset of leaf expansion (Kudo et al. 2015). It has been reported that earlywood vessels in deciduous ring-porous hardwoods developed before bud break (Suzuki et al. 1996; Takahashi et al. 2013; Guada et al. 2019); whereas, first vessel of diffuse-porous species developed after leaf expansion (Suzuki et al. 1996; Takahashi et al. 2013). Kitin and Funada (2016) assumed that earlywood vessels in ring-porous species are formed in early spring before bud burst to supply sap to growing leaves and shoots. Our findings on both ring-porous and diffuse-porous species agreed with previous studies on the timing of wood formation and onset of leaf expansion.
As for the timing of the end of radial growth of B. ermanii, it was observed approximately 1 month earlier than for the other species in both years (Fig. 3). The results were based on only a few individuals of each species, although the cessations of radial growth of B. pendula and B. pubescens at a certain site were reported to occur within 2 weeks, respectively (Schmitt et al. 2004). The earlier cessation of radial growth of B. ermanii in each of the 2 years examined may suggest that this species exhibits a unique growth property.
Chronologies
The cores from each of the three tree species were successfully cross-dated and used to develop the master residual chronologies (Fig. 4). The span of the tree-ring width chronology of B.ermanii was 56 years, 104 years for F. crenata, and 80 years for Q. crispula (Table 1, Fig. 4). Mean correlations between trees calculated for all possible pairs of individuals in B.ermanii, F. crenata, and Q. crispula were 0.32, 0.40 and 0.34, respectively. The common period for chronologies of the three tree species whose EPS exceeded 0.85 was from 1957 to 2013. We analyzed the correlation between chronologies and meteorological data from 1962 to 2013 (56 years), the period for which the daily meteorological data records of Takayama were available.
The residual tree-ring width chronologies. Shaded areas show the number of trees in chronologies; thick dotted lines indicate EPS for the residual chronologies (the central value of the 31-year running windows). Thin dotted horizontal lines at EPS = 0.85 represent the reliability threshold presented by Wigley et al. (1984)
Climate responses of the radial growth
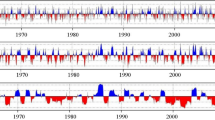
The correlation coefficient between moving averages of 31 days with a 1-day lag and the chronologies are shown in Figs. 5, 6 and 7. The values exceeded dotted lines indicate significant correlation (p < 0.05) for the central day of the 31-day moving average for the climate data used in the analysis. The periods which revealed significant (p < 0.05) correlation with ring widths are indicated in Fig. 8. The periods that showed significant correlations can be categorized into several stages associated with the phenology of wood formation (Fig. 8).
The climate responses of B. ermanii tree-ring width. The correlations between the residual chronology and 31-day moving average of maximum temperature, mean temperature, minimum temperature, precipitation and sunshine duration at 1-day intervals. Each value indicates the central day of the 31-day interval. The dotted lines represent the level of statistical significance (p = 0.05)
The climate responses of F. crenata tree-ring width. The correlations between the residual chronology and 31-day moving average of maximum temperature, mean temperature, minimum temperature, precipitation and sunshine duration at 1-day intervals. Each value indicates the central day of the 31-day interval. The dotted lines represent the level of statistical significance (p = 0.05)
The climate responses of Q. crispula tree-ring width. The correlations between the residual chronology and 31-day moving average of maximum temperature, mean temperature, minimum temperature, precipitation and sunshine duration at 1-day intervals. Each value indicates the central day of the 31-day interval. The dotted lines represent the level of statistical significance (p = 0.05)
The seasonal changes in the phenology of both leaves and xylem, and in the climate responses and moisture conditions at the study site in central Japan. The wood formation data were from observations of the 2014–2015 growing season (see Fig. 3). The climate response indicated the period of significant correlation between residual chronology and moving average climate variables (see Figs. 5, 6 and 7). The timing of the start of leaf expansion (SLE) and end of leaf fall (ELF) during 2004–2011 are from Nagai et al. (2013). Leaf phenology photos were taken at the TKY site at midday of each month over 2014–2015 and provided by the Phenological Eyes Network (http://www.pheno-eye.org). The average monthly soil water content and precipitation were from local data at the Takayama field station of the River Basin Research Center, Gifu University (http://www.green.gifu-u.ac.jp/takayama/)
During the period of division of cambial cells in the previous growing season the maximum temperature (19 June–21 July), mean temperature (18 June–19 July) and minimum temperature (17 June–19 July) were positively correlated with ring width of Q. crispula. There was no correlation between climate data and ring width of the other two species.
During the period just after the end of radial growth (12 August–11 September), sunshine duration was positively correlated with ring width of B. ermanii.
During the period from early October to early November of the previous year, which was when cessation of cambium division occurred, the ring width of all the species was negatively correlated with minimum temperature (B. ermanii: 9 October–10 November; F. crenata: 14 September–13 November; Q. crispula: 30 September–6 November) and mean temperature (B. ermanii: 8 October–18 November; F. crenata: 8 October–11 November; Q. crispula: 20 September–6 November). Furthermore, the ring width of F. crenata and Q. crispula was negatively correlated with precipitation (F. crenata: 20 August–13 November; Q. crispula: 4 October–11 November).
During the period from the beginning of March to the end of April, which was just before the division of cambial cells, precipitation was positively correlated with the ring width of F. crenata (1 March–30 April) and Q. crispula (29 February–4 May). The mean temperature was positively correlated with the ring width of F. crenata (16 March–20 April) and Q. crispula (26 March–26 May).
During the division of cambial cells in the current year, the ring width of B. ermanii was positively correlated with precipitation for both 29 April–12 June and 28 July–14 September. At the same time, ring width was negatively correlated with maximum temperature (22 July–4 September) and sunshine duration (15 July–4 September). For F. crenata, there was no significant correlation in the current growth period. For Q. crispula, ring width was positively correlated with precipitation (3 July–12 August). Ring width was positively correlated with sunshine duration (18 August–21 September), while the end of radial growth was observed (Fig. 8).
Our results showed that the temperature during the period of previous radial growth was positively correlated with the ring width of Q. crispula. Leaves were still green during this period (Fig. 8). These facts indicated that the temperature during the growth period of the previous years will affect the ring width of the following years in Q. crispula. A similar positive correlation was reported between the ring width of Q. crispula on Kunashir Island and maximum temperature of both the current and previous summer (Jacoby et al. 2004). Analysis of intra-annual δ13C of the tree rings formed after the labeling revealed that earlywood contained photoassimilate from the previous summer and autumn on the deciduous species Larix gmelinii (Kagawa et al. 2006). These findings suggest that the photosynthetic products of the previous growing period were used for radial growth of the following year for Q. crispula.
Sunshine duration during the period just after the end of radial growth was positively correlated with the ring width of B. ermanii (Fig. 8). The leaves were still green during this period (Fig. 8). Thus, the photosynthetic products after radial growth cessation were utilized for the following year’s growth and sunshine duration might affect photosynthetic production. Although there have been reports in which the climatic factors of the previous summer to autumn were significantly positively correlated with the ring widths of B. ermanii (e.g., Wang et al. 2013; Deck et al. 2017), the accurate wood formation periods are unknown.
For all the species, the minimum and mean temperature from late September to early November, while after the end of radial growth, was negatively correlated with ring width. This period coincides with the period from the beginning of leaf coloring to the end of leaf fall (Fig. 8). According to the observation of dark respiration and the photosynthetic rate of the leaves of B. ermanii and Q. crispula at the TKY site, the light-saturated photosynthetic rates (Amax), maximum carboxylation rate (Vcmax) and electron transport rate (Jmax) diminished when leaf coloring occurred, and the dark respiration was almost constant from summer to autumn (Noda et al. 2015). In another study at the adjacent site, the yearly variations in cumulative temperature lower than 18 °C (chilling degree days, CDD18) were significantly correlated with the timing of leaf fall, and the timing was delayed by the minimum and mean temperature rises (Nagai et al. 2013). By considering all these past findings and our present data at the TKY site, we suggest that the high temperature extends the period of yellow leaves and accelerates the respiration rate, thereby increasing the respiration that consumes the photosynthetic products used for the following year’s radial growth. Our previous research reported similar climatic responses for F. crenata at Hakkoda 2 (WI: 53.5) and Hachimantai 1 (WI: 55.7), where temperature conditions are the same as at Takayama (WI: 54.9), revealing negative correlations with the mean temperature of the previous October or November (Shen et al. 2018). Therefore, we suggest that the negative influence of temperature in autumn on the following year’s radial growth could occur for any deciduous species in a cool temperate forest in Japan.
Temperature and precipitation during the period just before the beginning of radial growth were positively correlated with the ring width of F. crenata and Q. crispula. This period was also just before leaf expansion (Fig. 8). The results indicated that the temperature and the precipitation before leaf expansion and cambial division increased the coming year’s ring width. We discussed the effects of temperature and precipitation on both leaf development and cambial activity. The start of leaf expansion (SLE) for canopy-dominated tree species (mainly consisting of Q. crispula and B. ermanii) in 2004 and 2011 was observed at day of year (DOY) 138.75 (approximately on May 18), and the standard deviation was 3.41 days (Nagai et al. 2013; Fig. 8). In the same study, the authors reported that a cumulative average temperature above 2 °C affected the timing of SLE (Nagai et al. 2013). Earlier leaf expansion would increase the vegetation period (e.g., Vitasse et al. 2009). Muraoka et al. (2010) examined the effects of canopy properties (Vcmax and leaf area index (LAI)) on gross primary production (GPP) at the Takayama site, and they found that changes in Vcmax during the leaf expansion period led to a remarkable variation in GPP. Our results and the previous observations suggest that temperature affected the leaf phenology which regulate photosynthesis (GPP), and consequently might positively affect the radial growth. In regard to cambial activity, studies on the effects of local heating on hardwood stems indicated that the start of cambial division was controlled by temperature (Begum et al. 2007; Kudo et al. 2014). Begum et al. (2008) found cambial reactivation occurs in stems of hybrid poplar (Populus sieboldii × P. grandidentata) when the maximum daily temperature exceeds 15 °C for 8–10 days under natural conditions. The timing of cambial reactivation can be predicted from the sum of maximum daily temperatures, in degrees, above a threshold value (Begum et al. 2018). Collectively, these findings indicate that temperature directly affects the division of cambium, and possibly prolongs the radial growth period. Therefore, high temperature during this period might have induced the start of the leaf and wood development, and possibly elongated the radial growth period and/or photosynthetic activity period.
The positive correlations between ring width of F. crenata and Q. crispula and precipitation in March and April were not attributable to water stress, since the highest soil water content was observed in March and April at the Takayama site (Fig. 8). March–April is the period of snow melt at the site (Noh et al. 2017). Similar responses were reported for F. crenata growing at four cooler sites in Japan (WI 53.5–60.9), revealing a positive correlation with precipitation in March or April of the current growing season, whereas 9 warmer sites (WI: 64.4–83.4) did not reveal a positive correlation between ring width of F. crenata and spring precipitation (Shen et al. 2018). These results highlight the importance of the influence of precipitation during snow-melt season on F. crenata and Q. crispula.
Precipitation in the early and late portions of the current growing period was positively correlated with the ring width of B. ermanii, whereas maximum temperature and sunshine duration during the late portion of the current growing period were also negatively correlated with ring width (Fig. 8). There was no correlation between climatic factors during the period of radial growth and ring width of F. crenata. Precipitation during the period from July to early August, the rainy season in Japan, was positively correlated with ring width of Q. crispula. Sunshine duration during the period from middle of August to middle of September was positively correlated with ring width of Q. crispula. These results indicate that climate responses to the current growing period differ among these three species, even in the same growth environment. The results for B. ermanii indicated that the ring width growth was limited by the water stress of August, the month when radial growth ends. There is a possibility that water stress affects the ending of cambial cell division. There was no limiting climate factor in the current growing season for F. crenata. Precipitation in the rainy season increased the ring width of Q. crispula. Sunshine duration during the period when the radial growth ends might affect the timing of the radial growth ending and consequently increase the ring width. Previous studies on B. ermanii have reported a positive correlation between summer temperatures and tree-ring widths at higher altitudes of their distribution (Takahashi et al. 2005; Wang et al. 2013; Deck et al. 2017). In the lower distribution limit of the eastern slope of Mt. Norikura (180 m higher than the current site), ring width of B. ermanii revealed positive correlation with precipitation and a negative correlation with the temperature of August (Takahashi et al. 2003). Our results on B. ermanii correspond to previous studies at the lower site. Therefore, water stress would be a main limiting factor of the radial growth of B. ermanii at the lower distribution limit and would affect the ending of radial growth. For F. crenata, dendrochronological studies at various sites in Japan reported that climate responses varied with local site conditions, but all of the sites showed no limitation of dryness in the current summer (Shen et al. 2018). Therefore, water stress would not be one of the main factors limiting the radial growth of F.crenata. For Q. crispula, the ring width of Q. crispula on Kunashir Island responded differently than in the present study—namely, it showed a positive correlation with the maximum summer temperature (Jacoby et al. 2004). However, there have been no other studies investigating the climate response of Q. crispula. Our results in Takayama and previous reports along environmental gradients highlight the difference in response to summer precipitation of these species. A study on the drought resistance of leaves among deciduous tree species based on the P–V curve classified B. ermanii as poor, F. crenata as intermediate, and Q. crispula as high (Maruyama 1996). The sensitive response of B. ermanii to precipitation at low altitudes can be attributed to the poor resistance. However, Q. crispula also responded to summer precipitation. These facts suggest that the drought resistance of leaves is not a main factor in the sensitivity of radial growth to water stress. The vessel organization, e.g., ring-porous or diffuse-porous, is also not a factor, since B. ermanii and F. crenata exhibited obvious differences in response to precipitation, despite the fact they are both diffuse-porous species. The cause of the differences in response to summer precipitation is still not clear. Further eco-physiological observations on the effect of water stress on photosynthesis and radial growth might clarify the causes of the difference in responses among these species.
Conclusion
In this study, we analyzed the responses of ring width to climate in three deciduous hardwood species growing in the same site in a cool temperature forest in Japan. We utilized moving correlation on averaged daily meteorological data to calculate the relationship between ring widths and climate. In addition, we observed radial growth phenology and included leaf phenology records from the Takayama site. Negative responses to mean and minimum temperature of previous autumn were revealed for the three species. We concluded that higher temperature may accelerate the respiration rate and/or prolong the timing of leaf fall, resulting in a decrease in consumption of photosynthetic products following the year’s radial growth. This study is, thus, the first to propose a mechanism to explain the negative effect of autumn temperature on the radial growth of deciduous hardwoods. A forecasted rising temperature in autumn can have a negative effect on most of the deciduous hardwood species in the cool temperate to subalpine region. The different species showed different responses to climate during the other phenology stages as well. Radial growth was limited by dryness during the growing season in B. ermanii, by mean temperature and precipitation just before the current growing season in F. crenata and by different climatic factors among various stages of phenology in Q. crispula. Under climate change scenarios, growth change might differ among species. The combination of dendrochronology, phenology of wood formation and phenology of leaves will enable us to determine the seasonal transition of growth stages and to discuss causal relationships between climate and radial growth. Further studies combining physiological and ecological observations would be helpful to clarify the mechanism of climate response, and to accurately evaluate the influence of climate change on tree radial growth.
Author contribution statement
Conception and design of study: YS, KY; Acquisition of data: YS, YH, KY, HM, TMS; Analysis: YS, EF, KY, YH; Drafting the manuscript: YS; Revising the manuscript critically for important intellectual content: KY, HM, TMS, EF, YH; Approval of the version of the manuscript to be published: KY, YS, HM, TMS, EF, YH.
References
Andrew GB (2008) A dendrochronology program library in R (dplR). Dendrochronologia 26:115–124. https://doi.org/10.1016/j.dendro.2008.01.002
Arvai M, Morgos A, Kern Z (2018) Growth-climate relations and the enhancement of drought signals in pedunculate oak (Quercus robur L.) tree-ring chronology in Eastern Hungary. Iforest 11:267–274. https://doi.org/10.3832/ifor2348-011
Beck W, Sanders TG, Pofahl U (2013) CLIMTREG: detecting temporal changes in climate–growth reactions—a computer program using intra-annual daily and yearly moving time intervals of variable width. Dendrochronologia 31:232–241. https://doi.org/10.1016/j.dendro.2013.02.003
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2007) Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Ann Bot 100:439–447. https://doi.org/10.1093/aob/mcm130
Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R (2008) Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiol 28:1813–1819. https://doi.org/10.1093/treephys/28.12.1813
Begum S, Kudo K, Rahman MH, Nakaba S, Yamagishi Y, Nabeshima E, Nugroho WD, Oribe Y, Kitin P, Jin HO, Funada R (2018) Climate change and the regulation of wood formation in trees by temperature. Trees 32:3–15. https://doi.org/10.1007/s00468-017-1587-6
Bergant K, Kajfež-Bogataj L (2005) N-PLS regression as empirical downscaling tool in climate change studies. Theor Appl Climatol 81:11–23. https://doi.org/10.1007/s00704-004-0083-2
Briffa K, Jones PD (1990) Basic chronology statistics and assessment. In: Cook ER, Kairiukstis LA (eds) Methods of dendrochronology. Kluwer Academic Publishers, Dordrecht, pp 137–152
Cook ER (1985) A time series analysis approach to tree ring standardization. Ph.D. dissertation, University of Arizona, Tucson
Cook ER, Peters K (1981) The smoothing spline: a new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree Ring Bull 41:45–54
Čufar K, Prislan P, De Luis M, Gričar J (2008) Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees 22:749–758. https://doi.org/10.1007/s00468-008-0235-6
Čufar K, De Luis M, Prislan P, Gričar J, Črepinšek Z, Merela M, Kajfež-Bogataj L (2015) Do variations in leaf phenology affect radial growth variations in Fagus sylvatica? Int J Biometeorol 59:1127–1132. https://doi.org/10.1007/s00484-014-0896-3
D’Arrigo RD, Yamaguchi DK, Wiles GC, Jacoby GC, Osawa A, Lawrence DM (1997) A kashiwa oak (Quercus dentata) tree-ring width chronology from northern coastal Hokkaido, Japan. Can J For Res 27:613–617. https://doi.org/10.1139/x96-220
Deck C, Wiles G, Frederick S, Matsovsky V et al (2017) Climate response of larch and birch forests across an elevational transect and hemisphere-wide comparisons, Kamchatka Peninsula, Russian Far East. Forests 8:315–326. https://doi.org/10.3390/f8090315
Fonti P, Solomonoff N, García-González I (2007) Earlywood vessels of Castanea sativa record temperature before their formation. New Phytol 173:562–570. https://doi.org/10.1111/j.1469-8137.2006.01945.x
Fritts HC (1976) Tree rings and climate. Academic Press, London
Fujimoto S (2007) Analysis of prediction methods for budburst days based on the phenological observation in 29 broad-leaved tree species for 10 years. J Jpn For Soc 89:253–261. https://doi.org/10.4005/jjfs.89.253
García-Suárez AM, Butler CJ, Baillie MGL (2009) Climate signal in tree-ring chronologies in a temperate climate: a multi-species approach. Dendrochronologia 27:183–198. https://doi.org/10.1016/j.dendro.2009.05.003
González-González BD, García-González I, Vázquez-Ruiz RA (2013) Comparative cambial dynamics and phenology of Quercus robur L. and Q. pyrenaica Willd. In an Atlantic forest of the northwestern Iberian Peninsula. Trees 27:1571–1585. https://doi.org/10.1007/s00468-013-0905-x
Guada G, Vázquez-Ruiz RA, García-González I (2019) Response patterns of xylem and leaf phenology to temperature at the southwestern distribution boundary of Quercus robur: a multi-spatial study. Agric For Meteorol 269:46–56. https://doi.org/10.1016/j.agrformet.2019.02.001
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull 43:69–78
Holmes RL (1994) Dendrochronology program library version 1994. Laboratory of Tree-Ring Research, University of Arizona, Tucson https://www.ltrr.arizona.edu/software.html. Accessed 24 Oct 2019
Horikawa Y (1972) Fagus crenata Blume. In: Atlas of the Japanese flora, an introduction to plant sociology of East Asia. Gakken, Tokyo, 37p
Hoshino Y (1998) Phytosociological studies of Quercus mongolica var. grosseserrata forest in Japan. Bull Fac Agric Tokyo Univ Agric Technol 32:1–99
Hoshino Y, Yonenobu H, Yasue K, Nobori Y, Mitsutani T (2008) On the radial-growth variations of Japanese beech (Fagus crenata) on the northernmost part of Honshu Island, Japan. J Wood Sci 54:183–188. https://doi.org/10.1007/s10086-007-0935-3
IPCC (2014) AR5 synthesis report: climate change 2014. https://www.ipcc.ch/report/ar5/wg2/. Accessed 1 June 2019
Jacoby G, Solomina O, Frank D, Eremenko N, D’Arrigo R (2004) Kunashir (Kuriles) oak 400——year reconstruction of temperature and relation to the Pacific Decadal Oscillation. Palaeogeogr Palaeocl 209:303–311. https://doi.org/10.1016/j.palaeo.2004.02.015
Jansons Ā, Matisons R, Šēnhofa S, Katrevičs J, Jansons J (2016) High-frequency variation of tree-ring width of some native and alien tree species in Latvia during the period 1965–2009. Dendrochronologia 40:151–158. https://doi.org/10.1016/j.dendro.2016.10.003
Japan Forestry Agency (2012) http://www.rinya.maff.go.jp/. Accessed 1 June 2019
Kagawa A, Sugimoto A, Maximov TC (2006) Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytol 171:793–804. https://doi.org/10.1111/j.1469-8137.2006.01780.x
Kira T (1949) Ringyo Kaisetsu series, 17. Nippon Ringyo Gijutsu Kyokai, Tokyo
Kirdyanov A, Hughes M, Vaganov E, Schweingruber F, Silkin P (2003) The importance of early summer temperature and date of snow melt for tree growth in the Siberian Subarctic. Trees 17:61–69. https://doi.org/10.1007/s00468-002-0209-z
Kitin P, Funada R (2016) Earlywood vessels in ring-porous trees become functional for water transport after bud burst and before the maturation of the current-year leaves. IAWA J 37:315–331. https://doi.org/10.1163/22941932-20160136
Kolář T, Giagli K, Trnka M, Bednářová E, Vavrčík H, Rybníček M (2016) Response of the leaf phenology and tree-ring width of European beech to climate variability. Silva Fenn 50:1520. https://doi.org/10.14214/sf.1520
Kudo K, Nabeshima E, Begum S, Yamagishi Y et al (2014) The effects of localized heating and disbudding on cambial reactivation and formation of earlywood vessels in seedlings of the deciduous ring-porous hardwood, Quercus serrata. Ann Bot 113:1021–1027. https://doi.org/10.1093/aob/mcu026
Kudo K, Yasue K, Hosoo Y, Funada R (2015) Relationship between formation of earlywood vessels and leaf phenology in two ring-porous hardwoods, Quercus serrata and Robinia pseudoacacia, in early spring. J Wood Sci 61:455–464. https://doi.org/10.1007/s10086-015-1487-6
Kujansuu J, Yasue K, Koike T, Abaimov AP et al (2007a) Climatic responses of tree-ring widths of Larix gmelinii on contrasting north-facing and south-facing slopes in central Siberia. J Wood Sci 53:87–93. https://doi.org/10.1007/s10086-006-0837-9
Kujansuu J, Yasue K, Koike T, Abaimov AP et al (2007b) Responses of ring widths and maximum densities of Larix gmelinii to climate on contrasting north-and south-facing slopes in central Siberia. Ecol Res 22:582–592. https://doi.org/10.1007/s11284-006-0062-4
Kuroda K (1986) Wound effects on cytodifferentiation in the secondary xylem of woody plants. Wood Res 72:67–118
Kuroda K, Kiyono Y (1997) Seasonal rhythms of xylem growth measured by the wounding method and with a band-dendrometer: an instance of Chamaecyparis obtusa. IAWA J 18:291–299. https://doi.org/10.1163/22941932-90001493
Maruyama Y (1996) Water content characteristics of major tree species produced in northern areas. Hoppo Ringyo 48:245–248
Miyawaki A (1985) Vegetation of Japan 6 Chubu. Shibundo, Tokyo (in Japanese)
Mosteller F, Tukey JW (1977) Data analysis and regression: a second course in statistics. Addison-Wesley series in Behavioral science, quantitative methods. Addison-Wesley, Reading
Muraoka H, Koizumi H (2009) Satellite Ecology (SATECO)—linking ecology, remote sensing and micrometeorology, from plot to regional scale, for the study of ecosystem structure and function. J Plant Res 122:3–20. https://doi.org/10.1007/s10265-008-0188-2
Muraoka H, Saigusa N, Nasahara KN, Noda H et al (2010) Effects of seasonal and interannual variations in leaf photosynthesis and canopy leaf area index on gross primary production of a cool-temperate deciduous broadleaf forest in Takayama, Japan. J Plant Res 123:563–576. https://doi.org/10.1007/s10265-009-0270-4
Nagai S, Saitoh TM, Kurumado K, Tamagawa I et al (2013) Detection of bio-meteorological year-to-year variation by using digital canopy surface images of a deciduous broad-leaved forest. Sola 9:106–110
Nagai S, Akitsu T, Saitoh TM, Busey RC et al (2018) 8 million phenological and sky images from 29 ecosystems from the Arctic to the tropics: the Phenological Eyes Network. Ecol Res 33:1091–1092. https://doi.org/10.1007/s11284-018-1633-x
Nechita C, Chiriloaei F (2018) Interpreting the effect of regional climate fluctuations on Quercus robur L. trees under a temperate continental climate (southern Romania). Dendrobiology 79:77–89. https://doi.org/10.12657/denbio.079.007
Noda HM, Muraoka H, Nasahara KN, Saigusa N, Murayama S, Koizumi H (2015) Phenology of leaf morphological, photosynthetic, and nitrogen use characteristics of canopy trees in a cool-temperate deciduous broadleaf forest at Takayama, central Japan. Ecol Res 30:247–266. https://doi.org/10.1007/s11284-014-1222-6
Noh NJ, Kuribayashi M, Saitoh TM, Muraoka H (2017) Different responses of soil, heterotrophic and autotrophic respirations to a 4-year soil warming experiment in a cool-temperate deciduous broadleaved forest in central Japan. Agric For Meteorol 247:560–570. https://doi.org/10.1016/j.agrformet.2017.09.002
Numata M, Miyawaki A, Itow D (1972) Natural and semi-natural vegetation in Japan. Blumea 20:435–496
Ohtsuka T, Saigusa N, Koizumi H (2009) On linking multiyear biometric measurements of tree growth with eddy covariance-based net ecosystem production. Glob Change Biol 15:1015–1024. https://doi.org/10.1111/j.1365-2486.2008.01800.x
Oladi R, Pourtahmasi K, Eckstein D, Bräuning A (2011) Seasonal dynamics of wood formation in Oriental beech (Fagus orientalis Lipsky) along an altitudinal gradient in the Hyrcanian forest, Iran. Trees 25:425–433. https://doi.org/10.1007/s00468-010-0517-7
Peng X, Du J, Yang B, Xiao S, Li G (2019) Elevation-influenced variation in canopy and stem phenology of Qinghai spruce, central Qilian Mountains, northeastern Tibetan Plateau. Trees 33:707–717. https://doi.org/10.1007/s00468-019-01810-z
Pérez-de-Lis G, Olano JM, Rozas V, Rossi S, Vázquez-Ruiz RA, García-González I (2017) Environmental conditions and vascular cambium regulate carbon allocation to xylem growth in deciduous oaks. Func Ecol 31:592–603. https://doi.org/10.1111/1365-2435.12789
R Development Core Team (2017) R: a language and environment for statistical computing. Version 3.3.3, R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. Accessed 28 July 2017
Roy J, Mooney HA, Saugier B (eds) (2001) Terrestrial global productivity. Academic, San Diego
Saigusa N, Yamamoto S, Hirata R, Ohtani Y et al (2008) Temporal and spatial variations in the seasonal patterns of CO2 flux in boreal, temperate, and tropical forests in East Asia. Agric For Meteorol 148:700–713. https://doi.org/10.1016/j.agrformet.2007.12.006
Saitoh TM, Nagai S, Saigusa N, Kobayashi H, Suzuki R, Nasahara KN, Muraoka H (2012) Assessing the use of camera-based indices for characterizing canopy phenology in relation to gross primary production in a deciduous broad-leaved and an evergreen coniferous forest in Japan. Ecol Inform 11:45–54. https://doi.org/10.1016/j.ecoinf.2012.05.001
Schmitt U, Jalkanen R, Eckstein D (2004) Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn 38:167–178. https://doi.org/10.14214/sf.426
Schweingruber FH (1988) Tree rings: basics and applications of dendrochronology. Kluwer Acad, Norwell, Mass
Schweingruberm FH (1996) Tree rings and environment. Dendroecology. Birmensdorf, Swiss Federal Institute for Forest, Snow and Landscape Research, Haupt, Berne
Seo JW, Eckstein D, Schmitt U (2007) The pinning method: from pinning to data preparation. Dendrochronologia 25:79–86. https://doi.org/10.1016/j.dendro.2007.04.001
Shen Y, Wakui S, Takehara Y, Hoshino Y, Utsumi Y, Kamata N, Nobori Y, Ichie T, Muraoka H, Saitoh T, Hirano Y, Yasue K (2018) Effects of climate on the radial growth of Japanese beech (Fagus crenata) at various sites in Japan. Mokuzai Gakkaishi 64:171–186. https://doi.org/10.2488/jwrs.64.171
Stokes MA, Smiley TL (1996) An introduction to tree-ring dating. University of Arizona Press, Tucson
Suzuki M, Yoda K, Suzuki H (1996) Phenological comparison of the onset of vessel formation between ring-porous and diffuse-porous deciduous trees in a Japanese temperate forest. IAWA J 17:431–444
Takahashi K, Okuhara I (2012) Comparison of climatic effects on radial growth of evergreen broad-leaved trees at their northern distribution limit and co-dominating deciduous broad-leaved trees and evergreen conifers. Ecol Res 27:125–132. https://doi.org/10.1007/s11284-011-0879-3
Takahashi K, Azuma H, Yasue K (2003) Effects of climate on the radial growth of tree species in the upper and lower distribution limits of an altitudinal ecotone on Mount Norikura, central Japan. Ecol Res 18:549–558. https://doi.org/10.1046/j.1440-1703.2003.00577.x
Takahashi K, Tokumitsu Y, Yasue K (2005) Climatic factors affecting the tree-ring width of Betula ermanii at the timberline on Mount Norikura, central Japan. Ecol Res 20:445–451. https://doi.org/10.1007/s11284-005-0060-y
Takahashi S, Okada N, Nobuchi T (2013) Relationship between the timing of vessel formation and leaf phenology in ten ring-porous and diffuse-porous deciduous tree species. Ecol Res 28:615–624. https://doi.org/10.1007/s11284-013-1053-x
Ueno K, Isono J, Imaizumi F, Inami A, Kanai R, Suzuki K, Kobayashi H, Tamagawa I, Saitoh T, Kondo H (2013) Data archive of meteorological data created through the Japanese Alps inter-university cooperative project. J Geo-CHIGAKU ZASSHI 122:638–650. https://doi.org/10.5026/jgeography.122.638
Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP (1999) Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400:149–151. https://doi.org/10.1038/22087
Vitasse Y, Delzon S, Dufrêne E, Pontailler JY, Louvet JM, Kremer A, Michalet R (2009) Leaf phenology sensitivity to temperature in European trees: do within-species populations exhibit similar responses? Agric For Meteorol 149:735–744. https://doi.org/10.1016/j.agrformet.2008.10.019
Wang X, Zhao X, Gao L (2013) Climatic response of Betula ermanii along an altitudinal gradient in the northern slope of Changbai Mountain, China. Dendrobiology 70:99–107. https://doi.org/10.12657/denbio.070.011
Wigley TML, Briffa KR, Jones PD (1984) On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteorol 23:201–213. https://doi.org/10.1175/1520-0450(1984)023%3c0201:OTAVOC%3e2.0.CO;2
Wolter KE (1968) A new method for marking xylem growth. For Sci 14:102–104
Yasue K, Funada R, Noda M, Fukazawa K (1994) Dendroclimatological study of Picea glehnii growing in the Teshio Experimental Fo@rest of Hokkaido University. Research Bulletins of the College Experiment Forests Hokkaido University
Yasue K, Funada R, Kondo T, Kobayashi O, Fukazawa K (1996) The effect of climatic factors on the radial growth of Japanese ash in northern Hokkaido, Japan. Can J For Res 26:2052–2055. https://doi.org/10.1139/x26-231
Yoshimura K, Itoh T, Shimaji K (1981) Studies on the improvement of the pinning method for marking xylem growth. II. Pursuit of the time sequence of abnormal tissue formation in loblolly pine. Mokuzai Gakkaishi 27:755–760
Acknowledgements
We thank Dr. Yoshitake and Prof. Tamagawa at River Basin Research Center, Gifu University for their support of the field investigations and provision of meteorological data (http://www.green.gifu-u.ac.jp/takayama/), and also Dr. Nagai at JAMSTEC and the PEN project (http://www.pheno-eye.org) for the canopy photographs. We also thank Dr. H. Kobayashi at Shinshu University for helpful comments and support with the knife marking analysis. We thank Dr. Fujiwara, Dr. Yamashita, and Dr. Kuroda at FFPRI for their help with the X-ray analysis. Part of this study was supported by MEXT/JSPS KAKENHI Grant Number JP 23380097 and by a Joint Usage/Research Grant from the River Basin Research Center 2016-G007, 2017-G008 and 2018-G002, Gifu University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Vospernik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, Y., Fukatsu, E., Muraoka, H. et al. Climate responses of ring widths and radial growth phenology of Betula ermanii, Fagus crenata and Quercus crispula in a cool temperate forest in central Japan. Trees 34, 679–692 (2020). https://doi.org/10.1007/s00468-019-01948-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-019-01948-w