Abstract
Key message
Stomatal regulation involves beneficial effects of pruning mulch and irrigation on leaf photosynthesis in Prunus yedoensis and Ginkgo biloba under moderate drought. G. biloba showed conservative water use under drought.
Abstract
Leaf photosynthesis is highly sensitive to soil water stress via stomatal and/or biochemical responses, which markedly suppress the growth of landscape trees. Effective irrigation management to maintain leaf photosynthesis and information on species-specific photosynthetic responses to soil water stress are essential for the sustainable management of landscape trees in Japan, in which summer drought often occurs. In order to investigate effective irrigation management, we used plants with moderate soil water stress as controls, and examined the effects of daily irrigation and pruning mulch on leaf photosynthesis in container-grown Ginkgo biloba and Prunus yedoensis, which are the first and second main tall roadside trees in Japan. Stomatal conductance was significantly increased by pruning mulch and daily irrigation, with similar increases in leaf photosynthesis being observed in P. yedoensis and G. biloba. In order to obtain information on species-specific photosynthetic responses to soil water stress, we compared the responses of leaf photosynthesis and leaf water status to reductions in soil water content (SWC) between the two species. G. biloba maintained a constant leaf water potential, leaf water content, maximum carboxylation rate, and electron transport rate with reductions in SWC, whereas reductions were observed in P. yedoensis. We concluded that pruning mulch and irrigation effectively offset the negative impact of moderate water stress on leaf photosynthesis in summer in P. yedoensis and G. biloba via stomatal regulation, and also that G. biloba maintained its photosynthetic biochemistry and leaf water status better than P. yedoensis under severe water stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban landscapes create different growth conditions from the surrounding rural and natural environments. A limited water supply due to a small volume of soil available and poor infiltration by paved roads, combined with low nutrient levels, the extremes of temperature, and high irradiance have been shown to markedly affect the growth of isolated urban trees in parking lots and along streets (Vico et al. 2014). Among the various environmental factors in urban landscapes, soil water stress has been identified as the main factor reducing growth and/or leading to the failure of transplanted urban trees (Kaushal and Aussenac 1989; Fini et al. 2009). Soil water stress is one of the most important factors negatively impacting leaf photosynthetic performance, which is an essential factor for determining tree growth, through stomatal regulation and/or photosynthetic biochemistry (e.g., Lambers et al. 2008; Chaves et al. 2009).
Although the climate in Japan is classified as humid subtropical in the summer, summer drought often occurs when the absence of precipitation is prolonged. Summer drought has become more serious due to the effects of urban and global warming; average air temperature and atmospheric relative humidity in Kyoto in August increased by 0.23 °C and decreased by 1.6 % per decade, respectively, between 1951 and 2012 (Japan Meteorological Agency); this rise in temperature was markedly larger than that of the global average in the same period (0.12 °C per decade between 1951 and 2012, IPCC 2014). Higher atmospheric temperatures and lower soil water content were reported in urban areas in Kyoto city than in rural areas in the summer (Kagotani et al. 2013). Summer drought has been shown to cause severe damage to landscape trees in Japan (Iijima et al. 1993; Ikeda and Hashimoto 1996). Osone et al. (2014) reported declines in leaf photosynthesis and stomatal conductance in urban trees in hot-dry summers than in average summers in Tokyo, Japan. Such declines may prevent landscape trees from playing important social, economic, and environmental roles including urban heat island mitigation and reducing energy use (Vico et al. 2014).
Supplemental irrigation management has been suggested to ameliorate the negative impact of soil water stress on landscape trees in summer (Fain et al. 1999; Vico et al. 2014). However, a smaller irrigation interval and amount of water are essential for the effective and sustainable management of landscape trees. One possible and promising management is mulching by organic materials; organic mulching is considered to be environmentally friendly and has been shown to have positive effects on plant growth and physiology (e.g., reviewed by Ferrini and Fini 2011), with increases in soil water content and/or decreases in evaporation by organic mulch (pine wood chip, pine bark) being reported for urban trees (Iles and Dosman 1999; Montague et al. 2000; Montague and Fox 2008). In this context, using pruning from landscape trees as mulch materials, as described previously by Youkhana and Idol (2009), may be particularly beneficial because it reduces waste materials and CO2 emission with their incineration and also increases soil water content. However, the effects of pruning mulch on tree photosynthetic performance by Japanese landscape trees have not yet been examined.
Information on the species-specific responses of urban trees to water stress is important for effective irrigation management for urban trees. Physiologically, it is widely recognized that the photosynthetic response and its underlying mechanisms to water stress markedly vary between species (Warren 2008; Medrano et al. 2009; Tomás et al. 2014). Nevertheless, information on urban tree physiological performance to water stress is limited because trees in urban landscapes are mainly selected for their esthetic qualities (Percival and Sheriffs 2002), which intensified after the 1980s in Japan (Kurihara et al. 2014). To the best of our knowledge, only a few studies have compared the species-specific physiological responses of urban trees to water stress in Japan; Osone et al. (2014) recently reported that Ginkgo biloba was less sensitive to summer drought under urban street conditions than Prunus yedoensis and Zelkova serrata. Environmental conditions in the field vary between study sites and species, and have led to difficulties in comparing species-specific responses; therefore, studies performed under controlled conditions will provide valuable information on the species-specific physiological responses of urban trees to drought. Comparisons and evaluations of the effects of soil water content on photosynthetic performance between landscape tree species may have a significant impact by improving leaf photosynthetic function and, thus, contributing to the more effective management of landscape trees.
The aims of the study were to test the hypotheses that
-
1.
The soil water content and leaf photosynthetic performance of landscape trees are increased by pruning mulch and irrigation under moderate soil water stress, and
-
2.
Photosynthetic responses and the leaf water status to soil water stress differ between tree species in a manner that depends on stomatal and/or biochemical responses.
We selected two landscape tree species, Ginkgo biloba (maiden hair tree) and Prunus yedoensis (Yoshino cherry). In 2009, 6.75 × 106 tall trees and 1.40 × 108 shrubs were planted as roadside trees in Japan, with G. biloba and P. yedoensis being the first (5.70 × 105, 8.4 %) and second (5.22 × 105, 7.7 %) main tall tree species (Kurihara et al. 2014). G. biloba is considered to be a good species for street planting (Handa et al. 1997; Bassuk et al. 2009) because it grows in a wide range of soil moisture from wet to very dry soil (Bassuk et al. 2009). On the other hand, urban Prunus trees are declining in Japan, partly due to inappropriate management including the cutting of roots (Satomura et al. 2005). We performed two experiments on container-grown G. biloba and P. yedoensis. In the first experiment, the effects of pruning mulch and daily irrigation on leaf photosynthesis were investigated from the rainy season to subsequent hot summer; the rainy season and summer season are from early June to late July and late July to late September, respectively, in a typical year in Kyoto city (Japan Meteorological Agency). In the second experiment, photosynthetic performance and the plant water status in relation to soil water content were monitored with reductions in soil water content in summer under controlled conditions.
Materials and methods
Site description and tree seedlings
This study was conducted in a glasshouse in Kyoto Institute of Technology (35°01′N, 135°41′E, 50 m of altitude). The climate of Kyoto city is warm temperate, with annual precipitation of 1490 mm and an annual mean temperature of 15.9 °C (Japan Meteorological Agency). Rainy season accounts for 29.5 % of annual rainfall, lasting from early June to late July, and is followed by a hot and clear summer season with daytime maximum temperatures regularly exceeding 30 °C, lasting to late September.
This study focused on two species of the deciduous broadleaved tall tree Ginkgo biloba L. and Prunus × yedoensis Matsum. (Figure 1a, b), the leaves of which begin to expand in April and fall in November. Stomatal density was higher in P. yedoensis than in G. biloba (230 and 110 stomata mm−2 for mature sun leaves, Fig. 1c, d).
Images of fully expanded mature leaves in June for G. biloba (a) and P. yedoensis (b), and microscopic images of stomata at the lower side of the leaves for G. biloba (c) and P. yedoensis (d). Arrows indicates stomata in G. biloba. Light photographs were obtained for the secondary replica using a digital microscope (VHX-1000, Keyence, Osaka, Japan)
Seedlings of the trees were grown under natural sunlight with a 50 % shading cloth in a glasshouse (7 m × 5 m × 5 m) in Kyoto Institute of Technology. In 2009, 150-cm tall, 3-year-old P. yedoensis seedlings (Nihon Kaki, Saitama, Japan) and 80-cm tall, 4-year-old G. biloba seedlings (Archinet, Tokyo, Japan) were obtained commercially. These seedlings were transplanted into 12-l Wagner pots with a diameter of 25.2 cm and depth of 30 cm (AlphaPurchase, Tokyo, Japan), filled with loamy soil:sand:muck soil = 2:2:1. Plants were irrigated daily and fertilized once a week with 500 mL of 1/500 nutrient solution (N:P:K = 6:10:5, Hyponex, USA) per container until the treatments were started. We selected nine healthy seedlings for each species, and pruned them in May 2010 so that they had similar numbers of leaves.
Irrigation and mulch treatments
Pruning mulch was made in 2009 from pruning of the G. biloba and P. yedoensis trees, which were planted on the campus of the Kyoto Institute of Technology. Pruned materials were dried for approximately 1 week and shredded into chips of approximately 2 cm in length using a chipper (Chipper & Shredder CSE 220-DC, Shindaiwa, Hiroshima, Japan), and their properties have been summarized in Table 1.
The effects of pruning mulch on evaporation from soil were tested in the glasshouse, using pots containing the same soil with the same thickness (5 cm) of pruning mulch as those used for plant growth. All pots were well-watered with 1.2 l of water on the first day of the experiment, and evaporation from soil was then estimated from weight lost from the pots for 4 days.
The pruning mulch and irrigation treatments were performed between June 19 and September 11, 2010 in the glasshouse with a 50 % shading cloth. Measurements were performed on the first day of the experiment (June 19) after all of the plants were well-watered. The irrigation and mulch treatments were started thereafter. We set the treatment of “irrigated once every 5 days with no mulch” as the control because Kyoto government irrigates roadside trees after 5 days without precipitation during the summer season. We compared the effects of the two treatments: (1) irrigated daily with 1.2 l of water with no mulch (well-watered) and (2) irrigated with 1.2 l of water once every 5 days with mulch (mulch) to control plants, for three plants for each treatment (n = 3). The thickness of the pruning mulch was 5 cm, which was previously determined to have no effect on soil oxygen levels (Hanslin et al. 2005). Atmospheric temperature, vapor pressure deficit (VPD), and PPFD in the glasshouse were monitored by a data logger (U23-002 and UA-002-64, Onset Computer Corporation, MA, USA) that was set 2 m above the floor, from which data were collected at 30-min intervals. The soil water content (SWC, g g−1) was measured 3 weeks (rainy season) and 9 weeks (summer season) after the onset of the treatments, and was determined by weighing the pots daily from the second to fifth days after irrigation using the following equation: SWC = remaining water/maximum amount of water, where remaining water = maximum amount of water — water loss. The maximum amount of water available per container (SWC of 1.0 g g−1) was obtained by subtracting the pot weight at the wilting point from that at field capacity for three sample plants of G. biloba and P. yedoensis after finishing the treatments, as described previously by Medrano et al. (2009). In the present study, SWC = 0 means the wilting point.
Leaf photosynthesis
The light-saturated CO2 assimilation rate (A sat) and stomatal conductance (g s) were measured using a Li-6400 photosynthesis system (Li-Cor, NE, USA). In 2010, the effects of irrigation and mulch on leaf photosynthesis were investigated by measuring photosynthetic parameters between June and September every 3 weeks (0, 3, 6, 9, and 12 weeks) for two attached fully expanded mature leaves from three plants 3 days after irrigation (n = 6). Photosynthesis chamber conditions were set as follows: light provided from red–blue LED, 1500 µmol m−2 s−1 PPFD, leaf temperature, 25.0 °C, VPD, 1.1 kPa, and 380 µmol mol−1 of CO2 supplied by a CO2 mixer, considering that leaf photosynthesis by G. biloba and P. yedoensis was light-saturated at 1500 µmol m−2 s−1 PPFD, and the mean daily atmospheric temperature and VPD between June and September in an average year in Kyoto city are 25.5 °C and 1.1 kPa, respectively, with the daytime (6:00–18:00) atmospheric temperature being 26.6 °C (monthly values varied from 24.0 to 29.2 °C, Japan Meteorological Agency). The lowest atmospheric CO2 concentration in the daytime was approximately 370 µmol mol−1 between June and September in Kyoto Institute of Technology in 1998 (Kawamukai et al. 2000), and was approximately 390 µmol mol−1 in 2009 in Nagoya city, Japan (Wada et al. 2011).
In summer (August) in Kyoto city, the maximum daytime temperature (33.3 °C) and VPD (3.7 kPa) are markedly higher than the average values of 25.5 °C and 1.1 kPa (Japan Meteorological Agency). The sensitivity of photosynthesis to this high temperature/VPD is an important trait for the acclimation of plants to the summer climate, and may be affected by the mulch or irrigation treatment. After 6 weeks of the treatments (the summer season), leaf photosynthesis was measured first at a leaf temperature of 25.0 °C and VPD of 1.1 kPa. Thereafter, they were increased to 35.0 °C and 3.1 kPa, and leaf photosynthesis was measured again after 30 min of acclimation. The chamber conditions were set at a light-saturated condition, 1500 µmol m−2 s−1 PPFD of light, and 380 µmol mol−1 of CO2.
Leaf properties
Leaf area, leaf dry mass per leaf area, and the leaf carbon isotope discrimination (Δ) were measured after 9 weeks of the treatments for the leaves from which photosynthesis measurements were performed. Leaf area was measured using a scanner (Canoscan 9950F, Canon, Tokyo, Japan) and image analysis software (Image-J, National Institute of Health, MD, USA). These leaves were then dried at 60 °C for 48 h using an oven (MOV-112, SANYO Electric, Osaka, Japan), weighed to determine the dry mass, and then stored at room temperature in order to analyze the stable carbon isotope ratio (δ13Cleaf). Leaf Δ is a good indicator of long-term leaf water-use efficiency, which was determined as described by Kagotani et al. (2013). Δ was calculated as δ13Cair − δ13Cleaf, where δ13Cair is the stable carbon isotope ratio of atmospheric CO2 measured using an isotope mass spectrometer (Finnigan MAT 252, Bremen, Germany). In the δ13Cair analysis, 2 l of air was collected at heights of 2 m from the floor of the glasshouse in the daytime, repeated at least three times on different dates. δ13Cleaf was measured using the combined system of an elemental analyzer (EA1108, Carlo-Erba, Italy), interface (Finnigan MAT conflo II, Bremen, Germany), and isotope mass spectrometer (Finnigan MAT Delta S, Bremen, Germany) corrected with standards (Tayasu et al. 2011).
Effects of severe soil water stress on leaf water status and leaf photosynthesis
We conducted the second experiment in 2014 in order to compare the leaf water status and photosynthesis of G. biloba and P. yedoensis in response to severe soil water stress. The same container-grown seedlings used in 2010 (n = 3) were pruned to have similar numbers of leaves. We removed small branches to reduce leaf numbers, and removed twigs from the perimeter of the canopy using reduction cuts in order to achieve similar tree heights. They were grown in the glasshouse following the experiments in 2010 without mulch materials. All plants were well-watered on 4 June, and irrigation was then stopped between 5 June and 7 July. Soil water content was monitored by weighing the pots as described previously. Volumetric soil water content was also measured by soil moisture sensors (ECH2O EC5, Decagon Devices, Pullman, WA, USA) with a data logger (EM50, Decagon Devices, Pullman, WA, USA). In order to obtain the plant water status, the midday leaf water potential was measured by a pressure chamber (Model 600, PMS Instrument Company, Albany, OR, USA) on two fully expanded mature leaves from three plants during soil water stress (n = 6). Regarding the measurement of relative leaf water content (RWC), two leaves were sampled from one plant in the morning and fresh weight (FW), turgid weight (TW), and dry weight (DW) were then determined. Relative water content (RWC, n = 6) was calculated according to Yamasaki and Dillenburg (1999), RWC = (FW − DW)/(TW − DW).
Severe water stress may affect photosynthetic biochemistry and, thus, alter photosynthetic parameters including A sat, dark respiration rate, CO2 compensation point, maximum carboxylation rate (V cmax), and CO2-saturated electron transport (J). These leaf photosynthetic parameters were estimated from the A/C i curve fit (Ethier 2004; Ethier et al. 2006) and light response curve model (Ögren and Evans 1993) using a photosynthesis system (Li-6400, Li-Cor, Lincoln, NE, USA). Leaf temperature and VPD were set at 25 °C and 1.7 kPa, respectively. Light response curves were obtained at 400 µmol mol−1 of CO2, while changing PPFD from 0 to 2000 µmol m−2 s−1, in which PPFD was increased stepwise from 400 to 2000 µmol m−2 s−1, and PPFD was then returned to 400 µmol m−2 s−1 and finally decreased stepwise to 0 µmol m−2 s−1. A sat and stomatal conductance (g s) were obtained at PPFD of 1500 µmol m−2 s−1. A/C i curves were obtained at PPFD of 1200 µmol m−2 s−1 with 12 different CO2 levels from 60 to 2000 µmol mol−1, in which CO2 was decreased stepwise from 400 to 60 µmol mol−1, returned to 400 µmol mol−1, and then increased stepwise to 2000 µmol mol−1.
Statistical analysis
A two-way ANOVA was performed to reveal the effects of the treatments (control, pruning mulch, and irrigation) on soil water content (SWC) and photosynthetic parameters for the pruning mulch and irrigation experiments. Intrinsic water-use efficiency (A sat/g s) for the three ranges of g s (<35, 35–65, and >65 % with respect to the species maximum) was calculated and then analyzed using the t test. In the severe water stress experiment, the mean values of photosynthetic parameters were calculated for SWC of <0.2, 0.2–0.4, and >0.6 g g−1, and then analyzed using ANOVA. Differences between means were tested by Tukey’s honest significant difference tests (p < 0.05). These statistical analyses were performed using R software (Rcmdr package, ver3.1.0).
Results
Environmental conditions
The treatments were conducted between 19 June and 11 September in 2010 (12 weeks) in the glasshouse. The rainy season was between 13 June and 17 July in 2010 in Kyoto city; 0–3 weeks of the treatments were conducted in the rainy season, with a daytime temperature of 30.0 °C and daytime VPD of 3.00 kPa on average in the glasshouse (n = 29, Fig. 2). At 0 week, daily cumulative PPFD was 6.1 mol m−2 day−1. Daytime temperature (35.1 °C), daytime VPD (3.22 kPa), and relative cumulative PPFD (1.34) in summer (from 4 to 12 weeks, n = 57) were significantly higher than those in the rainy season (t test, p < 0.05, Fig. 2).
Meteorological data during the experiment, obtained by data loggers at 30-min intervals. a Mean daytime (from 6:00 to 17:45) temperature and vapor pressure deficit (VPD). b Relative ratios of daily cumulative PPFD with respect to that on week 0 (6.1 mol m−2 day−1). Values are the mean ± SE of seven daily mean values with 48 data points
Effect of mulch and irrigation on soil water content and leaf photosynthesis
Pruning mulch had a significant effect on the evaporation rates in G. biloba and P. yedoensis (Table 1), with evaporation rates being reduced by 66 and 69 %, respectively.
The effects of the treatments on soil water content (SWC) were observed in the summer rainy season (from 19 June to 17 July) as well as in the summer dry season (from 18 July to 12 September), with similar effects being noted between species (Table 2). Mulch and irrigation increased SWC by 5–10 and 37–44 %, respectively (Fig. 3a).
Mean values ± SE of (a), soil water content (SWC), and gas exchange parameters such as (b), the light-saturated leaf photosynthesis rate (A sat), and (c), stomatal conductance (g s) in the rainy and summer seasons for G. biloba and P. yedoensis. Regarding SWC, values were estimated for three plants for 4 days (n = 12), and for the gas exchange parameters, they were estimated for two leaves from three plants for 2 or 3 weeks (n = 12 or 18). Asterisks indicate that values are significantly different from those of the controls (ANOVA, Tukey’s test) with *p < 0.05; **p < 0.01; ***p < 0.001; and n.s. not significant. Regarding gas exchange measurements, photosynthesis chamber conditions were set at 1500 µmol m−2 s−1 PPFD of light, 25.0 °C of leaf temperature, 1.1 kPa of VPD, and 380 µmol mol−1 of CO2
In both species, the treatments had no effects on leaf gas exchange parameters in the rainy season (Table 2). In contrast, the treatments significantly affected leaf gas exchange parameters in both species in summer, with similar effects being observed between species (Table 2). Increases of 9–15 and 21–26 % were observed in the light-saturated leaf photosynthetic rate (A sat) by mulch and irrigation, respectively (Fig. 3b). Stomatal conductance (g s) was increased by 18–20 and 63–70 % by mulch and irrigation, respectively (Fig. 3c).
Improvements in A sat/g s are an important acclimation process to soil water stress, and are highly species-dependent (Medrano et al. 2002, 2009). We compared averaged A sat/g s between G. biloba and P. yedoensis for three g s groups; <35, 35–65, and >65 % of the species maximum value (Fig. 4), considering that g s may be used as an indicator of physiological water stress (Medrano et al. 2009). A sat/g s was significantly higher in G. biloba than in P. yedoensis, when g s was <35 and 35–65 % of the species maximum (t test).
Differences in photosynthetic water-use efficiency (A sat/g s) for three groups of stomatal conductance (g s) between species. g s values were calculated as % with respect to the maximum values of G. biloba and P. yedoensis. Values are mean ± SE for three plants, in which data were pooled for two leaves in the summer season (6, 9, and 12 weeks, 4–34 replicates). Asterisks indicate that values were significantly different between species (ANOVA, t test) with ***p < 0.001 and n.s. not significant. Photosynthesis chamber conditions are the same as those in Fig. 3
Leaf area and leaf dry mass per leaf area (LMA) were not affected by the treatments, and were 29.4 (4.6) and 25.3 (5.4) cm2 and 78 (5) and 101 (7) g m−2 for G. biloba and P. yedoensis, respectively. In G. biloba seedlings, the effects of the treatments on leaf carbon isotope discrimination (Δ) were not clear (Table 3). Δ increased in P. yedoensis with the irrigation treatment.
Sensitivity of leaf photosynthesis to a temporal increase in leaf temperature/VPD
The sensitivity of leaf photosynthesis by G. biloba and P. yedoensis to a temporal high leaf temperature/VPD (35 °C/3.1 kPa) was estimated by comparing their gas exchange parameters with those measured at moderate temperature/VPD (25 °C/1.1 kPa) in the summer. The mulch or irrigation treatment had no significant effects on the sensitivity of leaf photosynthesis to the high leaf temperature/VPD (Table 4). In contrast, a highly significant species effect was noted (Table 4), with the sensitivity of A sat and g s being higher in G. biloba than in P. yedoensis (Fig. 5a, b). The decrease observed in A sat was 17–24 % in G. biloba, but it was very small in P. yedoensis (Fig. 5a). The decrease in g s was also higher in G. biloba (by 41–54 %) than in P. yedoensis (24–31 %, Fig. 5b).
Sensitivity of photosynthesis to a temporal high leaf temperature/VPD (33 °C/3.3 kPa) in G. biloba and P. yedoensis. Decreases in leaf gas exchange parameters were estimated by comparing them with those measured at a moderate temperature/VPD (25 °C/1.1 kPa) in the summer. a The light-saturated leaf photosynthesis rate (A sat), and b stomatal conductance (g s). Values are mean ± SE for two leaves from three plants (n = 6). The difference between treatments was analyzed by ANOVA using Tukey’s test, with n.s. meaning no significant difference being obtained
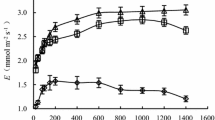
Effects of severe soil water stress on the leaf water status and photosynthesis
SWC was reduced from 1.0 to 0.09 g g−1 and from 1.0 to 0.04 g g−1 for G. biloba and P. yedoensis, respectively, during the experiment performed in 2014 (Fig. 6). SWC linearly correlated with volumetric soil water content in both species (r 2 = 0.9, p < 0.05), in which the volumetric soil water contents for G. biloba and P. yedoensis were 0.242 and 0.225 cm3 cm−3 at field capacity, and 0.098 and 0.103 cm3 cm−3 at the wilting point, respectively. Midday leaf water potential (Ψ day) was constant against changes in SWC in G. biloba (Fig. 6a), but decreased with reductions in SWC in P. yedoensis. A similar result was obtained for changes in relative leaf water content (RWC) against SWC (Fig. 6b); RWC was constant in G. biloba, but decreased with reductions in SWC in P. yedoensis.
Changes in midday leaf water potential (a) and relative leaf water content, RWC (b) with soil water content (SWC) during the severe water stress experiment. Values are mean ± SE for two leaves from three plants (n = 6). Asterisks indicate that values are significantly different from those at SWC of 1.0 g g−1 (field capacity, t test); *p < 0.05; **p < 0.01; ***p < 0.001; and n.s. not significant. Open and closed symbols show G. biloba and P. yedoensis, respectively
The responses of A sat, g s, and the CO2 compensation point to SWC were similar between species; under low SWC (<0.2 g g−1), A sat and g s were decreased by 41–69 %, while the CO2 compensation point was increased by 30–52 % (Table 5). The responses of the dark respiration rate, CO2-saturated electron transport rate (J), and maximum carboxylation rate (V cmax) to SWC were obtained for P. yedoensis only; the dark respiration rate was increased by 33 %, while J and V cmax were decreased by 31 % under low SWC.
Discussion
Effects of pruning much and irrigation on leaf photosynthesis
It is widely recognized that soil water content (SWC) is one of the most significant factors affecting leaf photosynthesis via a series of physiological mechanisms including stomatal regulation and/or photosynthetic biochemistry (Chaves and Oliveira 2004; Flexas et al. 2004; Chaves et al. 2009). SWC is strongly affected by evapotranspiration, which is the sum of soil evaporation and plant transpiration. Pruning mulch forms a physical layer between the soil and atmosphere, thereby preventing sunlight from reaching the soil surface and decreasing gas exchange, which ultimately results in decreases in soil surface temperature and evapotranspiration. Reduced soil evaporation (Table 1, Qin et al. 2013) and/or enhanced soil water retention (Cook et al. 2006) are among the benefits of organic mulch, resulting in an increase in SWC for field-grown urban trees (Iles and Dosman 1999; Montague and Fox 2008). Although the increase in SWC is partly compensated by plants through increased transpiration via the feedback mechanisms of stomata (Balwinder-Singh et al. 2011), we achieved a significant enhancement in SWC by the mulch treatment (Table 2; Fig. 3a), which is consistent with previous findings.
The responses of leaf photosynthesis to the pruning mulch and irrigation treatments were only obtained in the summer (Table 2; Fig. 3b, c), which is related to high SWC (Fig. 3a). This result suggests that photosynthetic responses to soil water stress are affected by atmospheric conditions, including irradiance levels, temperature, and VPD. In the summer, higher irradiance levels in daytime (by 30 %, Fig. 2b) together with higher daytime VPD (+0.22 kPa) involve a higher evaporative demand than that in the rainy season, during which the effects of SWC on stomatal conductance may be more significant. High VPD in the summer may induce high ABA levels in the leaf (McAdam and Brodribb 2015); high SWC has been shown to induce reductions in leaf ABA levels (Tardieu and Simonneau 1998), which may moderate the impact of high VPD on ABA levels. In the pruning mulch treatment, factors other than SWC, including a decrease in soil temperature (Iles and Dosman 1999; Cook et al. 2006), may benefit leaf photosynthesis by preventing a dysfunction in the root with the high summer temperatures. The effects of pruning mulch on leaf photosynthesis may be obtained at near wilting point as well as SWC of ~0.7 g g−1 (Fig. 3a) due to the enhancement of SWC as well as decrease in soil temperature as discussed above.
The concurrent increases observed in A sat and g s for G. biloba and P. yedoensis in the summer by pruning mulch and irrigation (Fig. 3b, c) suggest that the increase in A sat was affected by stomatal opening in both species, which supports previous findings of increases in the g s of field-grown urban shrub trees by bark mulch and irrigation (Montague et al. 2007). In the summer, this stomatal opening by pruning mulch and irrigation may be accompanied by an increase in CO2 supply to the carboxylation site. The enhancement induced in photosynthetic function by the pruning mulch and irrigation treatments suggests that the activity and health of roadside trees will be improved by these treatments, and thus, may contribute to maintaining tree canopies during the hot-dry summer.
The increase observed in leaf carbon isotope discrimination (Δ) in P. yedoensis by irrigation suggests that long-term water-use efficiency was decreased (Hanba et al. 2003) by the increase in SWC, thereby supporting the findings of Kagotani et al. (2013) in which Δ was larger in urban P. yedoensis grown at sites with higher SWC.
Difference in leaf photosynthetic and hydraulic responses between G. biloba and P. yedoensis
g s values were smaller in G. biloba than in P. yedoensis in the summer in all the treatments tested when they were measured at moderate leaf temperature/VPD (Fig. 3c). The lower g s in G. biloba than in P. yedoensis was also observed at SWC of >0.6 g g−1 in the second experiment (Table 5). These results suggested that, under summer conditions, the stomata of G. biloba acted to minimize water loss from the leaf. The decrease observed in g s and A sat in response to a temporal high temperature/VPD was markedly larger in G. biloba than in P. yedoensis with both treatments in the summer (Table 4; Fig. 5a, b), which supports previous finding showing that the sensitivity of stomata to VPD was the highest in G. biloba among the 13 species of C3 plants including tree and herbaceous species (Franks and Farquhar 1999). This result again suggests that G. biloba has more effective stomatal control than P. yedoensis to minimize water loss from the leaf under conditions of high evaporative demand. G. biloba had higher A sat /g s at low g s than P. yedoensis (Fig. 4), which is analogous to the evergreen woody shrub Limonium species with higher A sat /g s at lower g s among Mediterranean plants (Galmés et al. 2007), suggesting effective stomatal regulation for water loss and a more suited photosynthetic machinery under water stress (Galmés et al. 2007; Medrano et al. 2009). Stomatal density was lower in G. biloba than in P. yedoensis (Fig. 1c, d), which is consistent with the findings of Medrano et al. (2009) who reported low stomatal density with high A sat /g s in Limonium species. Effective stomatal control with low stomatal density enables plants to prevent water loss and maintain leaf turgor under water stress conditions, thereby leading to the high-drought resistance of these plants.
Our severe water stress experiment showed that G. biloba actually maintained leaf turgor under a wide range of SWC; the midday leaf water potential and leaf water content in G. biloba was almost constant with significant decreases in SWC, while they were decreased significantly in P. yedoensis (Fig. 6a, b). Under mild water stress (SWC > 0.6 g g−1), g s was smaller in G. biloba than in P. yedoensis (Table 5). These results, together with the smaller g s (Fig. 3c) and higher A sat/g s at low g s (Fig. 4) in G. biloba in the first experiment, suggested that, under mild soil water stress, G. biloba shows “conservative water use,” in which it maintains the midday leaf water potential with low g s, while P. yedoensis exhibits “opportunistic water use,” which allows for midday decreases in the leaf water potential in response to soil water stress with high g s (Tardieu and Simonneau 1998; Moreno-Gutiérrez et al. 2012; Hochberg et al. 2013). Osone et al. (2014) reported higher soil-to-leaf hydraulic conductance in G. biloba than in P. yedoensis grown in streets. Under more severe water stress (SWC < 0.4 g g−1), the effects of high leaf hydraulic conductance on the plant water relationship may become significant; it contributes to maintaining the high midday leaf water potential and leaf turgor in G. biloba (Fig. 6), and may also enable G. biloba to keep a similar g s to that in P. yedoensis (Table 5).
In the severe water stress experiment, some responses of leaf biochemistry were different between species; V cmax and J were down-regulated as SWC decreased in P. yedoensis only (Table 5). Declines in biochemical components, including the activities or levels of enzymes for carboxylation and electron transport, RuBP carboxylation capacity, and RuBP regeneration, have been shown to induce decreases in V cmax and J by reducing SWC, with this response being strongly species-dependent (Bota et al. 2004; Galmés et al. 2011). Based on these results, we concluded that the photosynthetic machinery is more suited to severe water stress conditions in G. biloba than in P. yedoensis. On the other hand, both species showed an increase in the CO2 compensation point with decreases in SWC (Table 5), which is often obtained in higher plants affected by changes in respiratory CO2 (Lawlor and Cornic 2002).
Conclusions
Pruning mulch and irrigation significantly ameliorated the negative impact of moderate summer water stress (0.7 g g−1) on leaf photosynthesis in P. yedoensis and G. biloba to a similar extent through increases in stomatal conductance via changes in soil water content (SWC). In the summer, G. biloba showed more effective stomatal control to prevent water loss from its leaves under high evaporative demand with high VPD/high irradiance levels. Additionally, G. biloba maintained a high midday leaf water potential and leaf water content against large variations in soil water content (from 0.08 to 1.0 g g−1) with less reliance of leaf biochemistry on SWC, which suggest higher drought resistance in G. biloba than in P. yedoensis. Tree species selected in consideration of their tolerance to summer water stress, together with the management methods to increase soil water retention may significantly increase the leaf photosynthesis and evaporation rates of urban trees, and, thus, may help to improve the growth and mortality of planted trees.
Author contribution statement
Y. Kagotani and Y. T. Hanba planned the experimental setup. T. Kiyomizu, K. Sasaki, and K. Nishida performed in vivo measurements in the severe water stress experiment. Y. Kagotani performed measurements in the mulch and irrigation experiment. Y. T. Hanba performed data analysis. Y. T. Hanba, A. Kume, and K. Nishida wrote the manuscript.
Abbreviations
- A sat :
-
Light-saturated CO2 assimilation rate
- Δ:
-
Carbon isotope discrimination
- g s :
-
Stomatal conductance
- J :
-
CO2-saturated electron transport rate
- PPFD:
-
Photosynthetic photon flux density
- RWC:
-
Relative leaf water content
- SWC:
-
Soil water content
- VPD:
-
Vapor pressure deficit
- V cmax :
-
Maximum carboxylation rate
References
Balwinder-Singh Eberbach PL, Humphreys E, Kukal SS (2011) The effect of rice straw mulch on evapotranspiration, transpiration and soil evaporation of irrigated wheat in Punjab, India. Agric Water Manag 98:1847–1855. doi:10.1016/j.agwat.2011.07.002
Bassuk N, Curtis DF, Marranca BZ, Neal B (2009) Recommended urban trees: site assessment and tree selection for stress tolerance. Urban Horticulture Institute, Department Of Horticulture, Cornell University, Ithaca
Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681. doi:10.1111/j.1469-8137.2004.01056.x
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384. doi:10.1093/jxb/erh269
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. doi:10.1093/aob/mcn125
Cook HF, Valdes GSB, Lee HC (2006) Mulch effects on rainfall interception, soil physical characteristics and temperature under Zea mays L. Soil Tillage Res 91:227–235. doi:10.1016/j.still.2005.12.007
Ethier GJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153. doi:10.1111/j.1365-3040.2004.01140.x
Ethier GJ, Livingston NJ, Harrison DL, Black TA, Moran JA (2006) Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant Cell Environ 29:2168–2184. doi:10.1111/j.1365-3040.2006.01590.x
Fain GB, Tilt KM, Gilliam CH, Ponder HG, Sibley JL (1999) Cyclic irrigation improves irrigation application efficiency and growth of sawtooth oak. J Arboric 25:200–203
Ferrini F, Fini A (2011) Sustainable management techniques for trees in the urban areas. J Biodivers Ecol Sci 1:1–20
Fini A, Ferrini F, Frangi P, Amoroso G, Piatti R (2009) Withholding irrigation during the establishment phase affected growth and physiology of norway maple (Acer platanoides) and linden (tilia spp.). Arboric Urban For 35:241–251
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 3:269–279. doi:10.1055/s-2004-820867
Franks PJ, Farquhar GD (1999) A relationship between humidity response, growth form and photosynthetic operating point in C3 plants. Plant Cell Environ 22:1237–1349. doi:10.1046/j.1365-3040.1999.00494.x
Galmés J, Flexas J, Savé R, Medrano H (2007) Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil 290:139–155. doi:10.1007/s11104-006-9148-6
Galmés J, Ribas-Carbó M, Medrano H, Flexas J (2011) Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J Exp Bot 62:653–665. doi:10.1093/jxb/erq303
Hanba YT, Kogami H, Terashima I (2003) The effect of internal CO2 conductance on leaf carbon isotope ratio. Isotopes Environ Health Stud 39:5–13. doi:10.1080/1025601031000102233
Handa M, Iizuka Y, Fujiwara N (1997) Ginkgo landscapes. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H (eds) Ginkgo Biloba A Global Treasure. Springer, Tokyo, pp 259–283
Hanslin HM, Sæbø A, Bergersen O (2005) Estimation of oxygen concentration in the soil gas phase beneath compost mulch by means of a simple method. Urban For Urban Green 4:37–40. doi:10.1016/j.ufug.2005.05.001
Hochberg U, Degu A, Fait A, Rachmilevitch S (2013) Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiol Plant 147:443–452. doi:10.1111/j.1399-3054.2012.01671.x
Iijima K, Suzuki K, Takahashi S, Kondo M (1993) On the damage of trees for landscaping caused by drought conditions at Kanto area in summer 1992. J Jpn Soc Reveg Technol 19:193–198. doi:10.7211/jjsrt.19.193
Ikeda T, Hashimoto H (1996) On decline of street trees of Elaeocarpus sylvestris POIRET var. ellipticus HARA in relation to water relations. J Jpn For Soc 78:478–480
Iles JK, Dosman MS (1999) Effect of organic and mineral mulches on soil properties and growth of faiview flame red maple trees. J Arboric 25:163–167
IPCC (2014) In: CoreWritingTeam, Pachauri RK, Meyer LA (eds) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, p 151
Kagotani Y, Fujino K, Kazama T, Hanba YT (2013) Leaf carbon isotope ratio and water use efficiency of urban roadside trees in summer in Kyoto city. Ecol Res 28:725–734. doi:10.1007/s11284-013-1056-7
Kaushal P, Aussenac G (1989) Transplanting shock in Corsican pine and cedar of Atlas seedlings: internal water deficits, growth and root regeneration. For Ecol Manag 27:29–40. doi:10.1016/0378-1127(89)90080-7
Kawamukai M, Nakazawa T, Shimizu S (2000) Continuous monitoring of the carbon dioxide concentration in the urban atmosphere in Kyoto. Bull Fac Text Fibers Kyoto Univ Ind Arts Text Fibers 24:53–56
Kurihara M, Takeda Y, Kubota S (2014) The roadside trees of Japan VIII. Tokyo
Lambers H, Chapin FS III, Pons TL (2008) Plant water relations, second edi. Springer Science + Business Media, New York
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
McAdam SAM, Brodribb TJ (2015) The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiol 167:833–843. doi:10.1104/pp.114.252940
Medrano H, Escalona JM, Bota J, Gulías J, Flexas J (2002) Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot Ann. doi:10.1093/aob/mcf079
Medrano H, Flexas J, Galmés J (2009) Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 317:17–29. doi:10.1007/s11104-008-9785-z
Montague T, Fox L (2008) Gas exchange and growth of transplanted and nontransplanted field-grown Shumard red oak trees grown with and without organic mulch. HortScience 43:770–775
Montague T, Kjelgren R, Rupp L (2000) Surface energy balance affects gas exchange and growth of two irrigated landscape tree species in an arid climate. J Am Soc Hortic Sci 125:299–309
Montague T, McKenney C, Maurer M, Winn B (2007) Influence of irrigation volume and mulch on establishment of select shrub species. Arboric Urban For 33:202
Moreno-Gutiérrez C, Dawson TE, Nicolás E, Querejeta JI (2012) Isotopes reveal contrasting water use strategies among coexisting plant species in a mediterranean ecosystem. New Phytol 196:489–496. doi:10.1111/j.1469-8137.2012.04276.x
Ögren E, Evans JR (1993) Photosynthetic light-response curves. Planta 189:182–190. doi:10.1007/BF00195075
Osone Y, Kawarasaki S, Ishida A, Kikuchi S, Shimizu A, Yazaki K, Aikawa S, Yamaguchi M, Izuta T, Matsumoto GI (2014) Responses of gas-exchange rates and water relations to annual fluctuations of weather in three species of urban street trees. Tree Physiol 34:1056–1068. doi:10.1093/treephys/tpu086
Percival GC, Sheriffs CN (2002) Identification of drought-tolerant woody periennials using chlorophyll fluorescence. J Arboric 28:215–223
Qin W, Chi B, Oenema O (2013) Long-term monitoring of rainfed wheat yield and soil water at the loess plateau reveals low water use efficiency. PLoS One 8:e78828. doi:10.1371/journal.pone.0078828
Satomura A, Imanishi J, Morimoto Y, Kojima A (2005) A study of diagnostic indices of vitality of Prunus jamasakura. J Jpn Soc Reveg Technol 31:15–20. doi:10.7211/jjsrt.31.15
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49:419–432. doi:10.1093/jxb/49.Special_Issue.419
Tayasu I, Hirasawa R, Ogawa NO, Ohkouchi N, Yamada K (2011) New organic reference materials for carbon- and nitrogen-stable isotope ratio measurements provided by Center for Ecological Research, Kyoto University, and Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology. Limnology 12:261–266. doi:10.1007/s10201-011-0345-5
Tomás M, Medrano H, Brugnoli E, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J (2014) Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Aust J Grape Wine Res 20:272–280. doi:10.1111/ajgw.12069
Vico G, Revelli R, Porporato A (2014) Ecohydrology of street trees: design and irrigation requirements for sustainable water use. Ecohydrology 7:508–523. doi:10.1002/eco.1369
Wada R, Pearce JK, Nakayama T, Matsumi Y, Hiyama T, Inoue G, Shibata T (2011) Observation of carbon and oxygen isotopic compositions of CO2 at an urban site in Nagoya using Mid-IR laser absorption spectroscopy. Atmos Environ 45:1168–1174. doi:10.1016/j.atmosenv.2010.10.015
Warren CR (2008) water deficits decrease the internal conductance to CO2 transfer but atmospheric water deficits do not. J Exp Bot 31:1–8. doi:10.1093/jxb/erm314
Yamasaki S, Dillenburg L (1999) Measurements of leaf relative water content in Araucaria angustifolia. Rev Bras Fisiol Veg 11:69–75
Youkhana A, Idol T (2009) Tree pruning mulch increases soil C and N in a shaded coffee agroecosystem in Hawaii. Soil Biol Biochem 41:2527–2534. doi:10.1016/j.soilbio.2009.09.011
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research, the Sumitomo Foundation (103230), Adaptable and Seamless Technology Transfer Program through Target-driven R&D (AS262Z01258N) and Discretionary expense of the President of Kyoto Institute of Technology. The leaf stable carbon isotope ratio was measured at the Center for Ecological Research, Kyoto University. We appreciate Drs. Ichiro Tayasu and Riyo Hirasawa for supporting the isotope measurements. We thank Dr Jiro Tatsumi for supporting our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by T. Koike.
Rights and permissions
About this article
Cite this article
Kagotani, Y., Nishida, K., Kiyomizu, T. et al. Photosynthetic responses to soil water stress in summer in two Japanese urban landscape tree species (Ginkgo biloba and Prunus yedoensis): effects of pruning mulch and irrigation management. Trees 30, 697–708 (2016). https://doi.org/10.1007/s00468-015-1312-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1312-2