Abstract
Key message
NH + 4 acts as a mild oxidative stressor, which triggers antioxidant cellular machinery and provide resistance to salinity.
Abstract
NH4 + nutrition in Carrizo citrange (Citrus sinensis L. Osbeck × Poncirus trifoliata L) plants acts as an inducer of resistance against salinity conditions. NH4 + treatment triggers mild chronic stress that primes plant defence responses by stress imprinting and confers protection against subsequent salt stress. In this work, we studied the influence of NH4 + nutrition on antioxidant enzymatic activities and metabolites involved in detoxification of reactive oxygen species (ROS) to clarify their involvement in NH4 +-mediated salt resistance. Our results showed that NH4 + nutrition induces in citrus plants high levels of H2O2, strongly inhibits superoxide dismutase (SOD) and glutathione reductase (GR) activities, and leads to higher content of oxidised glutathione (GSSG) than in control plants in the absence of salt, thus providing evidence to confirm mild stress induced by NH4 + nutrition. However, upon salinity, plants grown with NH4 + (N-NH4 + plants) showed a reduction of H2O2 levels in parallel to an increase of catalase (CAT), SOD, and GR activities compared with the control plants. Moreover, N-NH4 + plants were able to keep high levels of reduced glutathione (GSH) upon salinity and were able to induce glutathione-S-transferase (GST) and phospholipid hydroperoxide glutathione peroxidise (PHGPx) mRNA accumulation. Based on this evidence, we confirm that sublethal concentrations of NH4 + might act as a mild oxidative stressor, which triggers antioxidant cellular machinery that can provide resistance to subsequent salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is amongst the most significant environmental factors responsible for substantial losses in agricultural production worldwide, and it is one of the serious problems confronting sustainable agriculture in irrigated production systems in arid and semiarid regions (Ravindran et al. 2007; Marschner 2012). Nearly 20 % of the world’s cultivated area and approximately half of the world’s irrigated lands are affected by salinity stress (Munns and Tester 2008). This is a critical problem especially for citrus plants, as they are one of the most globally important horticultural crops that are considered to be salt-sensitive (Al-Yassin 2005). Damages caused by high salinity are often associated with three different mechanisms (Ievinsh 2006): ion toxicity caused by the excessive accumulation of Na+ and Cl− in the cytoplasm, leading to an ionic imbalance. Second, even if massive ion compartmentalisation occurred in the vacuole, the cytosol water potential must also be lowered to balance the low external water potential, thus allowing water intake in the plant cell and preventing macromolecular damage. Moreover, high cellular NaCl concentration induces oxidative stress in plants through enhanced generation of ROS (Khanna-Chopra and Selote 2007), which is considered the primary event for a variety of stress conditions (Foyer and Noctor 2005). It is known that oxidative stress results from the disruption of the cellular homeostasis of ROS production. ROS accumulation induces oxidative damage in membrane lipids, nucleic acids, and proteins (Mittler 2002). A causal relationship has been established between high or increased activities of antioxidant enzymes and the degree of protection from salt-associated oxidative damage (Mittova et al. 2004; Stepien and Klobus 2005). Moreover, ROS have been implicated in a plethora of physiological processes in plants, from seed germination to cell death (Arc et al. 2011; Filippou et al. 2011). In addition, the imposition of abiotic and biotic stresses causes the overproduction of ROS, which ultimately inflicts a secondary oxidative and nitrosative stress, leading to various signalling responses (Mittler et al. 2011). ROS steady-state levels are often altered in plants under various stress conditions and play a dual role: at mild concentrations, ROS act as signal molecules involved in acclimatory signalling and trigger tolerance against various stress conditions and at high concentrations, ROS orchestrate programmed cell death (Asada 2006). H2O2 has been suggested to be a signalling molecule in defence and adaptive responses such as increase tolerance to chilling in maize (Prasad et al. 1994), tolerance to paraquat in cucumber seedlings (Xia et al. 2009), and tolerance to chilling in tomato plants (Zhou et al. 2012).The damaging effects of ROS have caused plant cells to cope with oxidative stress by triggering complex redox homeostatic antioxidative mechanisms. These ROS-scavenging antioxidative mechanisms include specific antioxidant enzymes such as CAT, SOD, ascorbate peroxidase (APX), peroxidase and some other low-molecular-weight antioxidants such as ascorbate and reduced glutathione (Iannone et al. 2012).
NH4 + nutrition is of interest as an alternative to that of nitrate. However, NH4 + nutrition turns out to be stressful to many plants, including some important crops, leading to a reduced growth (Britto and Kronzucker 2002). In addition to growth reduction, several characteristics of plant metabolism–lower content of mineral cations and organic anions and higher levels of amino acids–are altered, resulting in the so-called ‘ammonium syndrome’. However, we demonstrated that Carrizo citrange (Citrus sinensis L. Osbeck × Poncirus trifoliata L) plants prefer to absorb NH4 + more than NO3 − if both N forms are present in the nutrient solution (Camañes et al. 2009). Although other studies have shown that some species develop toxic symptoms when only NH4 + nutrition is applied (Gerendás et al. 1997; Lasa et al. 2001), the Carrizo citrange plants displayed optimal growth when they were grown with NH4 + as the sole nitrogen source (Fernández-Crespo et al. 2012). Besides, in Fernández-Crespo et al. (2012) it was also demonstrated that NH4 + treatment confers protection against subsequent salt stress by reducing Cl− uptake and decreasing its toxicity, by priming abscisic acid and polyamines, and enhances H2O2 and proline basal content and they concluded that N-NH4 + treatment triggers mild chronic stress, which may account for the noted stress-induced morphogenetic responses (SIMRs). SIMR is a part of a general acclimation strategy that is characterised by the blockage of cell division in the main meristematic tissues, the inhibition of elongation, redirected outgrowth of lateral organs (Potters et al. 2009), an increase in the antioxidants that prevent damage caused by ROS and the accumulation of foliar molecules that act as modulators of stress signals (Gould and Lister 2006). Activation of the response related to SIMRs due to NH4 + toxicity led to the “acclimation stage”, which leads to better adaptation to subsequent salt stress. Preliminary stress exposure or stress imprinting is indeed necessary to induce priming, which makes the plants more resistant to future biotic or abiotic stress (Conrath et al. 2006; Bruce et al. 2007; Galis et al. 2009). Recent studies regarding NH4 + inducer effect indicate that NH4 + or one of its assimilation products (e.g. glutamine or glutamate) may serve as a stress signal and that NH4 + -grown plants operate metabolic pathways which induce the accumulation of ROS (Misra and Gupta 2006). Several studies demonstrated that NH4 + nutrition is able to improve the depressive effects of high salinity in barley (Kant et al. 2007) and in halophytes (Kudo and Fujiyama 2010; Hessini et al. 2011). Hessini et al. (2013) observed the benefits of NH4 + nutrition on the halophyte Spartina alterniflora under saline conditions which seems to be associated with high antioxidant enzyme activities, together with low MDA content, electrolyte leakage, and H2O2 concentration. Further studies need to be done to elucidate how NH4 + nutrition enhances plants ability to improve a subsequent salt stress situation and the relationship of this induced resistance with ROS metabolism. For this reason, the main aim of this work was to investigate the biochemical mechanisms underlying NH4 +-mediated resistance to high salt concentrations in Carrizo citrange plants and its relationship with the antioxidant cellular machinery.
Materials and methods
Plant material, growth conditions and nutrition treatments
Carrizo citrange seeds (Beniplant, Valencia, Spain) were allowed to germinate in vermiculite in a growth chamber under the following environmental conditions: light/dark cycle of 16/8 h, temperature of 20/24 °C and RH of 70 %. Seeds were irrigated twice a week with distilled water, and after 6 weeks, seedlings were irrigated for two months with Hoagland solution lacking nitrogen (Hoagland and Arnon 1950) complemented with either 1 mM NH4NO3 (control treatment) or 5 mM of N-NH4 + [(NH4)2SO4)] (NH4 + treatment). Prior to the experiments, 3-month-old plants with a single shoot were selected for uniformity of size and transferred to an aerated, complemented Hoagland solution for 7 days in hydroponic culture devices. Salt stress was induced by adding 90 mM NaCl to the hydroponic solution and was renewed twice weekly. Samples were taken for individual analysis at 14 days after addition of salt to the hydroponic solution.
DAB staining and H2O2 quantification
N-NH4 + and control plants were exposed to 90 mM NaCl for 2 h, and the salt-stressed leaves were stained in 1 mg of DAB per millilitre at pH < 3 for 24 h in the dark and subsequently destained in 95 % ethanol. Later, samples were rehydrated with distilled water. DAB staining intensities were quantified from digital photographs (Nikon Eclipse 11000, Tokyo) by the number of dark-brown DAB pixels relative to total pixels corresponding to plant material, using the GIMP software (http://www.gimp.org/).
Antioxidant enzymatic activity determination
Superoxide dismutase assay
Samples (0.05 g of tissue) were resuspended in 200 µL extraction buffer (Tris–HCl, pH 8.5) and a cocktail of protease inhibitors 1X (Roche) and vortexed for 30 s on three occasions. Extracts were centrifuged at 955×g for 3 min at 4 °C and the supernatant was used for protein quantification and gel electrophoresis analysis. The protein concentration was quantified for each extract using the Bradford protein assay from Bio-Rad. Protein samples were separated on 10 % native acrylamide gel electrophoresis as described in Gamero-Sandemetrio et al. (2013).
Catalase assay
Samples (0.05 g of tissue) were homogenised in 0.2 mL of 50 mM phosphate buffer at pH 7.0 and a mix of protease inhibitors (200 µM phenylmethylsulfonyl fluoride (PMSF), 20 M µTPcK, 200 µM pepstatin A) and centrifuged at 15,300×g for 10 min at 4 °C. Catalase activity was assayed in microplates by adding 1–10 µL of supernatant to 0.2 mL of 50 mM phosphate buffer pH 7.0 and 80 mM H2O2 (Jakubowski et al. 2000). The absorbance decrease of H2O2 was measured at 240 nm, and enzyme activity was calculated using an extinction coefficient of 43.66 M−1cm−1. The protein concentration was quantified for each extract using the Bradford protein assay from Bio-Rad. Catalase activity was expressed as µmol of H2O2 min−1 mg of protein−1 (U/mg prot).
Glutathione reductase assay
Samples (0.1 g of tissue) were homogenised in 0.5 mL of 50 mM MES/KOH buffer at pH 6.0 and centrifuged at 20,800×g for 10 min at 4 °C. The supernatant was diluted (10–40 µL) in a final volume of 0.2 mL of 50 mM Hepes buffer at pH 8.0 with 0.5 mM EDTA. GR activity was assayed in microplates and was measured spectrophotometrically as NADPH oxidation at 340 nm in the presence of 0.25 mM NADPH (Murshed et al. 2008). GR reaction was started by the addition of 5 µL of 20 mM GSSG to each well. The specific activity was calculated from the 6.22 mM−1 cm−1 extinction coefficient. GR activity was defined as micromoles of substrate consumed per minute per milligram of protein. The protein concentration was quantified for each extract using the Bradford protein assay from Bio-Rad.
Glutathione determination
Measurements of total glutathione (GSH, reduced form; GSSG, oxidised form) were carried out using 5,5′-dithiobis-(2-nitrobenzoic) acid (DTNB), according to the method of Griffith (1980). 0.1 g of tissue was homogenised in 1 mL ice-cold 8 mM HCl with 1.3 % (w/v) 5-sulphosalicylic acid. Samples were centrifuged at 18,000×g for 15 min at 4 °C, and supernatants were used for glutathione determination. For total glutathione determination, the supernatant was directly diluted in 0.2 mL of 0.4 M Mes, 0.1 M sodium phosphate buffer pH 7.4, 2 mM EDTA, pH 6.0. GSSG content was measured after the removal of GSH by 2-vinyl pyridine derivatisation for 1 h at room temperature. 0.2 mL of sample was mixed with 0.12 mL of NADP (0.4 mg mL−1), glucose-6-phosphate (0.16 mg mL−1), glucose-6-phosphate dehydrogenase (3 µg mL−1), glutathione reductase (1 mU) and 0.48 mL of 0.2 mM DTNB. The mixture was incubated at room temperature for 20 min under agitation and dark conditions, and absorbance was measured at 412 nm. For calculation of glutathione contents a standard curve prepared with GSSG was used. Glutathione levels are expressed as µmol per mg FW.
Quantitative RT-qPCR analysis
Gene expression by quantitative real-time PCR (RT-qPCR) was performed using RNA samples extracted from leaf tissue using the Total Quick RNA cells and tissues kit (Talent; http://www.spin.it/talent). Citrus leaf tissue samples for RNA isolation were collected at 2 h and 14 days after NaCl treatment. Leaf tissue from five plants each of the NH4 + treated and untreated plants was collected. For quantitative Real time PCR experiments, 1.5 μg of total RNA was digested using one unit of RNase-Free DNase (Promega; http://www.promega.com) in 1 μl of DNase buffer and up to 10 μl of Milli-Q water and was incubated for 30 min at 37 °C. After the incubation, 1 μl of RQ1 DNase stop buffer was added, and the solution was incubated again at 65 °C for 10 min to inactivate the DNase. Highly pure RNA was used for the RT reaction. The RT reaction was performed by adding 2 μl of RT buffer, 2 μl of 5 mM dNTP, 2 μl of 10 μM oligo(dT)15 primer (Promega), 1 μl of 10 U μl−1 RNasin RNase inhibitor (Promega) and 1 μl of Omniscript reverse transcriptase (QIAGEN; http://www.qiagen.com/). The reaction mixture was incubated at 37 °C for 60 min. Forward and reverse primers (0.3 μM) were added to 12.5 μl of QuantiTect™ SYBR Green PCR reaction buffer (QIAGEN), as were 2 μl of cDNA and Milli-Q sterile water up to 25 μl total reaction volume. Quantitative PCR was carried out using the Smart Cycler II instrument (Cepheid; http://www.cepheid.com). A list of the primers used in the RT-qPCR is shown in Table 1, the GAPDH gene expression was used as an internal standard. Melting-curve analysis was performed at the end of the PCR reaction to confirm the products’ purity. Differences in cycle numbers during the linear amplification phase between samples containing cDNA from treated and untreated plants were used to determine differential gene expression. The corresponding RT-qPCR efficiencies were calculated according to the equation E = 10[−1/slope] and E was in all case in the range of 1.8–2.2. Triplicate analyses were performed on all occasions using the cDNA samples derived from three independent experiments.
Statistical analysis
Statistical analysis was carried out using the StatGraphics software (Statpoint Technologies, Inc., Warrenton, Virginia, USA). The data are expressed as the means and standard error. Mean values were compared by an LSD (least significant difference) test. All experiments were repeated at least three times.
Results
NH4 + nutrition induces H2O2 accumulation in Carrizo citrange plants
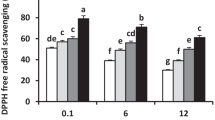
In this work, we used DAB staining to establish how NH4 + treatment affects cellular oxidative stress. H2O2 staining based on the in vivo reaction of H2O2 with DAB allows a rapid estimation of the H2O2 steady-state levels in leaves (Thordal-Christensen et al. 1997). Our results indicate that the N-NH4 +-treated plants show higher basal levels of H2O2 than the control plants in the absence of salt stress (Fig. 1). Increased H2O2 levels were noted at 2 h after treatment with 90 mM NaCl for both the control and N- NH4 + conditions. However, the highest levels of H2O2 were observed in the N-NH4 + plants treated with NaCl. Interestingly, after 14 days of salt addition, the control plants showed higher H2O2 steady-state levels than the N-NH4 +-treated plants, and these plants did not show significant changes between the salt-treated and untreated plants after 14 days.
H2O2 staining, estimated by using DAB staining in the leaves of the control plants and the N-NH4 + Carrizo citrange plants treated with NaCl (90 mM) for 2 h and 14 days. a Quantitative H2O2 measurement on the basis of brown pixels from digital photographs. b Representative pictures of H2O2 production. Data show a representative experiment that was repeated three times; each point is the average of a pool of 10 plants ± standard error (SE) (n = 10). Letters indicates statistically significant differences between treatments within each time points (p < 0.05)
NH4 + nutrition modify antioxidants enzymatic activity in salt stress conditions
To establish how NH4 + nutrition affects the response of citrus plants to salt-induced oxidative stress, several antioxidant enzyme activities were determined after 2 h and 14 days and after salt stress for both the control and the N-NH4 +-treated plants. Among different cellular free radical scavengers, superoxide dismutase (SOD) plays an important role as an antioxidant defence and protects cellular components from being oxidised by ROS. When acclimating to increased levels of oxidative stress, SOD concentrations typically increase with the degree of the stress condition. Therefore, we analysed SOD activity as a marker of oxidative stress under our experimental conditions (Fig. 2). The basal SOD activities differed between the control and the N-NH4 +-treated plants. Both of the SOD (Cu/Zn-SOD and Mn-SOD) isoforms displayed less activity in the N-NH4 + plants in the absence of salt stress when compared to the control plants, suggesting a possible antioxidant function for NH4 +. In the control plants, Mn-SOD and Cu/Zn-SOD activity remained unaffected after both 2 h and 14 days of salt stress. Similar results were observed for the N-NH4 + plants after 2 h salt treatment for both of the SOD isoforms (Fig. 2a, b). However, SOD activity levels in the N-NH4+ salt-treated plants were significantly lower than in salt-treated control plants at 2 h. Interestingly, after 14 days of salt stress, the N-NH4 + plants displayed increased Mn-SOD and Cu/Zn-SOD activities, but these increases were not observed in the salt-treated control plants.
Effect of NH4 + treatment on Mn-SOD (a) and Cu, Zn-SOD (b) in the Carrizo citrange plants treated with NaCl (90 mM) for 2 h and 14 days. Data show the average values of three independent experiments from a pool of 30 plants per experiment ± SE (n = 30). Letters indicates statistically significant differences between treatments within each time points (p < 0.05)
Another antioxidant enzyme is catalase (CAT), which plays an important role as an H2O2 scavenger. To determine how NH4 + nutrition affects CAT activity, leaf samples were analysed for the control and N-NH4 + plants (Fig. 3a). In the absence of salt, significant differences between treatments were noted: the N-NH4 + plants showed higher CAT activity than the control plants. After 14 days, N-NH4 + plants exhibited a 23 % increase in CAT activity in response to salt stress; however, no such increase was observed in the control plants upon salt stress.
Effect of NH4 + treatment on CAT (a) and GR (b) activities in the Carrizo citrange plants treated with NaCl (90 mM) for 2 h and 14 days. Data show the average of three independent experiments from a pool of 30 plants per experiment ± SE (n = 30). Letters indicates statistically significant differences between treatments within each time points (p < 0.05)
We also observed that NH4 + treatment induced a strong inhibition of glutathione reductase (GR) activity compared with the control plants (Fig. 3b). Under control conditions, plants did not show significant variation in the GR activity after 2 h of NaCl exposure. However, NH4 + treatment significantly increased the GR activity after 2 h of salt addition. Interestingly, after 14 days of salt treatment, control plants showed a significant decrease in the GR activity, while the N-NH4 +salt-treated plants were able to increase GR activity 56 % when compared to the salt-treated control plants. N-NH4 + plants are capable of increasing GR activity in presence of salt stress, although the initial activity was much lower than the control plants.
NH4 + nutrition induces changes in glutathione metabolism
To evaluate the contributions of antioxidant metabolites on NH4 + salt mediated-resistance, glutathione content was evaluated (Fig. 4). In the absence of salt, N-NH4 + plants displayed higher GSH and GSSG basal content without significant changes in the GSSG oxidation state. At 14 days after salt stress, we observed that GSH and GSSG content significantly decreased in both control and t N-NH4 + plants. However, N-NH4 + plants displayed higher GSH content if compared with control salt-treated plants (Fig. 4a). Moreover, GSSG oxidation state was higher in control plants after 14 days of salinity stress (Fig. 4b).
Effect of NH4 + treatment on GSH content in the Carrizo citrange plants treated with NaCl (90 mM) for 2 h and 14 days. a GSH (white bars) and GSSG content (black bars). b GSSG oxidation state [100 GSSG/(GSH + GSSG)]. Data show the average of three independent experiments from a pool of 30 plants per experiment ± SE (n = 30). Different letters indicates statistically significant differences in GSH content between treatments within each time points (p < 0.05). Different italic letters indicates statistically significant differences in GSSG content between treatments within each time points (p < 0.05)
These results point to the fact that the oxidised cellular environment displayed by control plants upon salinity stress may be due to their inability to synthesize GSH upon stress situation. Nevertheless, the GSSG oxidation state remained unaffected in the presence of salt stress in N-NH4 + plants (Fig. 4b), probably due to the significant increase in GSH content (Fig. 4a) that may attenuate NaCl cell disturbances.
NH4 + nutrition induces oxidative stress-related gene expression in salt response
To establish whether NH4 + nutrition affects the oxidative stress-related gene expression, the gene expression of glutathione-S-transferase (GST), the phospholipid hydroperoxide glutathione peroxidase (PHGPx) and ascorbate peroxidase (APX2) were analysed by RTq-PCR as markers of oxidative stress (Fig. 5). GST mRNA accumulation did not differ between the control and the N-NH4 +-treated plants either before or after salt treatment (Fig. 5a). However, GST mRNA accumulation was induced by salinity after 14 days in both treatments, and the highest GST mRNA accumulation was observed in the N-NH4 +-treated plants. We also checked PHGPx gene expression and observed that NaCl induced PHGPx accumulation after 2 h in the control plants. Although the PHGPx gene expression was unaffected in the absence of salt, NH4 + treatment significantly enhanced this expression after 14 days of salt stress (Fig. 5B). We also analysed APX2 gene expression (Fig. 5c). NH4 + treatment induced APX2 mRNA accumulation in the absence of salt stress, especially transcript accumulation after 14 days. NaCl treatment also induced APX2 mRNA accumulation in the control plants. However, the combination of both treatments, N-NH4 +-NaCl plants, exhibited a strong reduction in mRNA accumulation upon salinity. In summary, the largest differences in oxidative stress gene markers were observed after long-term salinity treatment, in which NH4 + nutrition significantly increased both GST and PHGPx gene expression and reduced APX gene expression. The obtained results for glutathione metabolism gene expression also correlated with higher GR activity and the maintenance of the GSSG oxidised state observed in the N-NH4 + salt-treated plants compared with the salt-treated control plants.
Effect of NH4 + treatment on the gene expression in Carrizo citrange plants upon salt stress. Total RNA was isolated from leaves at 2 h and 14 days after the addition of NaCl (90 mM) and was converted into cDNA and subjected to a RT-qPCR analysis. The results were normalised to the GAPDH gene expression measured in the same samples. The relative level of a GST, b PHGPx and c APX2 genes expression were analysed in the control and the N-NH4 + plants. The data show the average of three independent experiments obtained with a pool of 30 plants per point ± SE (n = 30). Letters indicates statistically significant differences between treatments within each time points (p < 0.05)
Discussion
In this study, the role of NH4 + nutrition on Carrizo citrange responses to NaCl-induced oxidative stress has been analysed. In a previous work, we found a differential response to salt stress between control and N-NH4 +-treated plants, which displayed strong salt resistance probably mediated by high levels of H2O2, reduced Cl− uptake, putrescine and proline content observed in N-NH4 + salt-treated plants. NH4 + treatment triggers mild chronic stress in Carrizo citrange plants which leads to the acclimation stage and to a better adaptation to subsequent salt stress (Fernández-Crespo et al. 2012). To gain further insight into other biochemical mechanisms related to NH4 +-mediated salt resistance in Carrizo citrange plants and its relationship with acclimation stage mediated by H2O2 accumulation, we studied the influence of this treatment on antioxidant enzymatic activities and metabolites involved in control of cellular redox stage. ROS have been proposed to be a central component of plant adaptation to both biotic and abiotic stresses. We show that both the control and N-NH4 +-treated Carrizo citrange plants display considerably increased H2O2 accumulation 2 h after salinity stress. It is noteworthy that the initial H2O2 levels were higher in the N-NH4 +-treated plants than in the control ones, supporting the idea that H2O2 acts as a stress signal in the N-NH4 +-treated plants. An increase in H2O2 content has been well-studied in acclimated plants, and exogenous H2O2 significantly increases the tolerance against salt stress mediated by induction of antioxidant defences (Gondim et al. 2012). Tanou et al. (2009) demonstrated that pre-exposure to H2O2 induces acclimation to subsequent treatment with NaCl in citrus plants. In our experimental procedure system, NH4 + treatment may acts as a mild stressor that induces H2O2 accumulation and leads the plants to the acclimation stage. However, at 14 days after salt stress NH4 + plants displayed lower H2O2 steady-state levels than control plants, probably due to a proper activation of the antioxidant machinery. These results are in concordance with the ones obtained by Hessini et al. (2013), who demonstrated that NH4 + nutrition benefits halophyte plants subjected to salt stress due to the efficiently activation of different antioxidants enzymes, resulting in physiologically acceptable levels of H2O2 in the leaves. To counteract the negative effect of H2O2 steady-state levels accumulated in stress situation the plants activate antioxidant cellular machinery. In this work, we observed that NH4 + nutrition provokes an inhibition of the two isoforms activity especially of Mn-SOD activity. This inhibitory effect was probably due to the mild stress induced by NH4 + treatment. These data are supported by the findings of Rodríguez-Serrano et al. (2009), who observed that the down-regulation of Mn-SOD and Cu/Zn-SOD in Cd stress conditions resulted in the overproduction of the H2O2 and O2− in Pisum sativum. However, N-NH4 + treated plants were able to increase the SOD activity upon salt stress. The up-regulation of SODs is implicated in combating oxidative stress caused by biotic and abiotic stress and plays a critical role in the survival of plants in stressful environments. Several studies have suggested that both the overexpression of Mn-SOD in transgenic Arabidopsis plants and Cu/Zn-SOD overexpression in transformed tomato plants produced increased salt tolerance (Wang et al. 2004, 2007). Catalases are the principal scavenging enzymes which can directly decompose H2O2 and are indispensable for ROS detoxification during stress (van Breusegem et al. 2001). In the present study, we show that salt-treated N-NH4 + plants exhibited an increase in CAT activity compared to the control plants. In previous work, we demonstrated that the salicylic acid (SA) accumulation occurred after 14 days of salt stress in the control plants, but not in the N-NH4 + plants (Fernández-Crespo et al. 2012). Several studies have suggested that SA binds directly to the catalase enzymes inhibiting its activity in several plants species (Sanchez-Casas and Klessig 1994; Horvath et al. 2002). These results support the ones obtained for CAT activity in our experimental system since control plants were unable to increase CAT activity after 14 days of salt stress; while NH4 + treatment leads to the correct functioning of the CAT antioxidant enzyme, which could avoid the injuries of ROS accumulation. For this reason, we conclude that the salinity tolerance induced by NH4 + nutrition may be partially due to the increase in SOD and CAT activities, allowing plants to resist the oxidative damage induced by salt. GR catalyses the reduction of GSH, molecule involved in many metabolic regulatory and antioxidant processes in plants. GR and GSH together play a crucial role in determining the tolerance of a plant for various stress situations (Chalapathi Rao and Reddy 2008). In the present work we observed that NH4 + treatment inhibited GR activity. The strong inhibition of GR activity might be caused by the toxic effect of NH4 + treatment on Carrizo citrange plants. Another study showed that GR activity was inhibited in Phaseolus vulgaris primary leaves after cooper accumulation, resulting in a shift from GSH towards its oxidised form GSSG (Cuypers et al. 2000). In despite of GR activity reduction by NH4 + treatment, we show that GR activity is enhanced in these plants after salt stress when compared with control plants after 2 h and 14 days. This correlation between GR activity and higher tolerance to salt stress under N-NH4 + nutrition is consistent with data from several crops where increases in GR activity during salt stress have been reported (Gueta-Dahan et al. 1997; Sumithra et al. 2006) and also over-expression of GR in plants which leads to increased resistance to oxidative stress (Kocsy et al. 2001).
Collectively, NH4 + nutrition induces a strong inhibition of the SOD and GR activities likely due the NH4 + toxic effect which also induces H2O2 accumulation. This increase in circulating peroxide could act as a trigger to signalling defence pathways and leads to Carrizo citrange plants to a better adapt to subsequent salt stress. Indeed, after 14 days of salt stress, N-NH4 + salt-treated plants that more efficiently activated the antioxidant machinery showed a higher capacity for ROS-scavenging compared with the control plants, demonstrating beneficial effect of NH4 + nutrition in citrus plants upon salinity conditions.
We also investigated the effect of NH4 + nutrition on the other components of glutathione pathway. In the control plants, we observed an increase of the GSSG oxidation state as consequence of salinity. However, in N-NH4 +-treated plants, the GSSG oxidation state remained unaffected by salt stress due to a significant increase in the total glutathione and GSH contents that allows for minimised NaCl-induced cell disturbances. Mittova et al. (2003) demonstrated that salt tolerance is linked to the ability to upregulate enzymes for glutathione synthesis and this activity is absent from the salt-sensitive species. Higher concentrations of glutathione would confer better antioxidative protection and would be considered to indicate acclimation. We further investigated the effect of NH4 + on GST gene expression, which have an important role in different detoxification process (Dixon et al. 2010). We noted that N-NH4 + nutrition in Carrizo citrange plants induced higher levels of GST mRNA accumulation after 14 days of salinity than in the control plants. It has also been found that GST overexpression enhances plant tolerance to various abiotic stresses (Gill and Tuteja 2010). Transgenic rice seedlings that co-express both GST and CAT displayed a greater increase in CAT and SOD activity and also showed markedly enhanced tolerance to salt stress compared with non-transgenic controls upon 200 mM NaCl treatment in a greenhouse (Zhao and Zhang 2006). Our results suggest that the higher GR activity, GST expression and GSH accumulation in the salt-treated N-NH4 + plants leads to an increase in the antioxidant capacity through the induction of a more reduced glutathione environment that minimises disturbances to the glutathione redox-based system.
In addition, we also investigated the effect of NH4 + nutrition on the expression of other oxidative marker genes, as PHGPx and APX2 involved in the oxidative stress response. Although APX is an important enzyme for removing intracellular H2O2, little is known about the role of APX2 gene expression in abiotic stress tolerance. In our experimental conditions, we found that NH4 + nutrition induces strong APX2 mRNA accumulation in the absence of salt. However, APX2 was strongly induced in control, but not in N-NH4 + plants 14 days after salt stress. Previous results in tobacco plants suggest that under stress conditions, APX was completely inactivated and that high CAT activity reduced H2O2 instead of APX in the chloroplasts (Shikanai et al. 1998). Thus, we can conclude that the higher CAT and SOD activities in N-NH4 + salt-treated plants seem to be enough to reduce the H2O2 steady levels without a concurrent induction of APX2. This fact would reveal a secondary role of APX2 activation in the NH4 + induced resistance against salinity. In this work, we also discovered that NH4 + treatment led to a greater induction of the PHGPx gene after 14 days of salt treatment. Chen et al. (2004) demonstrated that plant PHGPx genes that are expressed under various stresses may function as cytoprotectors against oxidative damage and play an important role in scavenging ROS such as lipid hydroperoxides (Chun-Juan et al. 2009).
In summary, we have provided evidence for several mechanisms underlying the resistance to salinity mediated by NH4 + treatment in Carrizo citrange plants, and also biochemical support for our previous hypothesis that NH4 + treatments would induce a mild stress condition that primes defence response system by stress imprinting, then conferring protection against subsequent salt stress. Moreover, we showed that NH4 +-induced acclimation stage significantly improves salinity tolerance and this enhancement of resistance was associated with a higher H2O2 basal content. Such evidences come from the analysis of the enzymatic and the non-enzymatic ROS scavenging machinery in both control and N-NH4 +-treated plants upon exposure to salinity. In the absence of salt, we observed higher levels of H2O2, stronger inhibition of the SOD and GR activities and higher GSSG content in the N-NH4 + plants than in the control plants. At early time points, 2 h after salt treatment, both the control and N-NH4 + treated plants displayed few changes on activation of antioxidant machinery against salinity stress. However, upon exposure for 14 days to salinity, N-NH4 +-treated plants showed a reduction in the H2O2 levels, increased enzymatic antioxidant activity and the induction of the GST and PHGPX genes. Moreover, N-NH4 + plants were able to maintain high levels of the GSH form upon exposure to salinity, which provided the cells with the ability to counteract the negative effects of oxidative stress syndrome. Together, these results reveals that sublethal concentrations of NH4 + can act as mild oxidative stressors that trigger the antioxidant cellular machinery thus providing resistance to subsequent salt stress.
Author contribution
All authors, except R. Gómez-Pastor R. are members of the Biochemistry and Biotechnology UJI. P. García-Agustín is the head of the group. E. Fernández-Crespo performed most of the experiments and analysing the results with the technical support of G. Camañes who participated in drafting the manuscript as well. qPCR procedures were developed by L. Scalschi and DAB staining and analysis of the photographs was performed with the E. Llorens contribution.
Abbreviations
- APX :
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DAB:
-
Diaminobenzidine
- FW:
-
Fresh weight
- GAPDH :
-
Glyceraldehyde-3-phosphate dehydrogenase
- GR:
-
Glutathione reductase
- GST :
-
Glutathione-S-transferase
- PHGPx :
-
Phospholipid hydroperoxide glutathione peroxidase
- ROS:
-
Reactive oxygen species
- SIMRs:
-
Stress induced morphogenetic response
- SOD:
-
Superoxide dismutase
References
Al-Yassin A (2005) Adverse effects of salinity on citrus. Int J Agri Biol 7:668–680
Arc E, Galland M, Cueff G, Godin B, Lounifi I, Job D et al (2011) Reboot the system thanks to protein post-translational modifications and proteome diversity: how quiescent seeds restart their metabolism to prepare seedling establishment. Proteomics 11:1606–1618
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 41:391–396
Britto DT, Kronzucker H (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Bruce JA, Matthes MC, Napier JA, Pickett JA (2007) Stressful ‘memories’ of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Camañes G, Cerezo M, Primo-Millo E, Gojon A, Garcia-Agustin P (2009) Ammonium transport and CitAMT1 expression are regulated by N in citrus plants. Planta 229:331–342
Chalapathi Rao ASV, Reddy AR (2008) Glutathione reductase: a putative redox regulatory system in plant cells.In: N.A. Khan, S. Singh, S. Umar (Eds) Sulfur assimilation and abiotic stresses in plants, Springer, The Netherlands, pp 111–147
Chen S, Vaghchhipawala Z, Li W, Asard H, Dickman MB (2004) Tomato phospholipid hydroperoxide glutathione peroxidase inhibits cell death induced by Bax and oxidative stresses in yeast and plants. Plant Physiol 135:1630–1641
Chun-Juan D, Xiao-Dong Y, Jin-Yuan L (2009) Enzymatic properties of a recombinant phospholipid hydroperoxide glutathione peroxidase from Momordica charantia and its complementation function in yeast. Biochemistry 74:620–628
Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse C, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19:1062–1071
Cuypers A, Vangronsveld J, Clijsters H (2000) Biphasic effect of copper on the ascorbate-glutathione pathway in primary leaves of Phaseolus vulgaris seedlings during the early stages of metal assimilation. Physiol Plant 110:512–517
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338–350
Fernández-Crespo E, Camañes G, Garcia-Agustin P (2012) Ammonium enhances resistance to salinity stress in citrus plants. J Plant Physiol 169:1183–1191
Filippou P, Antoniou C, Fotopoulos V (2011) Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal Behav 6:270–277
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell Environ 28:1056–1071
Galis I, Gaquerel E, Pandey SP, Baldwin IT (2009) Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant, Cell Environ 38:617–627
Gamero-Sandemetrio E, Gómez-Pastor R, Matallana E (2013) Zymogram profiling of superoxide dismutase and catalase activities allows Saccharomyces and non-Saccharomyces species differentiation and correlates to their fermentation performance. Appl Microbiol Biotechnol 97:4563–4576
Gerendás J, Zhu Z, Bendixen R, Ratcliffe RG, Sattelmacher B (1997) Physiological and biochemical processes related to ammonium toxicity in higher plants. Z Pflanzernähr Bodenkd 160:239–251
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gondim FA, Gomes-Filho E, Costa JH, Mendes Alencar NL, Prisco JT (2012) Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol Biochem 56:62–71
Gould KS, Lister C (2006) Flavonoid functions in plants. In: Andersen Oslash M, Markham KR (eds) Flavonoids: chemistry, biochemistry, and applications, CRC Press, Boca Raton, pp 397–441
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Ann Biochem 106:207–212
Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G (1997) Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta 203:460–469
Hessini K, Cruz C, Gandour M, Debez A, Koyro H-W, Huchzermeyer B, Abdelly C (2011) Ammonium nutrition improves salt tolerance of Spartina alterniflora. Eur J Plant Sci Biotechnol 5:33–36
Hessini K, Hamed KB, Gandour M, Mejri M, Abdelly C, Cruz C (2013) Ammonium nutrition in the halophyte Spartina alterniflora under salt stress: evidence for a priming effect of ammonium? Plant Soil 370:163–173
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agr Expt Sta Circ 347:1–32
Horvath E, Janda T, Szalai G, Paldi E (2002) In vitro salicylic acid inhibition of catalase activity in maize: differences between the isozymes and a possible role in the induction of chilling tolerance. Plant Sci 163:1129–1135
Iannone MF, Rosales EP, Groppa MD, Benavides MP (2012) Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf discs exposed to paraquat. Biol Trace Elem Res 146:246–255
Ievinsh G (2006) Biological basis of biological diversity: physiological adaptations of plants to heterogeneous habitats along a sea coast. Acta Universitatis Latviensis 710:53–79
Jakubowski W, Bilinski T, Bartosz G (2000) Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic Biol Med 28:659–664
Kant S, Kant P, Lips H, Barak S (2007) Partial substitution of NO3 − by NH4 + fertilization increases ammonium assimilating enzyme activities and reduces the deleterious effects of salinity on the growth of barely. J Plant Physiol 164:303–311
Khanna-Chopra R, Selote DS (2007) Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than -susceptible wheat cultivar under field conditions. Environ Exp Bot 60:276–283
Kocsy G, Galiba G, Brunold C (2001) Role of glutathione in adaptation and signaling during chilling and cold acclimation in plants. Physiol Plant 113:158–164
Kudo N, Fujiyama H (2010) Responses of Halophyte Salicornia bigelovii to different forms of nitrogen source. Pedosphere 20(3):311–317
Lasa B, Frechilla S, Lamsfus C, Aparicio-Tejo PM (2001) The sensitivity to ammonium nutrition is related to nitrogen accumulation. Sci Hort 91:143–152
Marschner P (ed) (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier/Academic Press, Amsterdam
Misra N, Gupta AK (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J Plant Physiol 163:11–18
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K et al (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mittova V, Theodoulou FL, Kiddle G, Gómez L, Volokita M, Tal M, Foyer CH, Guy M (2003) Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett 554:417–421
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55:1105–1113
Munns R, Tester M (2008) Mechanism of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murshed R, Lopez-Lauri F, Sallanon H (2008) Microplate quantification of enzymes of the plant ascorbate-glutathione cycle. Anal Biochem 383:320–322
Potters G, Pasternak TP, Guisez Y, Jansen MAK (2009) Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell Environ 32:158–169
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Ravindran KC, Venkatesa K, Balakrishan V, Chellappan KP, Balasubramanian T (2007) Restoration of saline land by halophytes for Indian soils. Soil Biol Biochem 39:2661–2664
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Río LA et al (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Sanchez-Casas P, Klessig DF (1994) A salicylic acid-binding activity and a salicylic acid-inhibitable catalase activity are present in a variety of plant species. Plant Physiol 106:1675–1679
Shikanai T, Takeda T, Yamauchi H, Sano S, Tomizawa K, Yokota A, Shigeoka S (1998) Inhibition of ascorbate peroxidase under oxidative stress in tobacco having bacterial catalase in chloroplasts. FEBS Lett 428:47–51
Stepien P, Klobus G (2005) Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant 125:31–40
Sumithra K, Jutur PP, Carmel BD, Reddy AR (2006) Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul 50:11–22
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G et al (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60:795–804
Thordal-Christensen H, Zang Z, Wei Y, Colling DB (1997) Subcellular localisation of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
van Breusegem F, Vranová E, Dat JF, Inzé D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Wang Y, Ying Y, Chen J, Wang X (2004) Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci 167:671–677
Wang Y, Wisniewski M, Meilan R, Uratsu SL, Cui MG, Dandekar A, Fuchigami L (2007) Ectopic expression of Mn-SOD in Lycopersicon esculentum leads to enhanced tolerance to salt and oxidative stress. J App Hort 9:3–8
Xia X-J, Wang Y-J, Zhou Y-H, Tao Y, Mao W-H, Shi K et al (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Zhao F, Zhang H (2006) Salt and paraquat stress tolerance results from coexpression of the Suaeda salsa glutathione S-transferase and catalase in transgenic rice. Plant Cell Tiss Org 86:349–358
Zhou J, Wang J, Shi K, Xia XJ, Zhou YH, Yu JQ (2012) Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol Biochem 60:141–149
Acknowledgments
This work was supported by Prometeo 2012/066. Loredana Scalschi is the recipient of a PhD fellowship from the Ministerio de Educación (Grant AP2008-01064). Eugenio Llorens is the recipient of a PhD fellowship from Pla de promoció de la investigació de la Universitat Jaume I (Ajudes predoctorals ref 2009/24). We thank Emilia Matallana (UV) for her corrections and suggestions.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Wang.
Rights and permissions
About this article
Cite this article
Fernández-Crespo, E., Gómez-Pastor, R., Scalschi, L. et al. NH4 + induces antioxidant cellular machinery and provides resistance to salt stress in citrus plants. Trees 28, 1693–1704 (2014). https://doi.org/10.1007/s00468-014-1078-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1078-y