Abstract
European pear is a flooding-sensitive species, and for its cultivation in lowland areas, it is necessary to carry out the grafting of scions of commercial pear varieties into rootstocks belonging to flooding-tolerant wild pear species. Flooding tolerance of Pyrus boissieriana—a type of wild pear—was studied as a promissory rootstock for commercial pear. For this purpose, 3-month-old plants of P. boissieriana were subjected for 30 days to control (C), well-irrigated treatment, short-term (15 days) flooding plus 15 days recovery (F + R) and long-term (30 days) continuous flooding (F). Physiological performance, plant morphological changes and biomass accumulation were assessed. Results showed that, although stomatal conductance, transpiration and photosynthesis were progressively decreased by flooding, when flooding was short term (i.e., 2 weeks, F + R treatment) plants were able to adequately recover their physiological activity (50–74 % with respect to controls). In contrast, when plants continued to be flooded (F treatment), the physiological activity became null and the plants died quickly after the water subsided. Adventitious rooting was the most conspicuous registered morphological response to flooding, despite that flooded plants had shorter shoots and roots than control plants. Leaf and root biomass were 63 and 89 % higher under short-term flooding (F + R) than under continuous flooding (F), condition in which plants did not survive. In conclusion, P. boissieriana appears to be a promising species for its use as rootstock of commercial pear in lowland areas prone to flooding of up to 2 weeks. However, if the flooding period is extended, plants of this species are at risk of perishing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The frequency and magnitude of flooding events is on the rise worldwide, a trend that is connected to human-induced greenhouse gas emissions (Arnell and Liu 2001; see also http://www.ippc.ch). Some horticultural crops—including the commercial European pear (Pyrus communis L.) varieties—are extremely vulnerable to the eventual occurrence of floods (Robbani et al. 2006; Schaffer et al. 2006). A strategy useful to cultivate such fruit tree species in lowland areas prone to suffering waterlogging seems to be grafting scions of commercial varieties into rootstocks belonging to flooding-tolerant wild relative species (Domingo et al. 2002; Lombard and Westwood 1987). Until now, some wild pears species—like P. calleryana and P. betulaefolia—have been successfully used by farmers as rootstock for commercial pear varieties cultivation in difficult sites subjected to drought, cold and/or salt stresses (Sisko et al. 2009; Tamura 2012). However, very few studies, apart from those by Robbani et al. (2006), have assessed the flooding tolerance of wild pear species to soil waterlogging. The Hyrcanian forest to the north of Iran is an important source of natural germplasm of Pyrus boissieriana Buhse (Vavilov 1994), a type of wild pear that occasionally experiences flooding episodes.
Flooding induces a sequence of changes in plants that could include alterations of physiological processes along with morphological adjustments related to their survival and growth under anaerobic conditions (Colmer and Voesenek 2009; Sairam et al. 2008; Striker et al. 2005, 2008, 2012). At the physiological level, flooding modifies the plant’s water relations and carbon fixation. Closing of stomata, reduction of transpiration and inhibition of photosynthesis are common responses that can occur for hours or days, depending on the tolerance to flooding of the species (Kozlowski and Pallardy 1984; Ashraf and Arfan 2005; Islam and MacDonald 2004; Núñez-Elisea et al. 1999; Ortuño et al. 2007; Striker et al. 2005). However, the reduction in physiological activity in flood-tolerant species is reverted when flooding subsides (Striker 2012a). So, the assessment of plant recovery when water subsides, an issue often overlooked in flooding experiments, is important when evaluating real flooding tolerance (Malik et al. 2002; Striker 2008, 2012b). In addition, flooding duration greatly influences plant tolerance to soil anaerobiosis (Armstrong 1979; Colmer and Voesenek 2009; Mukassabi et al. 2012).
Plant morphology is altered as a consequence of flooding stress. Flooded woody saplings/plants have short stems as a result of the negative impact of anaerobiosis on their growth (de Oliveira and Joly 2010; Kozlowski 1984, 1997), in contrast to the high plasticity for shoot elongation observed in flooding-tolerant herbaceous plants (Chen et al. 2011; Imaz et al. 2012; Manzur et al. 2009). In addition, the lack of oxygen in the soil may constrain root aeration and, in consequence, reduce root elongation, leading to decreased root growth when plants grow under flooding conditions (Colmer 2003; Colmer and Voesenek 2009). Adventitious rooting is one of the most conspicuous responses of plants tolerant to flooding (Jackson 2008; Kozlowski 1997; Larson et al. 1991; Vidoz et al. 2010). Adventitious roots help plants to continue with water and nutrient uptake under flooding conditions, replacing in some way the functions of the original root system (Kozlowski and Pallardy 1984).
The objective of this paper was to evaluate the tolerance to flooding of P. boissieriana by identifying the morphological and physiological responses conferring its potential success as rootstock of commercial pear. For this purpose, 3-month-old plants of P. boissieriana were subjected to well-irrigated (control) and two flooding treatments of different durations: 15 and 30 days. Variables of plant biomass accumulation, shoot and root length, adventitious rooting, carbon fixation, stomatal conductance and leaf transpiration were assessed.
Materials and methods
Plant material and experimental design
Pyrus boissieriana Buhse is a species of wild pear endemic to northern Iran and Turkmenistan (Zamani and Attar 2010). Traditionally, this species has been used for medicinal purposes as the extracts from its leaves have antioxidant, antihyperglycemic and diuretic properties (Shahaboddin et al. 2011). Nevertheless, considering the tolerance of this species to abiotic stress, such as flooding, its potential as rootstock for commercial pear has never been addressed.
Seeds of P. boissieriana were collected from distantly spaced trees in a forest ecosystem at Jozak-Darkesh, northeastern Iran (36°35′N, 52°02′E) during autumn 2011. The collected seeds were buried in black nylon pots filled with sand and then stratified at 0–4 °C for a month (cold stratification) under laboratory condition. This procedure helped to alleviate seed primary dormancy by simulating the natural cold temperatures experienced by seeds during winter in their natural environment. After this, the germinated seeds were transplanted to 4 L plastic pots filled with sand and topsoil (1:1 v/v) from the forest where the seeds were collected and transferred to the experimental garden at the Faculty of Natural Resources and Marine Sciences (Tarbiat Modares University). Seedlings were irrigated twice a week to field capacity and allowed to grow for 3 months under natural irradiance and photoperiod. After this period, plants presented with 12 ± 1.9 cm height and 3.3 ± 0.5 mm diameter (at 1 cm above soil surface for the main shoot).
Flooding treatments
Three treatments were applied to 3-month-old plants for 30 days, following a fully randomized design with four to five homogeneous replicates: (1) control—watered twice a week to field capacity; (2) flooding plus recovery (F + R)—water level maintained 3 cm above the soil surface during 15 days, and allowing plants to grow during a subsequent period of 15 days in well-drained (irrigated as controls) conditions, to assess their recovery; and (3) continuous flooding (F)—plants were flooded as in (2) during all the experimental period. Both flooding treatments represented short- and long-term flooding, which can occur naturally. Flood water was replaced once a week, and root zone temperature was checked to ensure that this parameter was similar between treatments. The experiment ran during early summer (late May to June) concurring with the start of the growing season of this species.
Physiological measurements
Net photosynthesis rate (A), stomatal conductance (g s ) and leaf transpiration rate (E) were measured in two–three expanded young leaves (above water level) per plant located in a similar plant position, using a portable infrared gas analyzer (Model LCpro+, ADC BioScientific Ltd., Hertfordshire, UK). Measurements were performed after 3, 7, 15 and 30 days from the beginning of treatments on the same plants, on clear days, approximately at midday (PPFD > 1,200 μmol m−2 s−1). At the same time, air temperature and relative humidity were monitored and used to calculate the air vapor pressure deficit (VPDair), which varied between 1.5 and 2.9 kPa (mean of 2.0 kPa) (Fig. 1a). In the 30 days’ duration flooding treatment, plants were allowed to recover. However, all of them wilted and died during the first week after flooding ceased, so it was not possible to continue with physiological measurements.
a Air vapor pressure deficit (VPDair), b net photosynthesis rate (A), c stomatal conductance (g s) and d transpiration rate (E) of 3-month-old plants of Pyrus boissieriana (wild pear) subjected to control conditions for 30 days (C, open squares), 15 days of flooding followed by 15 days at control conditions (F + R, half–closed squares), and to continuous flooding for 30 days (F, closed squares). Different letters indicate significant differences (p < 0.05) among treatments until the day of measurement based on the Tukey test. Values are mean ± SE of five replicates (four replicates for net photosynthesis in the F treatment on the last measurement date)
Morphological and plant biomass measurements
Shoot length and maximum root length were carefully measured at the end of the experiment using a ruler. The number of adventitious roots per plant was counted; they were easily differentiated from seminal roots, as they had white color and spongy appearance and grew from the junction between the shoot and the root (Kozlowski 1997; Striker 2012a, 2012b). The area per leaf was estimated from the average of the five expanded leaves located on the upper position of the plants (as in Li et al. 2011). The area of each leaf was determined from digital images of the same scanned leaves by using the software ImageTool 3.0 (Wilcox et al. 2002). Harvest was carried out at the end of the experiment when shoots were separated from roots. Shoots were also dissected into leaves and stems. Afterward, all plant materials were oven dried at 70 ºC for 3 days until of constant mass and then weighed.
Statistical analysis
Physiological parameters were analyzed by repeated measures analysis of variance (rmANOVA), with flooding treatment as the between-subject main effect and time as the within-subject factor (Von Ende 1993). Data sets were checked to satisfy the hypothesis of sphericity of the covariance matrices through Mauchly’s test (Von Ende 1993), as rmANOVA is particularly susceptible to the violation of this assumption. Contrasts between plants under the different treatments on individual dates were performed with Tukey tests. Morphological and biomass data were evaluated through one-way ANOVAs with subsequent Tukey tests. All statistical analyses were made with SPSS statistical package (version 16.0, SPSS Inc., Chicago, USA). All results are presented as untransformed means of five replicates ± standard error.
Results
Physiological responses as affected by flooding
Repeated measures ANOVA indicated significant flooding (treatment effect: P < 0.001) and time effects (p < 0.01) on net photosynthesis rate (A), stomatal conductance (g s) and transpiration rate (E). The time effect was related to variations in the air vapor pressure deficit—as a measure of the atmospheric evaporative demand—on each measurement date (Fig. 1a). The treatment–time interaction was significant (p < 0.001), showing that the negative effects of flooding increased over time (Fig. 1b–d). In this respect, decreases in stomatal conductance, and consequently in transpiration rate, were evident after 7 days of flooding (p < 0.001). From that date, stomata of flooded plants remained completely closed until flood water was released (see F + R treatment in Fig. 1c, d). Thus, in the continuous flooding treatment (F), flooded plants showed negligible stomatal conductance until day 30, while in the treatment in which flooding subsided on day 15 (F + R), plants were able to partially recover (i.e., 50 % with respect to control plants) their stomatal conductance and transpiration by the end of the experiment (Fig. 1c, d; p < 0.01). The net photosynthetic responses during the experiment were in line with the ones expected for stomatal behavior (Fig. 1b). Thus, carbon fixation of flooded plants dropped to half that of controls on day 7 and was almost null after 15 days of flooding, concurring with the full stomata closing registered on that day (p < 0.05 in all cases). This negative effect persisted until the end of the experiment in permanently flooded plants (F). Conversely, in the treatment where plants were allowed to grow under drained conditions after 15 days of flooding (F + R), net photosynthesis increased until it reached values of 74 % with respect to controls (p < 0.01). This evidences the ability of this species to partially recover its physiological activity after a flooding of up to 15 days (see day 30 in Fig. 1b). It should be mentioned that all plants subjected to the F treatment (30 days flooding duration) survived during the flooding period, but when allowed to recover all of them wilted and died during the first week after flooding.
Morphological changes and plant biomass accumulation in response to flooding
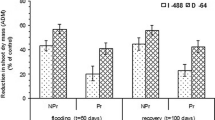
Plant morphology was altered as a consequence of flooding stress. In this respect, in both flooding treatments (F and F + R), plants registered ca. 23 % lower shoot length and ca. 23 % lower root maximum length in comparison to those attained by control plants (p < 0.05 in all cases; Fig. 2a, b). On the other hand, the leaf area of young expanded leaves was 42 % smaller with respect to controls under continuous flooding (F), whereas in the treatment where flooding was discontinued on day 15, and plants were allowed to recover (F + R), the reduction of this parameter was only 24 % (Fig. 2c). In addition, flooding triggered the generation of adventitious roots in this species (Fig. 2d). Plants that were grown under continuous flooding (F) attained 8.5 ± 1.3 adventitious roots per plant, while those allowed to recover after flooding (F + R) doubled the number of adventitious roots per plant (17.5 ± 2.5 adventitious roots; Fig. 2d; p < 0.01). As expected, non-flooded plants (i.e., control) did not develop adventitious roots throughout the experiment.
Morphological characteristics at the end of the experiment of 3-month-old plants of Pyrus boissieriana (wild pear) subjected to control conditions for 30 days (C), 15 days of flooding followed by 15 days at control conditions (F + R) and continuous flooding for 30 days (F). a Shoot length; b maximum root length; c area per leaf and d number of adventitious roots. Different letters indicate significant differences (p < 0.05) among treatments based on the Tukey test. Values are mean ± SE of five replicates
Total plant biomass was negatively affected by flooding treatments, but of a different magnitude (Fig. 3). In this regard, the total biomass of continuously flooded plants (F) was 63 % lower in comparison to that of controls (p < 0.001) as a result of a lower leaf (59 %) and root (74 %) biomass (p < 0.001 in both cases; Fig. 3). In contrast, under F + R treatment, the biomass accumulation of plants was less affected and they attained ca. 40 % lower biomass with respect to controls (p < 0.01), but 78 % higher total biomass than under continuous flooding (F; p < 0.05; Fig. 3). Such a lower impact on biomass gained by plants in the F + R treatment with respect to those subjected to continuous flooding (F) was the consequence of a higher leaf (63 %) and root (89 %) biomass. This better performance of plants under F + R treatments—when compared to permanently flooded plants—was in line with that expected from stomata reopening and the subsequent increases in net photosynthesis registered during the recovery phase (Fig. 1b–d). In all cases, stem biomass did not change among treatments (p = 0.49). Finally, although the harvested plant biomass on day 30 was entirely composed of living tissues, all extra plants under F treatment, which were allowed to recover, died during the first week after flooding.
Biomass of 3-month-old plants of Pyrus boissieriana (wild pear) at the end of the experiment after being subjected to control conditions for 30 days (C), 15 days to flooding followed by 15 days at control conditions for recovery (F + R) and continuous flooding for 30 days (F). Different letters indicate significant differences (p < 0.05) among treatments based on the Tukey test. Note the different scales for total biomass (right, Y axis) and plant compartments (left, Y axis). Values are mean ± SE of five replicates
Discussion
Stomata closing registered on flooded plants after 1 week of treatment was closely related to the reduction in leaf transpiration (Fig. 1c, d) and probably directed to maintain the water homeostasis of plants, as seen in Persea americana (Schaffer et al. 1992), Mangifera indica (Larson et al. 1989) and citrus spp. (Ortuño et al. 2007; Vu and Yelenosky 1991). Importantly, stomatal closure was effective in reducing water loss (75 % lower than controls), but without provoking a proportional reduction of carbon fixation (40 % lower than controls), which dropped to its minimal values a week after full stomata closure (compare Fig. 1b and Fig. 1c, d). These results differed from the behavior observed in citrus and Annona spp. rootstocks, which decreased their photosynthesis within 24–48 h of flooding (Núñez-Elisea et al. 1999; Ojeda et al. 2004; Phung and Knipling 1976; Schaffer et al. 2006), thereby suggesting that P. boissieriana was relatively tolerant to flooding during the stress period per se.
Monitoring physiological responses during the recovery period after flooding is crucial when assessing flooding tolerance (Ortuño et al. 2007; Striker 2008; 2012b). It is known that, in flood-tolerant plants, when the flood waters drain away, the stomata reopen and the rate of photosynthesis increases (Davies and Flore 1986; Kozlowski 1997; Larson et al. 1989; Schaffer et al. 2006); however, the capacity for stomatal reopening varies among species and flooding duration. For instance, recovery of stomatal conductance to pre-flood values required 18 days for Vaccinium corymbosum (highbush blueberry), but more than 18 days for V. ashei (rabbiteye blueberry, Davies and Flore 1986). Likewise, the closed stomata of Carya illinoensis (pecan) reopened following an 8-day flooding period, but did not reopen after a 15-day flooding period (Smith and Ager 1988). In our experiment, P. boissieriana achieved a high recovery of photosynthesis and an intermediate recovery of stomatal conductance after 15 days of flooding, but not after 30 days of flooding, when plants died soon after water subsided (see day 30 in Fig. 1). This evidences the ability of this species to adapt to short-term flooding, viewed from its physiological responses during its recovery. On the other hand, when flooding was extended to 30 days, plants wilted and died during the first week of recovery, which indicated the intolerance of this species to prolonged flooding. This result could be related to an imbalance between the potentials for transpiration and water uptake, which can be inferred from a much affected root biomass with respect to shoot biomass (compare C and F treatments in Fig. 3) due to prolonged flooding, as seen previously in earlier studies (Nakai et al. 2009; Li et al. 2011).
Flooding stress altered plant morphology of wild pear as expected: flooded plants were shorter and with smaller leaves than controls (Fig. 2), but with numerous adventitious roots at the root–shoot junction helping to withstand the hypoxic conditions (Robbani et al. 2006). Such adventitious rooting is an essential response when considering this species as potential rootstocks for pear, given that in fruit trees the tolerance to waterlogging is mediated by the rootstock and not the scion (Domingo et al. 2002; Schaffer et al. 1992). In addition, it is known that the number of adventitious roots is positively correlated to flooding tolerance (Sairam et al. 2008; Vidoz et al. 2010; Yamamoto et al. 1995), and our results showed that plants subjected to long-term flooding (F) ended with only half the adventitious roots than those that grew under short-term flooding (F + R). This was because adventitious roots entered into senescence after 2 weeks of flooding. So, these results add further evidence to claim that this species tolerates only short-term flooding. The reason why adventitious roots are so important under flooding is that they replace the original root system in the uptake of water and nutrients during flooding (Colmer and Voesenek 2009; Kozlowski and Pallardy 1984; Sairam et al. 2008).
Biomass accumulation concurred with that expected from the morphophysiological responses discussed above. Short-term flooding reduced the aerial biomass as a result of a low mass of leaves but without affecting stem mass, which agrees with smaller leaves registered under flooding (Fig. 2c) as well as with findings of previous reports on other fruit species, like Mangifera indica (Larson et al. 1991) and Annona spp. (Fu et al. 2012). This is a reasonable response as leaf growth is a very sensitive parameter to stress by flooding (Kozlowski and Pallardy 1984). Importantly, as root biomass was in proportion affected similarly to shoots, the shoot to root ratio was quite similar between control and flooded (F + R) plants (1.63 vs. 1.52, respectively), which seems to be important to restore the water homeostasis of plants after the flood water drains (de Oliveira and Joly 2010; Striker 2012a). Furthermore, this sharply contrasts with the increased shoot to root ratio commonly observed in flood-sensitive woody plants as a result of root growth inhibition (Kozlowski 1984), a characteristic of flood-intolerant species, which P. boissieriana did not show under short-term flooding.
In conclusion, the results of this research indicate that P. boissieriana is a promising species to be tested as rootstock for pear cultivation in soils prone to experiencing flooding episodes of up to 2 weeks. This statement is supported by the important adventitious rooting generated during flooding and the high capacity to recover its photosynthesis and (to a lesser extent) stomatal conductance when water subsided, all these responses being distinctive of flooding-tolerant species (de Oliveira and Joly 2010; Kawase 1981; Larson et al. 1991; Robbani et al. 2006; Striker et al. 2005; Tamura 2012; Visser and Voesenek 2004). However, when flooding was experimentally extended up to 30 days, the physiological activity of permanently flooded plants became null, and during the first days after water release, all leaves wilted and the plants perished (Núñez-Elisea et al. 1999). Therefore, wild pear can tolerate short-term flooding—although its growth is reduced—but it does not survive extended flooding periods. So, its use as rootstock in sites prone to long-term waterlogging (>2 weeks) is not recommended. As a practical result, our findings also highlight the need for considering the expected flooding duration/regime of target sites when aiming at successful introduction of this species as potential rootstock for pear. Further experimental investigation on the tolerance of P. boissieriana as rootstock in combination with different commercial pear varieties as scions is desirable to deepen our knowledge on this exciting topic.
References
Armstrong W (1979) Aeration in higher plants. Adv Bot Res 7:225–332
Arnell N, Liu C (2001) Climatic Change 2001: hydrology and water resources. Report from the Intergovernmental Panel on Climate Change. http://www.ipcc.ch/ (Verified on September 7th 2012)
Ashraf M, Arfan M (2005) Gas exchange characteristics and water relations in two cultivars of Hibiscus esculentus under waterlogging. Biol Plant 49:459–462
Chen X, Visser EJW, de Kroon H, Pierik R, Voesenek LACJ, Huber H (2011) Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytol 190:409–420
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell Environ 26:17–36
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Func Plant Biol 36:665–681
Davies FS, Flore JA (1986) Flooding, gas exchange and hydraulic conductivity of highbush blueberry. Physiol Plant 67:545–551
De Oliveira VC, Joly CA (2010) Flooding tolerance of Calophyllum brasiliense Camb. (Clusiaceae): morphological, physiological and growth responses. Trees 24:185–193
Domingo R, Pérez–Pastor A, Ruiz–Sánchez MC (2002) Physiological responses of apricot plants grafted on two different rootstocks to flooding conditions. J Plant Physiol 159:725–732
Fu X, Peng S, Yang S, Chen Y, Zhang J, Mo W, Zhu J, Ye Y, Huang X (2012) Effects of flooding on grafted annona plants of different scion/rootstock combinations. Agric Sci 3:249–256
Imaz JA, Gimenez DO, Grimoldi AA, Striker GG (2012) The effects of submergence on anatomical, morphological and biomass allocation responses of tropical grasses Chloris gayana and Panicum coloratum at seedling stage. Crop Pasture Sci. doi:10.1071/CP12335
Islam MA, MacDonald SE (2004) Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees 18:35–42
Jackson MB (2008) Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot 101:229–248
Kawase M (1981) Anatomical and morphological adaptation of plants to waterlogging. HortScience 16:30–34
Kozlowski TT (1984) Responses of woody plants to flooding. In: Kozlowski TT (ed) Flooding and plant growth. Academic Press, Orlando, pp 129–163
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol. Monograph No. 1 www.heronpublishing.com/tp/monograph/kozlowski.pdp
Kozlowski TT, Pallardy SG (1984) Effects of flooding on water, carbohydrate and mineral relations. In: Kozlowski TT (ed) Flooding and plant growth. Academic Press, Orlando, pp 165–193
Larson KD, Schaffer B, Davies FS (1989) Flooding, leaf gas exchange and growth of mango in containers. J Am Soc Hortic Sci 116:156–160
Larson KD, Schaffer B, Davies FS (1991) Flooding, leaf gas exchange and growth of mango in containers. J Am Soc Hort Sci 116:156–160
Li H, Lei G, Zhi Y, Bridgewater P, Zhao L, Wang Y, Deng Z, Liu Y, Liu F, An S (2011) Phenotypic responses of Spartina anglica to duration of tidal immersion. Ecol Res 26:395–402
Lombard PB, Westwood MN (1987) Pear rootstocks. In: Rom RC, Carlson RF (eds) Rootstocks for fruit crops. New York, Wiley, pp 145–183
Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M (2002) Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol 153:225–236
Manzur ME, Grimoldi AA, Insausti P, Striker GG (2009) Escape from water or remain quiescent? Lotus tenuis changes its strategy depending on depth of submergence. Ann Bot 104:1163–1169
Mukassabi TA, Polwart A, Coleshaw T, Thomas PA (2012) How long can young Scots pine seedlings survive waterlogging? Trees 26:1641–1649
Nakai A, Yurugi Y, Kisanuki H (2009) Growth responses of Salix gracilistyla cuttings to a range of substrate moisture and oxygen availability. Ecol Res 24:1057–1065
Núñez-Elisea R, Schaffer B, Fisher J, Colls AM, Crane JH (1999) Influence of flooding on net CO2 assimilation, growth, and stem anatomy of Annona species. Ann Bot 74:771–780
Ojeda MG, Schaffer B, Davies FS (2004) Flooding, root temperature, physiology, and growth of two Annona species. Tree Physiol 24:1019–1025
Ortuño MF, Alarcón JJ, Nicolás E, Torrecillas A (2007) Water status indicators of lemon trees in response to flooding and recovery. Biol Plant 51:292–296
Phung HT, Knipling EB (1976) Photosynthesis and transpiration of citrus seedlings under flooded conditions. HortScience 11:131–133
Robbani M, Banno K, Kakegawa M (2006) Differential flooding tolerance of some dwarfing pear rootstock clones selected from the progenies of Pyrus betulaefolia and P. calleryana. J Japan Soc Hort Sci 75:297–305
Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC (2008) Physiology and biochemistry of waterlogging tolerance in plants. Biol Plant 52:401–412
Schaffer B, Andersen PC, Ploetz RC (1992) Responses of fruit crops to flooding. In: Janick J (ed) Horticultural reviews. Wiley, New York, pp 257–313
Schaffer B, Davies FS, Crane JH (2006) Responses of subtropical and tropical fruit trees to flooding in calcareous soil. HortScience 41:549–555
Shahaboddin ME, Pouramir M, Moghadamnia AA, Parsian H, Lakzaei M, Mir H (2011) Pyrus biossieriana Buhse leaf extract: an antioxidant, antihyperglycaemic and antihyperlipidemic agent. Food Chem 126:1730–1733
Sisko M, Javornik B, Siftar A, Ivancic A (2009) Genetic relationships among Slovenian pears assessed by molecular markers. J Am Soc Hort Sci 134:97–108
Smith MW, Ager PL (1988) Effects of soil flooding on leaf gas exchange of seedling pecan trees. HortScience 23:370–372
Striker GG (2008) Visiting the methodological aspects of flooding experiments: quantitative evidence from agricultural and ecophysiological studies. J Agron Crop Sci 194:249–255
Striker GG (2012a) Flooding stress on plants: anatomical, morphological and physiological responses. In: Mworia JK (ed) Botany. InTech, Rijeka, pp 3–28
Striker GG (2012b) Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants. Ecol Res 27:984–987
Striker GG, Insausti P, Grimoldi AA, Ploschuk EL, Vasellati V (2005) Physiological and anatomical basis of differential tolerance to soil flooding of Lotus corniculatus L. and Lotus glaber Mill. Plant Soil 276:301–311
Striker GG, Insausti P, Grimoldi AA (2008) Flooding effects on plant recovery from defoliation in the grass Paspalum dilatatum and the legume Lotus tenuis. Ann Bot 102:247–254
Striker GG, Izaguirre RF, Manzur ME, Grimoldi AA (2012) Different strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biol 14:50–55
Tamura F (2012) Recent advances in research on Japanese pear rootstocks. J Japan Soc Hort Sci 81:1–10
Vavilov NI (1994) Origin and geography of cultivated plants. D. Love (translator). Cambridge University Press, Cambridge
Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P (2010) Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J 63:551–562
Visser EJW, Voesenek LACJ (2004) Acclimation to soil flooding—sensing and signal-transduction. Plant Soil 254:197–214
Von Ende CN (1993) Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 113–137
Vu JCV, Yelenosky G (1991) Photosynthetic responses of citrus trees to soil flooding. Physiol Plant 81:7–14
Wilcox D, Dove B, Mc David D, Greer D (2002) Image Tool for Windows. Version 3.0. The University of Texas Health Science Center, San Antonio
Yamamoto F, Sakata S, Tenazawa K (1995) Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol 15:713–719
Zamani A, Attar F (2010) Pyrus longipedicellata sp. nov. (Rosaceae) from central Alborz, Iran. Nordic J Bot 28:484–486
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Adams.
Rights and permissions
About this article
Cite this article
Parad, G.A., Zarafshar, M., Striker, G.G. et al. Some physiological and morphological responses of Pyrus boissieriana to flooding. Trees 27, 1387–1393 (2013). https://doi.org/10.1007/s00468-013-0886-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-013-0886-9