Abstract
Genetic differences in individual-tree biomass partitioning, growth efficiency, and stem relative growth rate (RGR) could confer intraspecific productivity differences and might strongly influence forest ecosystem carbon storage. We examined the relationship between genotype productivity (stem volume), whole-tree biomass partitioning, growth efficiency (stem wood production per unit leaf area), and stem RGR among nine different loblolly pine (Pinus taeda L.) genotypes from three different genetic groups of contrasting inherent genetic homogeneity: three open-pollinated (half-sib) families, three mass-control pollinated (full-sib) families, and three clonal varieties. We hypothesized that genotype productivity would be positively associated with increased partitioning to stem wood relative to other plant parts, higher stem RGR, and enhanced growth efficiency. After 3 years under plantation conditions, genotypes showed significant differences in stem volume, percent stem wood, percent branch wood, and partitioning to fine roots, yet no differences in stem RGR or growth efficiency. Furthermore, genotypic differences in stem volume were independent of genotypic differences in biomass partitioning, and overall, we found no evidence to support the hypothesized relationships. Even so, the observed variation in biomass partitioning has implications for forest C sequestration as genotypes which partition more biomass to long-lived biomass pools such as stems, may sequester more C. Moreover, the lack of a genetic relationship between stem volume and belowground partitioning suggests that highly productive genotypes may be planted without compromising belowground C storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pine plantations in the southeast USA currently cover roughly 11.9 million hectares (Zhang and Polyakov 2010) or roughly 21 % of the total timberland area of the region. The vast majority of these plantations (>80 %) are established with loblolly pine (Pinus taeda L.), and virtually all plantations are established with genetically improved seedlings ranging from open-pollinated families, which possess substantial tree-to-tree genetic variation, to clonal varieties, which possess no tree-to-tree genetic variation (McKeand et al. 2003). This range of genetic variation allows land managers to balance the gains and risks of planting less genetically diverse, yet potentially more productive genotypes (we broadly refer to genotypes as selected individuals with a specific and known genetic ancestry) (Bridgwater et al. 2005). In addition, roughly 50 years of genetic improvement has proven an important tool for increasing the productivity, diseases resistance, and sustainability of loblolly pine plantations (Li et al. 1999; McKeand et al. 2003; Carter and Foster 2006; Fox et al. 2007). With a large land base and rapid growth rates spurred by the combined use of genetically improved seedlings and intensive management, loblolly pine plantations have undoubtedly played and will likely continue to play an important role in carbon (C) sequestration (Ryan et al. 2010; Aspinwall et al. 2012). Yet, despite the fact that substantial gains in productivity (i.e., stem wood volume or dry mass) associated with genetic improvement have long been observed (see Talbert et al. 1985; Li et al. 1999), the physiological and morphological basis of enhanced productivity within genetically improved loblolly pine remains poorly understood (Martin et al. 2001, 2005).
Leaf-level physiological traits (i.e., net photosynthesis, intrinsic water-use efficiency), which show considerable spatial and temporal variability, have shown inconsistent correlations with genotype productivity (Yang et al. 2002; McGarvey et al. 2004; Aspinwall et al. 2011a). In some cases, differences in productivity among loblolly pine genotypes have been attributed to shifts in biomass partitioning (Bongarten and Teskey 1987; Li et al. 1991). While the physiological processes which determine biomass partitioning are complex, being primarily determined by source-sink C transport (Poorter and Villar 1997), and C respired, retranslocated or volatilized, whole-tree allometric relationships may provide insight into the underlying component traits which determine phenotypic variability in whole-plant development and productivity. In addition, genetic differences in stem relative growth rate (RGR) over time can be important for understanding variation in phenology, physiology, C gain, and productivity (Ledig and Perry 1969; Drew and Ledig 1980). When combined, RGR and biomass partitioning data provide important details with regard to how plants balance growth and biomass partitioning in a way that matches the physiological functions performed by individual organs (Poorter et al. 2011).
Likewise, some studies in loblolly pine have suggested that genetic differences in stem wood production are the result of enhanced growth efficiency (stem wood production per unit leaf area) (McCrady and Jokela 1996; Svensson et al. 1999). However, some loblolly pine clones have shown no relationship between growth efficiency and productivity (Tyree et al. 2009a; Stovall et al. 2012a). Nonetheless, growth efficiency depends on foliar photosynthetic capacity, respiration, and biomass partitioning (Albaugh et al. 1998), and may offer insight into the physiological and morphological variables underlying intraspecific productivity differences.
Understanding which traits are associated with higher productivity may also be important for developing desirable loblolly pine ideotypes; model trees which possess phenotypic characteristics correlated with yield (Martin et al. 2001, 2005; Nelson and Johnsen 2008). From a management perspective, understanding genotypic differences in these traits may optimize productivity by aiding planting decisions under different silvicultural regimes or changing climatic conditions (McKeand et al. 2006). From a C sequestration perspective, genotype-mediated shifts in partitioning toward greater stem wood could result in maximization of C storage in harvestable wood products, whereas greater partitioning to roots could increase belowground carbon storage, potentially mitigating the negative effects of rising atmospheric CO2 (Curtis et al. 1994; King et al. 1999; Johnsen et al. 2004). If genotypes with high stem wood production do not necessarily partition less biomass belowground, such genotypes may be planted without compromising the potential for belowground C storage.
The objective of this study was to explicitly examine the relationship between genotype productivity (individual-tree stem volume), biomass partitioning, growth efficiency, and stem RGR. We hypothesized that genotype productivity will be positively associated with (1) changes in whole-tree allometry which result in greater investment in stem biomass relative to other biomass components, particularly foliage and belowground components (tap roots, coarse roots and fine roots), (2) enhanced growth efficiency (stem biomass per unit leaf area), and (3) greater stem RGR over time. To test this hypothesis, we grew nine different loblolly pine genotypes, possessing varying degrees of inherent genetic homogeneity, under field conditions for 3 years and quantified stem growth, whole-tree biomass partitioning, growth efficiency, and stem RGR.
Materials and methods
The study site was located at the North Carolina State University Hofmann Forest in Onslow County, NC, USA (34°49.4′N, 77°18.2′W) on a flat, very poorly drained soil with loamy sedimentary deposits and moderate permeability overlain by thick accumulation of organic matter (fine-loamy, siliceous, semi-active, thermic Umbric Paleaquults) (USDA, NRCS available at http://websoilsurvey.nrcs.usda.gov/ accessed 03/24/2010). Mean annual precipitation (1971–2000) is 1,435 mm and is evenly distributed throughout the year. Mean summer (July) and winter (January) temperature are 26.7 and 7.6 °C, respectively (National Climate Data Center, NOAA, available at http://cdo.ncdc.noaa.gov/climatenormals/clim20/nc/314144.pdf accessed 24 March 2010). Other details on the site can be found in Aspinwall et al. (2011b).
In January 2006, the study was established as a randomized complete block design consisting of 20 replications of 9 genotypes: 3 half-sib (open-pollinated) families (HS1, HS2, HS3), 3 full-sib (mass-control pollinated) families (FS1, FS2, FS3), and 3 clones (C1, C2, C3). Each tree within a replication was considered a single-tree plot (Aspinwall et al. 2011a). C1 and C3 were planted as both bare-root and container-grown stock to examine planting stock effects on initial growth and development. After 2 years, there were no significant differences in ground-line diameter or tree height between bare-root and containerized trees of C1 (P = 0.48 and 0.80 for ground-line diameter and height, respectively) and C3 (P = 0.19 and 0.34 for ground-line diameter and height, respectively). Given that all other seedlings were planted bare-root, only bare-root trees were included in our analysis of height, diameter, and volume growth. Seedlings were hand planted at a 3.0 × 6.1-m spacing (~539 trees ha−1). Prior to planting, the site was fertilized with nitrogen (N), phosphorus (P), and boron (B) at elemental rates of 40, 100, and 1 kg ha−1, respectively. In spring 2006 and 2007, competing vegetation was controlled with a combined spray application of imazapyr and sulfometuron methyl at a rate 2.8 and 1.9 L ha−1, respectively. Competing vegetation growing between rows of measurement trees was controlled using a 2.44 m wide drum chopper in winter 2008.
Because loblolly pine genetic improvement operations currently produce a variety of highly productive genotypes from each of these three genetic ‘groups’ [i.e., open-pollinated (or half-sib) families, mass-control pollinated (full-sib) families, and clonal varieties], with each ‘group’ containing varying amounts of inherent genetic homogeneity (Bridgwater et al. 2005; McKeand et al. 2006), the genotypes selected for this study represent the range of possible planting options. Full-sib family (FS3) was a cross of two female parents of half-sib families (HS1 and HS3). The female parent of HS1 was also one of the parents of FS1. All half-sib and full-sib families were second-generation selections from the Georgia-South Carolina Coastal Plain known to exhibit excellent stem form, productivity and rust resistance. Clonal material originated from somatic tissue culture (somatic embryogenesis; SE) of the best individuals produced from selected full-sib families originating from the Georgia-South Carolina Coastal Plain. Somatic embryogenesis is a clonal propagation technique which involves removal of embryos from immature seed cones, initiation of embryonic tissue, multiplication of tissue masses, maturation of embryonic tissue masses, germination of a cotyledon, and eventually, development of a plantlet (Gupta and Durzan 1987; Pullman et al. 2003).
From January 2007 to January 2009, tree height (m) and ground-line diameter (cm) were measured monthly or semi-monthly on all measurement trees. Productivity at the end of the study period was assessed in terms of individual-tree outside bark stem wood volume (m3), calculated following Sherrill et al. (2011). In January 2008, after two growing seasons, four complete replications (n = 44) were harvested to estimate above- and belowground biomass production and partitioning. Ground-line diameter and height were measured and each tree was felled onto a tarp. The aboveground portion was then separated into foliage, branches, and stem. Each component was processed following Aspinwall et al. (2011b). Briefly, total fresh mass of each component was measured in the field and a subsample representing ~25 % of the component fresh mass was weighed and dried to a constant mass at 70 °C. Total dry mass (kg) of each component was calculated as the product of total fresh weight and subsample moisture content (%). Following the aboveground harvest, a 0.5 m3 (1 m2 × 50 cm in depth) volume of soil, centered on the cut stem, was carefully excavated so that taproot, coarse root, and fine root biomass could be estimated. We excavated this soil volume based on several ‘test’ excavations of adjacent trees root systems which revealed that excavation of larger volumes did not yield significant increases in tree root biomass. Furthermore, given that our design consisted of single-tree plots, we decided not to expand our area of excavation, thereby limiting the possibility of roots entering the soil volume from adjacent trees. Given the results of our ‘test’ excavations, the age and size of the trees, the wide (3 m) within-row spacing, and our standardized volume of excavation, we feel confident that we excavated the bulk (>90 %) of the target trees root system with limited interference from surrounding trees. Since production and turnover of fine roots may show considerable temporal variability within a given year (King et al. 2002), fine root biomass during the winter months may not represent total fine root production. Nonetheless, these data do allow for comparison of the size of the fine root component relative to other plant parts (King et al. 2002, 2005). Total dry mass for each belowground component and each sample tree was determined by drying all biomass to a constant mass at 70 °C. Biomass partitioning to each component was calculated as a percentage of total tree dry mass. In January 2009, after the third growing season, two more replications (n = 22) were harvested using the same protocol to account for age-related changes in allometry.
At each harvest, a subsample of fresh needles (~25 % of individual-tree needle biomass) was scanned (Epson v700 scanner, Epson Inc., Long Beach, CA, USA) and needle surface area was calculated using ImageJ software (NIH Image software v1.62 http://rsbweb.nih.gov/nih-image). The scanned needles were dried to a constant mass at 70 °C, and the fresh area of the scanned needles was divided by the dry mass to estimate projected specific leaf area (SLA, m2 kg−1 foliage dry mass). Total projected leaf area (m2) was calculated as the product of the total needle dry mass and SLA.
To compare biomass partitioning patterns among genetic groups and genotypes, allometric relationships were developed by fitting a model of the natural logarithm of plant dry mass (foliage, stem wood, coarse roots, etc.) against the natural logarithm of another biomass component:
where Y is the dry mass of the respective biomass component (i.e., stem) and X is another biomass component (i.e., roots), a and k are intercept and slope regression coefficients, and ε is the random error. The slope coefficient, k, describes the partitioning of biomass to Y relative to X.
Equation (1) was also used to estimate stem dry mass production where Y represents stem dry mass and X represents ground-line diameter. When back-transforming to estimate stem dry mass, corrections for logarithmic bias were made on all stem dry mass estimates (Baskerville 1972). Estimates of stem biomass over time were used to calculate individual-tree stem RGR using the following equation:
where W 2 is the total stem dry mass (g) at time two and W 1 total stem dry mass (g) at time one. The difference between t 2 and t 1 represents the number of days between measurements.
There were no significant differences in the intercept or slope parameters among genetic groups (P = 0.21, P = 0.40), or among genotypes (P = 0.83, P = 0.91) for the allometric relationship relating tree ground-line diameter to stem dry mass. Therefore, one allometric equation was developed for estimating individual-tree stem dry mass (ln(Y) = −4.79 + 2.62 × ln(X), R 2 = 0.93, MSE = 0.07, P < 0.01) which was subsequently used for estimating stem RGR over time.
Data analysis
Prior to analysis, data were tested for homogeneity of variance and normality. To account for the correlation among observations measured on the same tree over time, a mixed model analysis of variance (ANOVA) with a correlated residual structure was used to determine the significance of the main and interactive effects of measurement date, replication, genetic group and genotype on tree height, ground-line diameter, and stem RGR. Akaike information criteria (AIC) and Bayesian information criteria (BIC) were used to select the residual structure which was most effective at minimizing the sum of squared error in the ANOVA. Because the genotypes in this study do not represent all clones, all half-sib or all full-sib families, we considered the genotype effect to be fixed. Measurement date was also considered a fixed effect. Replication and the replication × genetic group interaction were considered random effects. When genotype effects were significant in the ANOVA, Tukey’s post hoc means separation tests were used for pairwise comparison of genotype means. Similarly, a mixed model approach was used to determine the significance of the main and interactive effects of age, genetic group, and genotype on biomass component dry mass and percent partitioning to different biomass components. All analyses were conducted in SAS PROC MIXED (SAS/STAT software version 9.2. SAS Institute Inc. 2002) and all tests were conducted at the P ≤ 0.05 significance level.
Analysis of covariance (ANCOVA), fit to the model described in Eq. (1), was used to test for allometric differences among experimental factors (ages, genetic groups, genotypes). Following Coyle and Coleman (2005) and Coyle et al. (2008), if the experimental factor significantly altered the allometric coefficient (k), a significant interaction occurred between the covariate and the factor, resulting in an adjusted allometric equation for each factor. If the interaction was not significant, the interaction term was removed, and the factor and covariate were tested. If the factor effect was significant and the interaction was not, an adjusted equation was produced with a common slope across factors, and different intercepts for each factor. Following ANCOVA, genetic group and genotype differences in a and k were tested using the ESTIMATE statement in SAS PROC MIXED (SAS/STAT software version 9.2. SAS Institute Inc. 2002). Using the same approach, ANCOVA was used to test for differences in the intercept and slope parameters describing the relationship between projected leaf area and stem dry mass where the slope represents growth efficiency (stem dry mass production per unit leaf area). Tests of intercept and slope differences were conducted at the P ≤ 0.05 significance level.
Results
Individual-tree growth over time
We found a significant date × genetic group effect for height growth; however, the genotype effect was significant with no date × genotype interaction (Table 1). Clone C1 consistently showed significantly lower height growth than all other genotypes (Fig. 1a). In contrast, full-sib FS1 and FS3, along with half-sib HS1 consistently showed the greatest height growth (Fig. 1b, c).
Genotypes showed significant differences in ground-line diameter growth and there was no significant date × genetic group or date × genotype effect which indicated that genotype differences were consistent over time (Table 1). Overall, FS1, FS3, HS1 and C2 showed the greatest diameter growth over time (Fig. 1d–f).
At the end of the study period, individual-tree stem volume was significantly different among genetic groups (P = 0.03) and genotypes (P < 0.01). Among genotypes, FS3 and C1 showed the highest and lowest volume growth, respectively (P < 0.01, Fig. 2). Full-sib and half-sib families showed significantly higher (both P < 0.04) stem volume (both 0.014 ± 0.001 m3) than clones (0.012 ± 0.001 m3).
Biomass production and partitioning
Tree age had the largest effect on standing dry mass for all biomass components (Table 2). The age × genetic group interaction was significant for all biomass components and total tree (one-sided) leaf area, but not SLA (Table 2; Fig. 3). Average SLA across both ages was 3.7 ± 0.02 m2 kg−1. The age × genetic group interaction was due to a lack of differences in total and component dry mass between genetic groups at age 2, whereas full-sib and half-sib families showed greater total and component dry mass than clones at age 3 (Fig. 3). Similarly, while there were no significant differences in total tree leaf area between genetic groups at age 2 (mean = 3.17 ± 0.4 m2), total tree leaf area of full-sib (8.61 ± 1.0 m2) and half-sib (8.15 ± 1.0 m2) families was significantly higher than total tree leaf area of clones (3.73 ± 0.8 m2) at age 3.
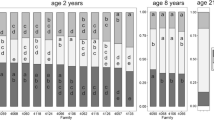
With exception to partitioning to fine roots, tree age had a significant effect on percent partitioning to all biomass components (Table 2). From age 2 to 3, the percentage of total tree dry mass partitioned to perennial components (branch, stem wood, and tap roots) increased while partitioning to foliage decreased (Fig. 4a). Interestingly, we found a significant age × genotype effect for percent stem wood (Table 2; Fig. 4b). At age 2, genotypes C3 and HS2 showed significantly higher percent stem wood than FS2. At age 3, C3 continued to show significantly higher percent stem wood than FS2, however, HS2 showed little increase in percent stem wood resulting in no difference between HS2 and FS2 (Fig. 4b). The overall genotype effect on percent branch wood was also significant across both ages with no significant age × genotype effect (Table 2; Fig. 4c). Genotypes C3 and FS2, which showed the highest and lowest percent stem dry mass, respectively, showed the lowest and highest percent branch dry mass, suggesting that these genotypes showed different patterns of partitioning to aboveground perennial organs. Despite significant genotypic variation in aboveground biomass partitioning, genotype mean percent wood and branch wood showed no relationship with genotype stem volume (P = 0.67 and P = 0.99, respectively).
a Biomass partitioning in age 2 and 3 loblolly pine trees growing in a plantation on the lower Coastal Plain of North Carolina. b Differences in partitioning to stem wood biomass among different loblolly pine genotypes. At each age, genotypes with the same letter are not significantly different at the P ≤ 0.05 significance level. c Differences in partitioning to branch biomass among different loblolly pine genotypes. Genotypes with the same letter are not significantly different at the P ≤ 0.05 significance level
Allometric relationships
Partitioning to total belowground biomass relative to total biomass increased significantly between age 2 and 3 (P = 0.01) (Table 3). The allometric coefficient describing partitioning to total aboveground biomass, foliage, stem wood, tap root and coarse root biomass, relative to total tree biomass, was not significantly different between age 2 and 3 trees although the intercept parameters were generally higher in age 3 trees (Table 3). Partitioning to branches and fine roots relative to total tree biomass did not differ between age 2 and 3 trees (P = 0.58, Table 3).
Partitioning to aboveground and belowground biomass components relative to total tree biomass was not significantly different among genetic groups (all P > 0.20). However, the intercept parameter was significantly different among genotypes for the relationship between branch and total tree biomass (P = 0.02) (Table 4). Moreover, genotypes showed significant differences in partitioning to fine roots relative to total tree biomass (P = 0.05). Nonetheless, differences in allometric parameters were not associated with genotypic differences in stem volume (all P > 0.40).
Partitioning to stem wood relative to branches was not significantly different between age 2 and 3 trees (P = 0.84), or among genetic groups (P = 0.79) or genotypes (P = 0.73). There were no significant differences in partitioning to stem wood relative to fine roots, coarse roots, or tap roots among genetic groups or genotypes (all P > 0.20). However, genetic groups showed significant differences in partitioning to stem wood relative to foliage (P = 0.02), with half-sib and full-sib families showing greater partitioning to stem wood than clones (Fig. 5). There were no differences in partitioning to stem wood relative to foliage among genotypes (P = 0.31). As a result, contrary to our expectation, more productive genotypes did not necessarily invest more biomass in stem wood relative to other above- and belowground components.
Stem relative growth rate over time
Over time, stem RGR was not significantly different among genetic groups (P = 0.08) or genotypes (P = 0.87) and there was no significant date × genetic group (P = 0.96) or date × genotype interaction (P = 0.99). Measurement date had the largest effect (P < 0.01) with peak stem RGR occurring in May 2007 (15.7 ± 0.5 mg g−1 day−1) and June 2008 (14.7 ± 0.7 mg g−1 day−1) (Fig. 6).
Growth efficiency
Overall, individual-tree projected leaf area explained 94 % of the variation in stem dry mass. The relationship between projected leaf area and stem dry mass was significantly different between age 2 and 3 trees (P < 0.01), with growth efficiency (i.e., the slope) increasing with age (Fig. 7). Nonetheless, genetic groups (P = 0.20) and genotypes (P = 0.59) showed no differences in the relationship between projected leaf area and stem wood dry mass indicating that there were no genetic effects on growth efficiency. These results did not support the hypothesized positive relationship between genotype growth efficiency and productivity.
Discussion
To further our understanding of genetic differences in the drivers of productivity, we grew a range of improved genotypes of loblolly pine under plantation conditions for 3 years and measured whole-tree biomass production and partitioning, growth efficiency, and stem RGR. We expected that genetic differences in biomass partitioning, stem RGR, and growth efficiency would result in correlated changes in genotype productivity. However, the lack of a genetically determined productivity–biomass partitioning relationship, along with no significant variation in stem RGR or growth efficiency, suggests that intraspecific productivity differences may not be easily explained by patterns of biomass partitioning or growth efficiency. Even so, the genetic variation demonstrated for biomass partitioning may have important implications for pine plantation C sequestration.
Genotype growth over time
Over time, we found no significant date × genotype interaction for tree height and ground-line diameter growth. Instead, differences in height and ground-line diameter over time were generally consistent among this group of intensively selected genotypes. Therefore, we found no evidence to suggest that growth phenology differed among genotypes. In contrast, Emhart et al. (2006) found that genetic differences in basal area growth among different loblolly pine and slash pine (Pinus elliottii Engelm. var. elliottii) families were mainly expressed during brief time periods in the spring and fall. Jayawickrama et al. (1998) also found that loblolly pine provenance and family differences in height and diameter growth were positively associated with a longer growing season, and lower productivity genotypes tended to reach maximal growth at an earlier date. On the other hand, in agreement with our findings, Cannell et al. (1978) found little evidence that more productive loblolly pine families had extended periods of seasonal growth.
Most importantly, these consistent growth differences over time resulted in significant genetic variation in biomass production. These genotypic differences in individual-tree dry mass production can result in substantial differences in stand-level above- and belowground dry mass production (Aspinwall et al. 2011b). Moreover, several studies in loblolly pine have demonstrated similar genetic variation in individual-tree total and component dry mass production (Bongarten and Teskey 1987; Li et al. 1991; Retzlaff et al. 2001) which suggests that genetic variability in dry mass production has important implications for loblolly pine plantation C cycling.
Genetic effects on biomass partitioning and allometric relationships
In agreement with King et al. (1999), whole-tree biomass partitioning was largely driven by ontogeny. However, when comparing patterns of partitioning among genotypes, we found that genotypes showed some significant differences in percent stem wood and branch dry mass (Fig. 4). Other studies in loblolly pine have found similar differences in biomass partitioning among different seed sources (Bongarten and Teskey 1987), and between contrasting full-sib families (Chmura et al. 2007) and clones (Tyree et al. 2009b). More recently, Stovall et al. (2012b) found substantial differences in biomass partitioning among ten different loblolly pine clones. In contrast, Retzlaff et al. (2001) and Li et al. (1991) observed little to no variation in stem dry mass partitioning among different loblolly pine provenances and half-sib families.
While our study included a limited number of genotypes, similar studies have typically included a comparable number of genotypes (sometimes fewer), often from within only one genetic group (Li et al. 1991; Retzlaff et al. 2001; Chmura et al. 2007). Moreover, genotype biomass partitioning studies are often conducted under greenhouse conditions (Bongarten and Teskey 1987; Li et al. 1991; Tyree et al. 2009b) whereas our study was conducted under plantation conditions (also see Stovall et al. 2012b). Our results suggest that genotypic differences in individual-tree biomass partitioning may have some consequences for stand-level productivity and C sequestration. For instance, genotypes which show greater partitioning to long-lived woody components may ultimately sequester more C. However, future studies should examine how resource availability and edaphic conditions influence genotype-specific patterns of biomass partitioning.
In general, the allometric coefficients in our study are within the range of those reported for loblolly pine seedlings (Ledig et al. 1970) and young plantation grown trees (Retzlaff et al. 2001). Apart from partitioning to individual-tree components, we hypothesized that more productive genotypes would show greater partitioning to stem wood relative to other aboveground and belowground components, as has been observed for different loblolly pine families (Li et al. 1991), seed sources (Bongarten and Teskey 1987), and cultural treatments (Albaugh et al. 1998). However, it was not apparent that increased or decreased partitioning to stem wood relative to foliage or belowground components was related to genotype productivity. Thus, our results do not support the hypothesis that more productive genotypes show greater partitioning to stem dry mass relative to other biomass components. Similarly, Retzlaff et al. (2001) found significant differences in dry mass production between two loblolly pine families, yet no differences in allometry. Stovall et al. (2012a) also found no relationship between loblolly pine clone productivity and biomass partitioning. We conclude that biomass partitioning patterns may be only partially responsible for genotype differences in productivity, consistent with previous reports for loblolly pine (Bongarten and Teskey 1987; Li et al. 1991; Stovall et al. 2012a). On the other hand, the lack of a genotype productivity–biomass partitioning relationship implies that there may be opportunities to plant genotypes which are capable of both high stem wood production and belowground C storage.
Stem relative growth rate
Overall, we found no evidence to suggest that productivity differences among genotypes were related to stem RGR. Therefore, in agreement with Ledig et al. (1970), Britt et al. (1991), and van den Driessche (1992), we conclude that absolute growth is more effective than RGR at explaining differences in productivity. Even so, incorporation of the seasonal dynamics of foliage production and senescence would have allowed us to calculate the true net assimilation rate, which may have provided a better understanding of genotype C gain and thus, productivity (Pallardy and Kozlowski 1979).
In addition to genotypic effects on growth and biomass partitioning, an unexpected, yet common observation which emerged from this study was that the selected clones, which were produced via somatic embryogenesis, showed lower productivity, lower partitioning to stem wood relative to foliage, and lower dry mass and leaf area production relative to full-sib and half-sib family seedlings. Clones, as a whole, showed little increase in foliage biomass from age 2 to 3 which may reflect a poorly developed root system with limited ability to supply the necessary resources for C fixation and aboveground growth. For example, Rahman et al. (2003a) found that in comparison to loblolly pine zygotic seedlings, SE clones had higher root:shoot ratios and produced thicker and shorter roots (less branching) which likely resulted in less water and nutrient uptake. McKeand and Allen (1984) also found that loblolly pine tissue culture plantlets had thick, unbranched roots resulting in inefficient nutrient uptake. Together, these results may suggest that the growth of SE clones in our study was hampered by a poorly developed root system. However, Rahman et al. (2003b) also found that seedling stomatal conductance was more responsive to changes in soil moisture while SE clones showed less stomatal control, and therefore, lower leaf water potentials. Problems with maturation and poorly developed root and shoot vascular connections may be the casual agent behind growth differences between tissue culture seedlings and zygotic seedlings (McKeand 1985; Pullman et al. 2003). Lastly, we emphasize that plantations established with loblolly pine SE clones are relatively young and the performance of these three SE clones is most likely genotype specific and should not be used to make broader inferences about SE clones in general.
Growth efficiency
Following Cannell et al. (1983) and Svensson et al. (1999), we hypothesized that greater genotype productivity would be the result of enhanced growth efficiency (stem wood production per unit leaf area). Other studies have shown enhancements in leaf area production as a result of increased resource availability that resulted in enhanced growth efficiency and higher productivity (Vose and Allen 1988; Colbert et al. 1990; Albaugh et al. 1998). Overall, our results did not support our hypothesis that more productive genotypes would exhibit higher growth efficiency. In fact, we found no significant genetic variation in growth efficiency which may be partly associated with the relatedness among some genotypes. Instead our results align with those of Tyree et al. (2009a) and Stovall et al. (2012a) who found significant variability in growth efficiency among different loblolly pine clones, yet no association between clone productivity and growth efficiency. The seemingly contradictory information among studies may suggest that growth efficiency per se is not a determinant of genotype productivity.
Overall, our results do not provide evidence for a common trait, or sets of traits, which determine productivity differences among loblolly pine genotypes. Alternatively, it has been generally suggested that loblolly pine productivity is primarily driven by the capacity or efficiency of the tree canopy to use light energy to convert C into biomass. This capacity or efficiency may be determined by different mechanisms such as crown morphology (i.e., leaf area distribution, leaf and branch angles), leaf area duration, C allocation, and leaf-level photosynthetic resource-use efficiency (McCrady and Jokela 1996, 1998; Emhart et al. 2007; Chmura and Tjoelker 2008). We found no significant variation in leaf-level photosynthetic capacity, quantum yield or dark respiration among these same genotypes growing under the same conditions (Aspinwall et al. 2011a). We also found no evidence of a tradeoff between genotype productivity and C allocation to foliar secondary biochemical compounds (Aspinwall et al. 2011c). Given that we have found no relationship between productivity and biomass partitioning in the present study, the arrangement, distribution, and duration of leaf area may be the critical factor governing light capture over time and ultimately, productivity (Cannell 1989; McCrady and Jokela 1996, 1998). Indeed, studies have suggested that the development and structure of tree crowns are key determinants of resource interception, use, and productivity (Cannell et al. 1978; Martin et al. 2001; Staudhammer et al. 2009). Emhart et al. (2007), in particular, found moderate individual-tree broad-sense heritabilities for crown structural traits and significant genetic correlations between crown traits and volume increment. Therefore, crown traits such as crown radius, crown length, and branch angle, in addition to leaf area duration, may be critical determinants of canopy light interception and genotype productivity.
In summary, we found that significant productivity differences among different loblolly pine genotypes were independent of biomass partitioning and growth efficiency. Further information regarding genotypic differences in crown structure and leaf area duration may provide insight into the drivers of intraspecific productivity differences in loblolly pine. Nonetheless, the genotypic differences in biomass partitioning demonstrated in this study may have important consequences for loblolly pine ecosystem C sequestration.
References
Albaugh TJ, Allen HL, Dougherty PM, Kress LW, King JS (1998) Leaf area and above- and belowground growth responses of loblolly pine to nutrient and water additions. For Sci 44:317–328
Aspinwall MJ, King JS, McKeand SE, Domec J-C (2011a) Leaf-level gas-exchange uniformity and photosynthetic capacity among loblolly pine (Pinus taeda L.) genotypes of contrasting inherent genetic variation. Tree Physiol 31:78–91. doi:10.1093/treephys/tpq107
Aspinwall MJ, King JS, McKeand SE, Bullock BP (2011b) Genetic effects on stand-level uniformity and above- and belowground dry mass production in juvenile loblolly pine. For Ecol Manage 262:609–619. doi:10.1016/j.foreco.2011.04.029
Aspinwall MJ, King JS, Booker FL, McKeand SE (2011c) Genetic effects on total phenolics, condensed tannins and non-structural carbohydrates in loblolly pine (Pinus taeda L.) needles. Tree Physiol 31:831–842. doi:10.1093/treephys/tpr073
Aspinwall MJ, McKeand SE, King JS (2012) Carbon sequestration from 40 years of planting genetically improved loblolly pine across the southeast United States. For Sci 58(5):446–456
Baskerville GL (1972) Use of logarithmic regression in the estimation of plant biomass. Can J For Res 2:49–53
Bongarten BC, Teskey RO (1987) Dry weight partitioning and its relationship to productivity in loblolly pine seedlings from seven sources. For Sci 33(2):255–267
Bridgwater F, Kubisiak T, Byram T, McKeand S (2005) Risk management with current deployment strategies for genetically improved loblolly and slash pines. South J Appl For 29:80–87
Britt JR, Mitchell RJ, Zutter BR, South DB, Gjerstad DH, Dickson JF (1991) The influence of herbaceous weed control and seedling diameter on six years of loblolly pine growth—a classical growth analysis approach. For Sci 37(2):655–668
Cannell MGR (1989) Physiological basis of wood production: a review. Scand J For Res 4:459–490
Cannell MGR, Bridgwater FE, Greenwood MS (1978) Seedling growth rates, water stress responses and root-shoot relationships related to eight-year volumes among families of Pinus taeda L. Silvae Genet 27(6):237–248
Cannell MGR, Sheppard LJ, Ford ED, Wilson RHF (1983) Clonal differences in dry matter distribution, wood specific gravity and foliage “efficiency” in Picea sitchensis and Pinus contorta. Silvae Genet 32(5–6):195–202
Carter MC, Foster CD (2006) Milestones and millstones: a retrospective on 50 years of research to improve productivity in loblolly pine plantations. For Ecol Manage 227:137–144. doi:10.1016/j.foreco.2006.02.014
Chmura DJ, Tjoelker MG (2008) Leaf traits in relation to crown development, light interception and growth of elite families of loblolly and slash pine. Tree Physiol 28:729–742. doi:10.1093/treephys/28.5.729
Chmura DJ, Rahman MS, Tjoelker MG (2007) Crown structure and biomass allocation patterns modulate aboveground productivity in young loblolly pine and slash pine. For Ecol Manage 243:219–230. doi:10.1016/j.foreco.2007.02.027
Colbert SR, Jokela EJ, Neary DG (1990) Effects of annual fertilization and sustained weed control on dry matter partitioning, leaf area, and growth efficiency of juvenile loblolly and slash pine. For Sci 36(4):995–1014
Coyle DR, Coleman MD (2005) Forest production responses to irrigation and fertilization are not explained by shifts in allocation. For Ecol Manage 208:137–152
Coyle DR, Coleman MD, Aubrey DP (2008) Above- and below-ground biomass accumulation, production, and distribution of sweetgum and loblolly pine grown with irrigation and fertilization. Can J For Res 38:1335–1348
Curtis PS, O’Neill EG, Teeri JA, Zak DR, Pregitzer KS (1994) Belowground responses to rising atmospheric CO2: implications for plants, soil biota and ecosystem processes. Plant Soil 165:1–6
Drew AP, Ledig FT (1980) Episodic growth and relative shoot:root balance in loblolly pine seedlings. Ann Bot 45:143–148
Emhart VI, Martin TA, White TL, Huber DA (2006) Genetic variation in basal area increment phenology and its correlation with growth rate in loblolly and slash pine families and clones. Can J For Res 36:961–971. doi:10.1139/X05-309
Emhart VI, Martin TA, White TL, Huber DA (2007) Clonal variation in crown structure, absorbed photosynthetically active radiation and growth of loblolly and slash pines. Tree Physiol 27:421–430. doi:10.1093/treephys/27.3.421
Fox TR, Jokela EJ, Allen HL (2007) The development of pine plantation silviculture in the southern United States. J For 105(7):337–347
Gupta PK, Durzan DJ (1987) Biotechnology of somatic polyembryogenesis and plantlet regeneration in loblolly pine. Biotech 5:147–151
Jayawickrama KJS, McKeand SE, Jett JB (1998) Phenological variation in height and diameter growth in provenances and families of loblolly pine. New For 16:11–25
Johnsen K, Teskey B, Samuelson L, Butnor J, Sampson D, Sanchez F, Maier C, McKeand S (2004) Carbon sequestration in loblolly pine plantations: methods, limitations, and research needs for estimating storage pools, In: Rauscher HM, Johnson K (eds) Southern forest science: past, present and future, USDA For Serv Gen Tech Rep SRS-75, pp 373–381
King JS, Albaugh TJ, Allen HL, Kress LW (1999) Stand-level allometry in Pinus taeda as affected by irrigation and fertilization. Tree Physiol 19:769–778. doi:10.1093/treephys/19.12.769
King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty P (2002) Belowground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol 154:389–398. doi:10.1046/j.1469-8137.2002.00393.x
King JS, Kubiske ME, Pregitzer KS, Hendry GR, McDonald EP, Giardina CP, Quinn VS, Karnosky DF (2005) Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2. New Phytol 168:623–636
Ledig FT, Perry TO (1969) Net assimilation rate and growth in loblolly pine seedlings. For Sci 15(4):431–438
Ledig FT, Bormann H, Wenger KF (1970) The distribution of dry matter growth between shoot and roots in loblolly pine. Bot Gaz 131(4):349–359
Li B, McKeand SE, Allen HL (1991) Nitrogen and family effects on biomass allocation of loblolly pine seedlings. For Sci 37(1):271–283
Li B, McKeand S, Weir R (1999) Tree improvement and sustainable forestry—impact of two cycles of loblolly pine breeding in the USA. For Genet 6(4):229–234
Martin TA, Johnson KH, White TL (2001) Ideotype development in southern pines: rationale and strategies for overcoming scale-related obstacles. For Sci 47(1):21–28
Martin TA, Dougherty PM, Topa MA, McKeand SE (2005) Strategies and case studies for incorporating ecophysiology into southern pine tree improvement programs. South J Appl For 29(2):70–79
McCrady RL, Jokela EJ (1996) Growth phenology and crown structure of selected loblolly pine families planted at two spacings. For Sci 42(1):46–57
McCrady RL, Jokela EJ (1998) Canopy dynamics, light interception, and radiation use efficiency of selected loblolly pine families. For Sci 44(1):64–72
McGarvey RC, Martin TA, White TL (2004) Integrating within-crown variation in net photosynthesis in loblolly and slash pine families. Tree Physiol 24:1209–1220. doi:10.1093/treephys/24.11.1209
McKeand SE (1985) Expression of mature characteristics by tissue culture plantlets derived from embryos of loblolly pine. J Am Soc Hortic Sci 110(5):619–623
McKeand SE, Allen HL (1984) Nutritional and root development factors affecting growth of tissue culture plantlets of loblolly pine. Physiol Plant 61:523–528
McKeand S, Mullin T, Byram T, White T (2003) Deployment of genetically improved loblolly and slash pine in the South. J For 101(3):32–37
Mckeand SE, Jokela EJ, Huber DA, Byram TD, Allen HL, Li B, Mullin TJ (2006) Performance of improved genotypes of loblolly pine across different soils, climates, and silvicultural inputs. For Ecol Manage 227:178–184
Nelson CD, Johnsen KH (2008) Genomic and physiological approaches to advancing forest tree improvement. Tree Physiol 28:1135–1143
Pallardy SG, Kozlowski TT (1979) Early root and shoot growth of Populus clones. Silvae Genet 28(4):153–156
Poorter H, Villar R (1997) The fate of acquired carbon in plants: chemical composition and construction costs. In: Bazzaz FA, Grace J (eds) Plant Resource Allocation. Academic Press, New York, pp 39–70
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2011) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. doi:10.111/j.1469-8137.2011.03952.x
Pullman GS, Johnson S, Peter G, Cairney J, Xu N (2003) Improving loblolly pine somatic embryo maturation: comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep 21:747–758
Rahman MS, Messina MG, Newton RJ (2003a) Performance of loblolly pine (Pinus taeda L.) seedlings and micropropagated plantlets on an east Texas site I. Above- and belowground growth. For Ecol Manage 178:245–255
Rahman MS, Messina MG, Newton RJ (2003b) Performance of loblolly pine (Pinus taeda L.) seedlings and micropropagated plantlets on an east Texas site II water relations. For Ecol Manage 178:257–270
Retzlaff WA, Handest JA, O’Malley DM, McKeand SE, Topa MA (2001) Whole-tree biomass and carbon allocation of juvenile trees of loblolly pine (Pinus taeda): influence of genetics and fertilization. Can J For Res 31:960–970
Ryan MG, Harmon ME, Birdsey RA, Giardina CP, Heath LS, Houghton RA, Jackson RB, McKinley DC, Morrison JF, Murray BC, Pataki DE, Skog KE (2010) A synthesis of the science on forests and carbon for US Forests. Issues Ecol 13:1–16
SAS/STAT software version 9.2. SAS Institute Inc. Copyright © 2002–2008. Cary, NC, USA
Sherrill JR, Bullock BP, Mullin TJ, McKeand SE, Purnell RC (2011) Total and merchantable stem volume equations for mid-rotation loblolly pine (Pinus taeda L.). South J Appl For 35:105–108
Staudhammer CL, Jokela EJ, Martin TA (2009) Competition dynamics in pure- versus mixed-family stands of loblolly and slash pine in the southeastern United States. Can J For Res 39:396–409. doi:10.1139/X08-184
Stovall JP, Fox TR, Seiler JR (2012a) Allometry varies among 6-year-old Pinus taeda (L.) clones in the Virginia Piedmont. For Sci (in press)
Stovall JP, Fox TR, Seiler JR (2012b) Short-term changes in biomass partitioning of two full-sib clones of Pinus taeda L. under differing fertilizer regimes over 4 months. Trees 26:951–961. doi:10.1007/s00468-011-0673-4
Svensson JC, McKeand SE, Allen HL, Campbell RG (1999) Genetic variation in height and volume of loblolly pine open-pollinated families during canopy closure. Silvae Genet 48:204–208
Talbert JT, Weir RJ, Arnold RD (1985) Costs and benefits of a mature first-generation loblolly pine tree improvement program. J For 83:162–166
Tyree MC, Seiler JR, Maier CA, Johnsen KH (2009a) Pinus taeda clones and soil nutrient availability: effects of soil organic matter incorporation and fertilization on biomass partitioning and leaf physiology. Tree Physiol 29:1117–1131. doi:10.1093/treephys/tpp050
Tyree MC, Seiler JR, Maier CA (2009b) Short-term impacts of nutrient manipulations on leaf gas exchange and biomass partitioning in contrasting 2-year-old Pinus taeda clones during seedling establishment. For Ecol Manage 257:1847–1858. doi:10.1016/j.foreco.2009.02.001
van den Driessche R (1992) Absolute and relative growth of Douglas-fir seedlings of different sizes. Tree Physiol 10:141–152. doi:10.1093/treephys/10.2.141
Vose JM, Allen HL (1988) Leaf area, stem wood growth, and nutrition relationships in loblolly pine. For Sci 34:547–563
Yang WQ, Murthy R, King P, Topa MA (2002) Diurnal changes in gas exchange and carbon partitioning in needles of fast- and slow-growing families of loblolly pine (Pinus taeda). Tree Physiol 22:489–498. doi:10.1093/treephys/22.7.489
Zhang D, Polyakov M (2010) The geographical distribution of plantation forests and land resources potentially available for pine plantations in the US. South. Biomass Bioenergy 34:1643–1654. doi:10.1016/j.biombioe.2010.05.06
Acknowledgments
This study was funded by the NCSU Donald Moreland Endowed Graduate Fellowship, the NCSU Cooperative Tree Improvement Program, and the Department of Forestry and Environmental Resources. Additional support was provided by the USDA Forest Service Eastern Forest Environmental Threat Assessment Center (EFETAC). We also thank the NCSU Tree Physiology Lab Group for editorial comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. E. Major.
Rights and permissions
About this article
Cite this article
Aspinwall, M.J., King, J.S. & McKeand, S.E. Productivity differences among loblolly pine genotypes are independent of individual-tree biomass partitioning and growth efficiency. Trees 27, 533–545 (2013). https://doi.org/10.1007/s00468-012-0806-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-012-0806-4