Abstract
To survive new microclimatic conditions of a forest gap environment, plant species must physiologically and structurally adjust. A morpho-anatomical, ultrastructural and ecophysiological study was performed at three different times in a forest gap that was created by illegal selective logging. The study followed the early successional Actinostemon verticillatus and the late-successional Metrodorea brevifolia, to elucidate the adaptive strategies of acclimation to gaps. Additionally, Schinus terebinthifolius was included in the study in order to test the plasticity of a pioneer species that grows on forest edges, where this species had higher values of leaf thickness, leaf mass area and succulence. M. brevifolia had succulent leaves, high leaf area and a thin cuticle. A. verticillatus presented the densest leaves and was the only species to show leaf morpho-anatomical plasticity. Ultrastructural and physiological differences were observed only in A. verticillatus and M. brevifolia leaves from the gap: increase in the stroma volume, oil droplets, plastoglobuli, photochemical and non-photochemical quenching. Photosynthetic efficiency showed that the early stages of gap formation are the most critical. Acclimation strategies of A. verticillatus suggest this species invests in the efficiency of photosynthesis by increasing its leaf thickness, leaf mass area and in water content maintenance by increasing the density of its leaves, at the expense of gas exchange, was compensated by a high density of stomata. M. brevifolia compensates for the higher cost of leaves and lower leaf plasticity with ultrastructural changes that are used to adjust the photosynthetic process, which promotes a shorter leaf payback time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The early stage of a forest gap represents a great challenge and/or opportunity for tree seedlings and other plants that remain after its formation. New microclimatic conditions that develop suddenly in a forest, such as during gap formation, can have high irradiance and temperatures, and low levels of moisture (Denslow 1987; Brown 1993) to which surviving species need to quickly adjust both physiologically and structurally. Thus, the species that will adapt and develop to the new conditions constitutes the paradigm of species diversity in tropical ecosystems (Denslow 1987).
In a recent review, Schliemann and Bockheimb (2011) list numerous works that emphasize the impacts of gaps on forest structure, nutrient cycling, microclimates, and forest management. Other studies have focused on photosynthetic light acclimation (Naidu and Delucia 1998; Yamashita et al. 2000) and the anatomical leaf changes that are involved during photosynthetic adjustments (Oguchi et al. 2003, 2006).
Leaf physiology and structural changes are one of the primary means for plants to respond to microclimatic changes in a gap. However, plants differ in their capacity of change phenotypically (phenotypic plasticity) in response to the environment (Via et al. 1995). Species of different successional groups demand different levels of light, temperature, and nutrients (Strauss-Debenedetti and Bazzaz 1996). According to Bazzaz and Pickett (1980), the survival strategies used to separate tree species into two successional groups (early and late) are supported by growth features and shade adaptation. The early successional group includes pioneer species, which are light demanding and their germination is inhibited by far-red light (shade avoidance syndrome) that dominates the understory. Species in the late-successional group are shade tolerant and exhibit a lower light acclimation potential (Yamashita et al. 2000).
Highly diverse environments, such as a tropical ecosystem, imply high levels of ecological redundancy (Peterson et al. 1998). Thus, in a tropical ecosystem, it is appropriate to use successional groups, where botanical identity is substituted for functionality instead of the species, which is based on intrinsic assumptions of the taxonomic characteristics (Quesada et al. 2009). Additionally, studying the phenotypic plasticity of different successional groups that are acclimating to gaps can serve as a good model to predict the behavior of forests to climate change. Thus, how to choose an appropriate method that can be used to predict the acclimation process integrating ecological and phenotypic plasticity studies?
Sultan (2004) enumerates three complex aspects of plasticity that have substantial ecological importance. The first is cross-generational plasticity, where the adaptive legacy is transmitted to the offspring in response to environmental conditions. The second is dynamic plasticity, which relates to the variation in ontogenetic trajectories and the rates of phenotypic response, namely, as the plastic response may depend on the timing of its expression. The third is the plasticity of anatomical and architectural traits, where the adaptive strategies are analyzed at the tissue and cellular level.
The present study focused on the plasticity of morpho-anatomical traits, adding insight into ultrastructural and ecophysiological characteristics and how phenotypic adjustments occur in the leaves of plants. This study used a forest gap created by illegal selective logging of commercial timber species (mostly Paratecoma peroba (Record) Kuhlm. and Myrocarpus frondosus Fr. All.) and followed the response of fully expanded leaves of understory saplings. The morpho-anatomical, ultrastructural and ecophysiological responses of leaves after gap formation were compared with leaves from a closed forest to clarify the adaptive strategies of acclimating to the gap. In addition, to test the acclimation capacity of a pioneer plant, we included a woody species that occurs on the edge of the forest.
Materials and methods
Study site
The study was carried out at the Guaxindiba Ecological Station, located in the city of São Francisco do Itabapoana (21o24′S, 41o04′W) in northern Rio de Janeiro, Brazil. This is the largest fragment (ca. 1200 ha) of lowland forest on tertiary sediments in Brazil, which is also known as tabuleiro Atlantic forest (RadamBrasil 1983), and classified as seasonal semi deciduous lowland forest based on its phytogeographic features (Veloso et al. 1991). According to Köppen (1948), the climate of this region is classified as Aw, and has an intense dry season from May to August (RadamBrasil 1983). Mean annual rainfall is approximately 1,000 mm; the wettest month is December and the driest is August (Villela et al. 2006). The mean annual temperature is 23 °C (RadamBrasil 1983) and the soils are ultisols (USDA classification) that have a low capacity of water retention and are poor in nutrients (Villela et al. 2006).
The forest fragment consists mainly of secondary semi deciduous forest and has a history of disturbance mainly from logging for commercial timber species, charcoal production, and plantation farming (Villela et al. 2006); consequently, the fragment has artificial canopy gaps of different sizes and ages.
Microclimatic measurements (Table 1) were made a closed area of forest (Forest) and in the gap studied, 2 months after it formed (Gap2m), at four cardinal points of each of five chosen individuals. Solar irradiance (μmol m−2 s−1) was measured with a LI-250A light meter (Li-Cor Inc.), and temperature (oC) and humidity (%) were measured with a therm-hygrometer HT-300 (Instrutherm). Statistical differences were determined by the Mann–Whitney U rank sum test Statistica 7, StatSoft, USA (Statsoft Inc. 1998).
Plant material and sampling
The sampling was carried out from February to April 2008, at the end of the wet season. Three woody species of different successional status were studied: a pioneer species, Schinus terebinthifolius Raddi (Anacardiaceae); an early successional species, Actinostemon verticillatus (Kl.) Baill. (Euphorbiaceae); and a late-successional species, Metrodorea brevifolia var. nigra Engl. (Rutaceae). Three individuals of each species were measured in each site (forest and forest gap); individuals were about 1–2.5 m tall.
All species responses were measured under field conditions and the forest gap used was created by illegal selective logging. The plant species were studied in a single forest gap (around 10 m in diameter) at three different times relative to when the gap was created: 1-day-old gap (Gap1d); 7-day-old gap (Gap7d); 2-month-old gap (Gap2m). As a control, all data obtained were compared to the closed forest (Forest), which lacked signs of logging. In addition, the pioneer S. terebinthifolius, which occurs exclusively along forest edges, was studied. In this part of the study, for comparison, leaves fully exposed to sun were classified as gap leaves and those exposed the least to sun were considered forest leaves.
For the general leaf measurements, anatomical measurements, and chloroplast analysis, fully expanded leaves from the third node were collected from the higher branches of three individuals in the forest and Gap2m sites. It is emphasized that in the Gap2m, only leaves that had been formed after the gap was created were collected. For the chlorophyll a fluorescence we choose fully expanded leaves, from the third node of the higher branches, and these same leaves were analyzed in the Gap1d and Gap7d.
Electron microscopy
Leaf fragments of all species studied were collected in the Forest and Gap2m sites, fixed for 2 h in a solution of 2.5 % glutaraldehyde and 4.0 % formaldehyde, buffered with 0.05 M sodium cacodylate, to pH 7.2, at room temperature. After being rinsed with the same buffer, the samples were post-fixed with 1.0 % osmium tetroxide in a 0.05 M sodium cacodylate buffer, at pH 7.2, for 1 h. Subsequently, the samples were dehydrated in an ascending series of acetone solutions. The material was infiltrated and embedded in epoxy resin (Polybed). Ultrathin sections (70 nm) were collected in copper grids (300 mesh), stained with 1.0 % uranyl acetate, followed by 5.0 % lead citrate. Sections were observed at 80 kV using a ZEISS TEM 900 transmission electron microscope. For scanning electron microscopy (SEM), the samples were fixed, post-fixed, dehydrated (as described previously) and rinsed in the same buffer. Afterwards, the samples were CO2 critical-point-dried (CPD 030 Baltec). Dried samples were adhered to stubs with carbon adhesive tape (3 M) and sputter coated with 20 nm of gold (SCD 050 Baltec). The material was observed using a ZEISS DSM962 microscope operating at 25 kV.
Morphological leaf measurements
The leaf area (LA) (cm2) was measured using an electronic leaf area meter (LiCor mod. LI-3100). Leaf discs were taken from fifteen leaves from each of the three individuals of S. terebinthifolius, A. verticillatus and M. brevifolia and hydrated, placing the leaf disks in distilled water for 24 h. Following this, the saturated mass of the discs was weighed on a digital balance (0,001 g) and their thickness (THIC) was measured using a digital caliper rule (0.001 ± mm). The discs were then dried at 55 °C for 72 h and their mass was measured. From this data, the succulence (SUC g m−2) was calculated based on the difference between the saturated mass and dry mass, divided by leaf disc area. The leaf mass area (LMA g m−2) was calculated by dividing leaf dry mass by disc area. Leaf density (DEN mg mm−3) was calculated by dividing LMA by thickness.
Anatomical leaf measurements
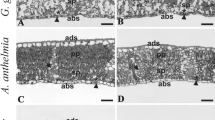
The leaf anatomy variables were made from the following: cuticle, adaxial surface, palisade parenchyma, spongy parenchyma, leaf blade, and stomata density with the aid of an Axioplan Zeiss (Oberkochen, Germany) light microscope coupled with a Hamamatsu C3077 digital camera and the software Analysis®-LINK/ISIS/ZEISS (Oxford, UK).
Chlorophyll a fluorescence
Chlorophyll a fluorescence parameters were determined at 9:00 a.m., 12:00 and 15:00 p.m. using a portable modulated pulse fluorimeter (MINI PAM, Walz, Germany). Three intact, totally expanded and healthy leaves from each of the three individuals of the species from the four sites (Forest, Gap1d, Gap2m and Gap7d) were kept in the dark for 30 min using clamps and exposed to a weak, modulated light beam (approximately 6 μmol m−2 s−1 at 660 nm), followed by exposure for 0.8 s to highly intense (10,000 μmol m−2 s−1) actinic white light (adapted from Genty et al. 1989; Van Kooten and Snel 1990). The chlorophyll a fluorescence emission parameters were recorded as minimal fluorescence (F 0), maximum fluorescence (F m), maximum quantum efficiency (F v/F m), maximum primary efficiency (F v/F 0) and the fluorescence quencher coefficients qP (photochemical quenching), qN and NPQ (non-photochemical quenching).
Photosynthetic pigments
Analyses of the content of chlorophyll a, chlorophyll b and carotenoids were carried out using an organic solvent extraction method with a dimethylsulfoxide (DMSO) reagent. Three leaf discs were taken at 12:00 p.m. from each of the three individuals. The discs were sliced and placed in Falcon™ tubes containing 5 ml of DMSO and were kept in the dark. The extracts were analyzed in a spectrophotometer at the wavelengths 480, 649 and 665 nm and quantified using the equations from Wellburn (1994) with values expressed in μmol cm−2.
Statistical analysis
The leaf and anatomical comparisons among the three species and the sites were made using Notched Box Plot (McGill et al. 1978). The analyses were done with the software Systat 12.02.00 (Systat 1992). Statistical analyses of chlorophyll a fluorescence parameters and pigments comparisons were performed using a one-way ANOVA followed by Tukey’s test (p < 0.05) (Zar 1999). The principal component analysis (PCA) was based on a comparison between the quantitative leaf characters of the species in the forest and Gap2m (Ludwig and Reynolds 1988); a statistical software package was used for the analysis (Statistica 7, StatSoft, USA Statsoft Inc. 1998).
The index of the phenotypic plasticity (Valladares et al. 2000) was calculated for the three species as the difference between the minimum and maximum mean value among the forest and Gap2m variables divided by the maximum mean value. The phenotypic plasticity index ranges from zero to one. For this, the results were presented as the median for each parameter and for the leaf traits (anatomical, morphological and physiological).
Results
Morphological leaf measurements
In general, the three species tended to have distinct values for all leaf variables (Fig. 1). The pioneer species, S. terebinthifolius, presented higher values for the leaf variables THIC, SUC and LMA, and the lowest values for highest leaf density (DEN) and higher leaf area (LA). The lowest values for THIC, SUC and LMA were observed in the early successional A. verticillatus leaves; however, this species had the DEN. Leaves from the late-successional M. brevifolia had LA and a tendency toward elevated succulence values.
Leaf morphology plasticity from forest and leaves formed after the gap formation. Notched box plot of leaf morphology features of S. terebinthifolius (St), A. verticillatus (Av) and M. brevifolia (Mb) measured in the forest and forest gap 2 months (Gap2m) after gap formation. THIC thickness (mm), SUC succulence (g m−2), LMA leaf mass area (g m−2), DEN Leaf density (mg mm−3), LA leaf area (cm2). They are notched at the median and return to full width at the lower and upper 95 % confidence interval values. Interquartile ranges define inner and outer fences. Asterisks are “outside values” and circles are “far outside values”. The medians are statistically different (α = 0.95, represented by different letters) when the confidence intervals do not overlap. Dark grey = forest; Light grey = Gap2m
When comparing the intraspecific difference (Fig. 1) between the studied sites (Forest and Gap2m), only A. verticillatus showed significant difference in the THIC, LMA and DEN variables, where leaves from the gap were thicker and had higher LMA values than those from the forest, while leaves from the forest were the densest. The leaf traits of S. terebinthifolius and M. brevifolia showed no significant differences for all studied variables.
Anatomical leaf measurements
The three species showed interspecific differences for all anatomical leaf measurements (Fig. 2). S. terebinthifolius leaves had the highest values for all of the variables studied, except the adaxial surface. A. verticillatus leaves presented the lowest values for the adaxial surface, palisade parenchyma, spongy parenchyma and leaf blade. M. brevifolia had a thinner cuticle and intermediate values for blade, spongy and palisade parenchyma.
Leaf anatomy plasticity from forest and leaves formed after the gap formation. Notched box plot of leaf anatomical features of S. terebinthifolius (St), A. verticillatus (Av) and M. brevifolia (Mb) measured in the forest and forest gap 2 months (Gap2m) after gap formation. They are notched at the median and return to full width at the lower and upper 95 % confidence interval values. Interquartile ranges define inner and outer fences. Asterisks are “outside values”. The medians are statistically different (α = 0.95, represented by different letters) when the confidence intervals do not overlap. Dark grey = forest; Light grey = Gap2m
For the intraspecific comparison between the studied sites (Forest and Gap2m), the adaxial surface and stomata density showed significant differences (Fig. 2), where the width of the adaxial surface of M. brevifolia was thicker in the forest site than the gap site and the stomata density of gap leaves from A. verticillatus was higher than the leaves collected in the forest. No significant differences were observed for S. terebinthifolius.
Chloroplast ultrastructure
The ultrastructural organization of the chloroplasts revealed a remarkable tendency toward the accumulation of starch, mostly accompanied by an increase in the number and size of oil droplets and plastoglobuli in leaves exposed to high irradiation. In S. terebinthifolius, where leaves were exposed to high irradiance for long periods each day, the chloroplasts exhibited one or two starch grains and an accumulation of oil droplets (Fig. 3a); however, no plastoglobuli was observed. There were no difference between leaves fully exposed to sun and leaves less exposed to sun (Fig. 3a–c). In both leaf types, there was an enormous accumulation of tannins occupying most of the lumen (Fig. 3a–c).
Leaf chloroplasts from forest and leaves formed after the gap formation. Transmission electron microscopy (TEM) of the mesophyll chloroplasts of S. terebinthifolius, A. verticillatus and M. brevifolia collected in the forest and forest gap, 2 months (Gap2m) after gap formation. Schinus terebinthifolius collected in the Forest (a) and Gap2m (b–c). Actinostemon verticillatus collected in the forest (d–e) and Gap2m (f–g, note the increase of the number and size of oil droplets, plastoglobuli, stroma volume and dislodgement of the stroma). h M. brevifolia from the forest. i–k M. brevifolia collected in the Gap2m presented an increase of starch grains, oil droplets and plastoglobuli and disarrangement of the thylakoid membrane (arrow), note a content pass through the chloroplast membrane to the lumen cellular (l). St Starch grain; Tn Tannin; OD Oil droplets; Pg Plastoblobuli. Scale bar a–b, d–e, h, k = 2 μm; c, i = 1 μm; f–g, j = 500 nm
Actinostemon verticillatus leaves from the forest exhibited chloroplasts of typical form, grana and stroma organization and presented few starch grains (Fig. 3d, e), while leaves from Gap2m (Fig. 3f, g) had an increased number of oil droplets and larger plastoglobuli, some changes grana organization of the thylakoids and a remarkable increase in stroma volume and relocation of the stroma.
The chloroplast from forest leaves of M. brevifolia had few oil droplets, typical grana and stroma organization and some small plastoglobuli (Fig. 3h). Leaves from Gap2m had a disarranged thylakoid membrane, and an increased number of starch grains, oil droplets and plastoglobuli (Fig. 3i, j). The plastoglobuli content passing through the chloroplast membrane into the cellular lumen is shown in Fig. 3k.
Chlorophyll a fluorescence and pigments content
The parameters maximum quantum yield of photosystem II (F v/F m) and the maximum primary yield of the photochemistry of photosystem II (F v/F 0) showed the same pattern of response for each of the studied species (Fig. 4). No significant differences were observed in S. terebinthifolius for both ratios. In general, both A. verticillatus and M. brevifolia presented no significant difference between the forest and Gap2m and lower values in the Gap1d and Gap7d for the three periods (9:00 a.m., 12:00 and 15:00 p.m.) measured. For Gap7d, M. brevifolia presented the lowest values during the three periods studied, while A. verticillatus had decreased values along the three periods. On the other hand, M. brevifolia presented a linear F v/F m and F v/F 0 response for the three periods, while A. verticillatus showed a remarkable slope at 12:00 p.m. in Gap1d.
Photosynthetic efficiency during the acclimation process. Mean values of the maximum quantum yield of photosystem II (F v/F m) and the maximum primary yield of photochemistry of photosystem II (F v/F 0) measured at 9, 12 and 15 h for different traits: forest and forest gap at 1 day (Gap1d), 7 days (Gap7d) and 2 months (Gap2m) after gap formation. Different letters indicate significant differences at p < 0.05 (HSD Tukey test). NS not significantly different
The qP value increased in function in exposed environments of forest gaps (Table 2); however, no significant differences were observed for S. terebinthifolius. The qP values for A. verticillatus showed no significant differences at 12:00 p.m. At 9:00 a.m.; the only difference was the higher qP values in Gap2m compared to the forest traits. At 15:00 p.m., Gap2m still had higher qP values, while no significant difference were found among the forest, Gap1d and Gap7d. The qP values for M. brevifolia presented the lowest values in the forest when compared to all other stages of the gap.
The NPQ values of S. terebinthifolius presented no significant difference for the three periods measured. A. verticillatus and M. brevifolia showed higher NPQ values in the gaps than in the forest for the three periods (Table 2).
The Chl a/b and carotenoids contents were measured at 12:00 p.m. (Table 3). Only M. brevifolia presented a significant difference in the Chl a/b rate, where Gap2m showed the highest values. There were no variations in the concentrations of carotenoids, due to the environment, for the species studied.
PCA and plasticity index
For PCA, the first two axes explain about 69 % of the total variation (Fig. 5a). The ordination along with the principal components 1 and 2 show the separation of the five groups of plants into distinct clusters where even intraspecific groups (Fig. 5a, dashed circles) composed of A. verticillatus and M. brevifolia were separated clearly as plants from the forest and plants from gaps. Individuals from S. terebinthifolius (SG and SF) could not be segregated.
In Fig. 5b, the first axis, which explains 40.01 % of the total variation, shows variables that are more important in separating the groups. Most of these variables that exhibit high factor loadings are related to morpho-anatomical leaf characteristics (Thickness, Succulence, Blade, Palisade, LSM, Chl a/b and Density). Seven variables (Spongy, Adaxial ep., Leaf area, F v/F 0, F v/F m, Cuticle and NPQ) were found to be highly associated with the second principal component, which explains 29.32 % of the variation (Fig. 5b).
The different leaf traits (morpho-anatomical and physiological) were compared in order to present the plasticity index (sensu Valladares et al. 2000) (Table 4). The highest median was for a morphological leaf trait (leaf area 0.60) and the lowest was for a physiological trait (F v/F m: 0.01). Cuticle, leaf area, F v/F 0 and carotenoids showed the highest plasticity index among anatomical, morphological and physiological traits, respectively, while sponginess and thickness showed higher disparity.
When comparing species, the highest plasticity index for the morphological and physiological traits was observed in A. verticillatus (0.47 and 0.20, respectively), while S. terebinthifolius had the lowest (0.27 and 0.15, respectively). For anatomical traits, S. terebinthifolius presented the highest plasticity index (0.32) and M. brevifolia the lowest (0.24).
Discussion
Morpho-anatomical plasticity
Leaves of the S. terebinthifolius were the THIC, and together with M. brevifolia were more SUC than the leaves of A. verticillatus. Leaf thickness is related to an improvement in the photosynthetic mesophyll cell layer because high irradiance promotes elongation of the palisade parenchyma and development of more subepidermal layers which causes an increase in thickness (Lambers et al. 1998; Niinemets et al. 1999). High photosynthetic performance requires an improvement in water apportion in leaf tissues, and the succulence of leaves represents a water storage mechanism that possibly promotes the efficient use of water during moments when water is not available (Lamont and Lamont 2000; Schwinning and Ehleringer 2001).
According to Oguchi et al. (2003, 2005), there is a significant relationship between LMA and THIC. Higher LMA and THIC are typically associated with species, genotypes, and phenotypes from xeric or exposed sites (Sobrado 1986; Myers et al. 1987; Abrams 1990). Higher LMA of S. terebinthifolius leaves might reflect better efficiency in water absorption by maintaining higher osmotic pressure and stomata conductance (Abrams et al. 1994).
The lowest LA values were found in S. terebinthifolius and the highest in M. brevifolia. This might represent two microclimatic extremes that these species colonize, where the reduction of leaf area in S. terebinthifolius corresponds to an adaptation to counteract the negative effects of overheating and high transpiration rates (Gates 1980). On the other hand, M. brevifolia prioritizes an investment in photosynthetic tissues, and, as a consequence, this species maintains a high leaf area to maximize the irradiation flux above the leaves.
The leaves of A. verticillatus had the lowest THIC, SUC and LMA and DEN that are commonly related to an increase in fibers and sclereids and thicker cell walls, which promotes changes in tissue elasticity (Niklas 1989) and increases water tolerance limitations (Niinemets 2001). Such modifications, according to Witkowski and Lamont (1991), also promote reduction in herbivory.
Only A. verticillatus presented plasticity in leaves that was related to the study sites (Forest and Gap2m), where it adjusted its leaf thickness and LMA in the high irradiance environment of the gap and grew the densest leaves in the forest. The high LMA and thickness of A. verticillatus leaves allow for a higher photosynthetic ratio, and for this, consequently, an increase in water flux is required. If the density of the leaves of this species causes a decrease in intercellular conductance of CO2 (Reich et al. 1999) then the uptake of water can increase (Niinemets 2001). In addition, the plasticity of the stomata density allows for efficient gas exchange, which counteracts the negative effects of an increase in leaf density.
The presence of thicker cuticles and high stomata density in S. terebinthifolius was probably a response to higher exposure to light (Cutler et al. 1982; Bastos et al. 1993; Rôças et al. 1997, 2001), where thicker cuticles act to minimize uncontrolled water loss (Schönherr and Riederer 1989) and high stomata density allows for efficient gas exchange (Lleras 1977), better control of transpiration, and possibly to avoid excessive water loss (Weyers and Meidner 1990). As with other late-successional species, M. brevifolia showed the thinnest cuticle, which is an adjustment to the shady environment of the forest.
The thicker palisade and spongy parenchyma found in S. terebinthifolius leaves represents a structural mechanism that maximizes light absorption and carbon fixation. The tubular shape of palisade cells aids in the penetration of direct sunlight, and thicker spongy parenchyma helps scatter the light, thus increasing the chance that light will be absorbed for photosynthesis (Vogelmann and Martin 1993; Vogemann et al. 1996). The palisade and spongy parenchyma are the two main characteristics responsible for an increase in blade thickness and are considered an improvement to the photosynthetic structural mechanism, because thicker mesophyll facilitates CO2 dissolution into the water within the cell wall, which decreases the resistance to CO2 diffusion from the intercellular space to the chloroplast stroma (Evans 1999; Terashima et al. 2001). M. brevifolia also presented high leaf blade values, mainly because of the thickness of the spongy parenchyma. These results associated with the higher SUC values could corroborate with the hypothesis of adaptation mechanisms with investments in tissues that inhibit water shortage and maximize gas exchange.
Ultrastructure, chlorophyll a fluorescence and pigments
It is known that changes in the grana organization of the thylakoids, an increase in stroma volume, oil droplets and plastoglobuli are commonly related to plants subjected to environmental conditions that increase oxidative stress on the photosynthetic apparatus (Molas 2002; Vitória et al. 2003, 2006; Munné-Bosch and Alegre 2004; Austin et al. 2006; Lage-Pinto et al. 2008). All of these ultrastructural changes were observed in the A. verticillatus and M. brevifolia chloroplasts from the Gap2m site. M. brevifolia also showed a noticeable increase of starch grains and plastoglobuli content. According to Molas (2002), disturbances and inhibitions of carbohydrate transport from leaves to the other parts of plant were accompanied by an increase in plastoglobuli content.
The F v/F m and F v/F 0 of the M. brevifolia revealed that maximum stress occurred 7 days after the gap formed, and that the photosynthetic process stabilized after 2 months (Gap2m). A similar response occurred for A. verticillatus, but this species showed more sensitivity at 12:00 p.m., suggesting a “midday depression” of net CO2 uptake, which is observed when the photon flux density (PFD) and leaf temperatures reached their daily maximum (Muraoka et al. 2000; Franco and Lüttge 2002; Lichtenthaler et al. 2005). In addition, thin leaves of A. verticillatus did not allow an efficient dissipation of large amounts of energy that occur in forest gaps.
According to Jakl and Bolhar-Nordenkampf (1991), values of F v/F m between 0.75 and 0.85 reflect an efficient photosynthetic apparatus, while a decrease in the F v/F 0 ratio is an indication of structural damage, which occurs in the thylakoids and affects the photosynthetic transport of electrons (Vernay et al. 2007). The trend of lower F v/F m and F v/F 0 in A. verticillatus, and mainly in the late-successional M. brevifolia in the early stage of gap formation, suggests problems in photosynthetic performance (Krause and Weis 1991; Krause et al. 2001) or a lower efficiency of PSII when exposed to high irradiance (Critchley and Russell 1994).
The reduction state of the primary stable quinone acceptor of PSII can be estimated as photochemical quenching (qP). The qP values for A. verticillatus and M. brevifolia increased in the exposed environments of forest gaps, where large amounts of absorbed energy were driven to photochemical reactions of photosynthesis (Krause and Weis 1991; Bolhàr-Nordenkampf and Öquist 1993).
Non-photochemical quenching (NQP) monitors the constant rate of heat loss. The increase in NPQ of A. verticillatus and M. brevifolia may indicate a control mechanism in the thylakoid membrane that adjusts thermal dissipation of excess excitation energy that exists because there is more light in a gap (Demmig-Adams et al. 1996). The chlorophyll a/b ratio of the M. brevifolia also had higher values in the gap, and, according to Brugnoli et al. (1998), NPQ is linearly related to the chlorophyll a/b ratio. A higher chlorophyll a/b ratio may indicate a higher ratio of PSI to PSII, which was observed by Takabayashi et al. (2005) as an adaptive stress mechanism.
The ultrastructural (M. brevifolia) and morpho-anatomy (A. verticillatus) differences found between the Gap2m and forest sites, and mainly the consequent stabilization in photosynthesis after 2 months, suggest some acclimation mechanisms that were sufficient enough to maintain the normal photosynthetic process.
The multivariate analysis distinguished the three species and two sub-clusters formed by the intraspecific variation between A. verticillatus and M. brevifolia, mostly as a consequence of the morpho-anatomical differences of leaf traits. The individuals of S. terebinthifolius could not be segregated. The plasticity index analysis (Valladares et al. 2000) was performed to simplify the comparison of leaf responsiveness to gap formation among the three species. Higher morphological and physiological plasticity in the early successional A. verticillatus suggests two important adaptations to high irradiance in this species; morphological plasticity implies an efficient capacity to resource acquisition of the plant (Crick and Grime 1987) and higher physiological plasticity is related to a greater capacity to exploit high light environments (Strauss-Debenedetti and Bazzaz 1991; Yamashita et al. 2000). In comparison to A. verticillatus, the lower anatomical plasticity in the late-successional M. brevifolia, in association with higher costs of leaf construction and maintenance (higher SUC), revealed limited leaf acclimation. This suggests that this species, as other later secondary, has a metabolism adapted to constantly low levels of flux resource (Bazzaz and Pickett 1980; Strauss-Debenedetti and Bazzaz 1991; Rabelo et al. 2012).
Conclusions
This study, based on field conditions, describes the leaf morpho-anatomy, ultrastructure and ecophysiology of tree species of different successional status, and how the status can favor the acclimation of species to forest gaps. All of the features studied reinforce how the pioneer species S. terebinthifolius is adjusted well to open environments; however, the absence of this species just inside the forest edge suggests other abilities are required, especially for germination and seedling establishment, to grow in the forest (Whitmore 1989, 1996).
Higher morphological and ecophysiological plasticity of the early successional A. verticillatus reveals the opportunistic behavior of this species to take advantage of high light environments when a forest gap is created. The differences in leaf acclimation of A. verticillatus suggest an investment in mechanisms that promote more efficient photosynthesis by increasing leaf thickness and LMA, and in water absorption by increasing leaf density at the expense of gas exchange, which is compensated by a high density of stomata. M. brevifolia compensates for the higher costs of leaves and the lowest leaf plasticity with ultrastructural changes that adjust the photosynthetic process and this, consequently, promotes shorter leaf pay-back time (time required to compensate for leaf construction) (Niinemets 2001). Complementary greenhouse studies of plastic response to single types of stress, such as irradiance or water stress, might shed additional light on the adaptive strategies of these species.
References
Abrams MD (1990) Adaptations and responses to drought in Quercus species of North America. Tree Physiol 7:227–238
Abrams MD, Kubiske ME, Mostoller SA (1994) Relating wet and dry year ecophysiology to leaf structure in contrasting temperate tree species. Ecology 75:123–133
Austin JR II, Frost E, Vidi P, Kessler F, Staehelin LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18:1693–1703
Bastos EO, Perazzolo M, Baptista JMR (1993) Variação do número de estômatos e micropêlos em Paspalum vaginatum SW em relação às condições abióticas numa marisma do estuário da Lagoa dos Patos, Rs-Brasil. Acta Botanica Brasilica 6:105–117
Bazzaz FA, Pickett STA (1980) Physiological ecology of tropical succession: a comparative review. Annu Rev Ecol Syst 11:287–310
Bolhàr-Nordenkampf HR, Öquist GO (1993) Chlorophyll fluorescence as a tool in photosynthesis research. In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf HR, Leegood RC, Long SP (eds) Photosynthesis and production in a changing environment: a field and laboratory manual. Chapman and Hall, London, pp 193–206
Brown N (1993) The implications of climate and gap microclimate for seedling growth conditions in a Bornean lowland forest. J Trop Ecol 9:153–168
Brugnoli E, Scatazza A, De Tullio MC, Monteverdi MC, Lauteri M, Augusti A (1998) Zeaxanthin and non-photochemical quenching in sun and shade leaves of C3 and C4 planta. Physiol Plant 104:727–734
Crick JC, Grime JP (1987) Morphological plasticity and mineral nutrient capture in two herbaceous species of contrasted ecology. New Phytol 107:403–414
Critchley C, Russell W (1994) Photoinhibition of photosynthesis in vivo: the role of protein turnover in photosystem II. Physiol Plant 92:188–196
Cutler DS, Alvin KL, Price CE (1982) The plant cuticles. Academic Press, London
Demmig-Adams B, Adams WW, Barker DH, Logan BA, Bowling DA, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Ann Rev Ecol Syst 18:431–451
Evans JR (1999) Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytol 143:93–104
Franco AC, Lüttge U (2002) Midday depression in savanna trees: coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 131:356–365
Gates DM (1980) Biophysical ecology. Springer-Verlag, New York
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Jakl T, Bolhar-Nordenkampf HR (1991) Energy conversion efficiency and energy partitioning of White lupins (Lupinus albus L). Biores Technol 36:193–197
Köppen W (1948) Climatologia con un estudio de los climas de la tierra. Fondo de Cultura Economica, México
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu rev plant physiol plant mol biol 42:313–349
Krause GH, Koroleva OY, Dalling JW, Winter K (2001) Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant, Cell and Environ 24:1345–1352
Lage-Pinto F, Oliveira JG, Da Cunha M, Souza CMM, Rezende CE, Azevedo RA, Vitória AP (2008) Chlorophyll a fluorescence and ultrastructural changes in chloroplast of water hyacinth as indicators of environmental stress. Environ Exp Bot 64:307–313
Lambers H, Chapin FS, Pons TL (1998) Plant physiological ecology. Springer-Verlag, Berlin
Lamont B, Lamont H (2000) Utilizable water in leaves of 8 arid species as derived from pressure-volume curves and chlorophyll fluorescence. Physiol Plant 110:64–71
Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 43:379–393
Lleras E (1977) Differences in stomata number per unit area within the same species under different micro-environmental conditions: a working hypothesis. Acta Amazonica 7:473–476
Ludwig JA, Reynolds JF (1988) Statistical ecology: a primer on methods and computing. John Wiley and Sons, New York
McGill R, Tukey JW, Larsen WA (1978) Variations of box plot. Amer Statist 32:12–16
Molas J (2002) Changes of chloroplast ultrastructure and total chlorophyll concentration in cabbage leaves caused by excess of organic Ni(II) complexes. Environ Exp Bot 47:115–126
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Muraoka H, Tang YH, Terashima I, Koizumi H, Washitani I (2000) Contributions of diffusional limitation, photoinhibition and photorespiration to midday depression in Arisaema heterophyllum in natural high light. Plant, Cell and Environ 23:235–250
Myers BJ, Robichaux RH, Unwin GL, Craig IE (1987) Leaf water relations and anatomy of a tropical rainforest tree species vary with crown position. Oecologia 74:81–85
Naidu SL, Delucia EH (1998) Physiological and morphological acclimation of shade-grown tree seedlings to late-season canopy gap formation. Plant Ecol 138:27–40
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area density, and thickness in trees and shrubs. Ecology 82:453–469
Niinemets Ü, Kull O, Tenhunen JD (1999) Variability in leaf morphology and chemical composition as a function of canopy light environment in co-existing trees. Int J Plant Sci 160:837–848
Niklas KJ (1989) Mechanical behavior of plant tissues as inferred from the theory of pressurized cellular solids. Am J Bot 76:929–937
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant, Cell Environ 26:505–512
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant, Cell Environ 28:916–927
Oguchi R, Hikosaka K, Hiura T, Hirose T (2006) Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate deciduous forest. Oecologia 149:571–582
Peterson G, Allen C, Holling C (1998) Ecological resilience, biodiversity and scale. Ecosystems 1:6–18
Quesada M, Arturo Sanchez-Azofeifa G, Alvarez-Anorve M, Stoner KE, Avila-Cabadilla L, Calvo-Alvarado J, Castillo A, Espiritu-Santo MM, Fagundes M, Fernandes GW, Gamon J, Lopezaraiza-Mikel M, Lawrence D, Morellato P, Powers JS, Neves F, Rosas-Guerrero V, Sayago R, Sanchez-Montoya G (2009) Succession and management of tropical dry forests in the Americas: review and new perspectives. Forest Ecol Manag 258:1014–1024
Rabelo GR, Klein DE, Da Cunha M (2012) Does selective logging affect the leaf structure of a late successional species? Rodriguesia 63:419–427
RadamBrasil (1983) Levantamento de recursos naturais. Ministério das Minas e Energia, Rio de Janeiro-Brazil
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969
Rôças G, Scarano FR, Barros CF (1997) Leaf anatomy plasticity of Alchornea triplinervia (Euphorbiaceae) under distinct light regimes in a Brazilian montane Atlantic rain forest. Trees 11:469–473
Rôças G, Scarano FR, Barros CF (2001) Leaf anatomical variation in Alchornea triplinervia (Spreng) Müll Arg (Euphorbiaceae) under distinct light and soil water regimes. Bot J Linn Soc 136:231–238
Schliemann SA, Bockheimb JB (2011) Methods for studying tree fall gaps: a review. Forest Ecol Manag 261:1143–1151
Schönherr J, Riederer M (1989) Foliar penetration and accumulation of organic chemicals in plant cuticles. Rev Environ Contam Toxicol 108:1–70
Schwinning S, Ehleringer JR (2001) Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J Ecol 89:464–480
Sobrado MA (1986) Aspects of tissue water relations and seasonal changes of leaf water potential components of evergreen and deciduous species coexisting in tropical dry forests. Oecologia 68:413–416
Statsoft, Inc. (1998) Statistica for windows, version 6.0, Tulsa, OK
Strauss-Debenedetti S, Bazzaz FA (1991) Plasticity and acclimation in tropical Moraceae of different successional positions. Oecologia 87:377–387
Strauss-Debenedetti S, Bazzaz FA (1996) Photosynthetic characteristics of tropical trees along successional gradients. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 162–186
Sultan SE (2004) Promising directions in plant phenotypic plasticity. Perspect Plant Ecol, Evol Syst 6:227–233
Systat (1992) Systat for Windows: graphics, version 7.01, 5th edition, Systat, Evanston
Takabayashi A, Kishine M, Asada K, Endo T, Sato F (2005) Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis. Proc Natl Acad Sci USA 102:16898–16903
Terashima I, Miyazawa S, Hanba YT (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO, diffusion in the leaf. J Plant Res 114:93–105
Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81:1925–1936
Van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Veloso HP, Rangel Filho ALR, Lima JCA (1991) Classificação da vegetação brasileira adaptada a um sistema universal. Instituto Brasileiro de Geografia e Estatística IBGE, Rio de Janeiro-Brazil
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575
Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH (1995) Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol Evol 10:212–217
Villela DM, Nascimento MT, Aragão LEOC, Gama DM (2006) Effect of selective logging on forest structure and cycling in seasonally dry. Brazilian forest J Biogeography 33:506–516
Vitória AP, Rodrigues APM, Da Cunha M, Lea PJ, Azevedo RA (2003) Structural changes in radish seedlings exposed to cadmium. Biol Plant 47:561–568
Vitória AP, Da Cunha M, Azevedo RA (2006) Ultrastructural changes of radish leaf exposed to cadmium. Environ Exp Bot 58:47–52
Vogelmann TC, Martin G (1993) The functional significance of palisade tissue: penetration of directional vs diffuse light. Plant, Cell Environ 16:65–72
Vogemann TC, Nishio JN, Smith WK (1996) Leaves and light capture: light propagation and gradients of carbon fixation within leaves. Trends Plant Sci 1:65–70
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Weyers JDB, Meidner H (1990) Methods in stomatal research. Longman Scientific and Technical, Lynn
Whitmore TC (1989) Canopy gaps and two major groups of forest trees. Ecology 70:536–538
Whitmore TC (1996) A review of some aspects of tropical rain forest seedling ecology with suggestions for further enquiry. In: Swaine MD (ed) The ecology of tropical forest seedlings UNESCO and Parthenon Publishing Group, Paris, pp 3–39
Witkowski ETF, Lamont BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
The authors are indebted to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoando de Pessoal de Nível Superior (CAPES) for their financial support. We thank the technicians Beatriz Ferreira Ribeiro and Giovanna Alves de Moraes of Laboratório de Biologia Celular e Tecidual da Universidade Estadual do Norte Fluminense (LBCT/UENF) for helping with laboratory tasks, and the Instituto Estadual do Ambiente (INEA) to the plant collecting license. This study was part of the PhD thesis of the first author, presented to the Programa de Pós-Graduação em Biociências e Biotecnologia da Universidade Estadual do Norte Fluminense (UENF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Shane.
In Memoriam: Ricardo A. Cruz.
Rights and permissions
About this article
Cite this article
Rabelo, G.R., Vitória, Â.P., da Silva, M.V.A. et al. Structural and ecophysiological adaptations to forest gaps. Trees 27, 259–272 (2013). https://doi.org/10.1007/s00468-012-0796-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-012-0796-2