Abstract
Individual tree-ring width chronologies and mean chronologies from Pinus tabuliformis Carr. (Chinese pine) and Sabina przewalskii Kom. (Qilian juniper) tree cores were collected and analyzed from two sites in the eastern Qilian Mountains of China. The chronologies were used to analyze individual and time-varying tree-ring growth to climate sensitivity with monthly mean air temperature and total precipitation data for the period 1958–2008. Climate–growth relationships were assessed with correlation functions and their stationarity and consistency over time were measured using moving correlation analysis. Individuals’ growth–climate correlations suggested increased percentages of individuals are correlated with certain variables (e.g., current June temperature at the P. tabuliformis site; previous June, December and current May temperature and May precipitation at the S. przewalskii site). These same climatic variables also correspond to the mean chronology correlations. A decreased percentage of individuals correlated with these climatic variables indicates a reduced sensitivity of the mean chronology. Moving correlation analysis indicated a significant change over time in the sensitivity of trees to climatic variability. Our results suggested: (1) that individual tree analysis might be a worthwhile tool to improve the quality and reliability of the climate signal from tree-ring series for dendroclimatology research; and (2) time-dependent fluctuations of climate growth relationships should be taken into account when assessing the quality and reliability of reconstructed climate signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investigating the relationships between climate proxies and the myriad of factors that affect them provides a great challenge for paleo-climatology (Briffa 1995; Esper et al. 2002). Such a challenge is especially acute when applying biological proxies to climate reconstruction due to the complexity of biological responses to climate forcing (e.g., tree-rings). As natural archives, tree-rings are the most important and widely used sources of long-term proxy data for paleo-environmental studies and climate reconstructions (Mann et al. 1998; Esper et al. 2002). The relationships between tree-ring proxies and climatic and ecological variables have been traditionally described using quasi-linear models that do not account for change over time. However, several studies have documented the dynamic nature of tree-ring growth responses to climate variations (Carrer and Urbinati 2006; Carrer 2011; Zhang and Wilmking 2010; Zhang et al. 2011).
In dendroclimatology, the uniformitarian principle implies that tree growth–climate relationships are stable over time (Fritts 1976), thus allowing dendroclimatologists to infer the character of past climates derived from tree-ring calibrations from recent observational data. Unstable relationships between tree growth and climate factors (Wilmking et al. 2004, 2005; D’Arrigo et al. 2007; Zhang et al. 2008; Carrer 2011), however, have indicated that tree growth response to climate forcing is inconsistent with the assumption of stability as invoked by the uniformitarian principle. This finding casts doubt on some tree-ring reconstructions of past climate that are based on mean chronologies. In addition, some tree-ring research has reported that tree-ring width and density variations have changed in response to air temperature, with decreasing sensitivity to temperature in recent decades (Briffa et al. 1998; Solberg et al. 2002; Lloyd and Bunn 2007). Given the fact that trees respond differentially to climate (e.g., Briffa et al. 1998; D’Arrigo et al. 2007; Zhang et al. 2008), it may be worthwhile to use individual tree chronologies instead of mean chronologies to investigate tree growth–climate relationships (Wilmking et al. 2004, 2005; Pisaric et al. 2007; Zhang and Wilmking 2010; Zhang et al. 2011).
In spite of possible advantages of using individual tree chronologies (as opposed to mean chronologies), relatively few studies have compared temporal responses from individual tree chronologies and a mean chronology in the eastern Qilian Mountains. To address this existing data gap, our study focuses on two climate-sensitive tree species—Pinus tabuliformis Carr. (Chinese pine) and Sabina przewalskii Kom. (Qilian juniper)—to examine how their tree-ring width responds to the climate change at the individual tree level in an effort to reconstruct the past climate in the Qilian Mountains.
The Qilian Mountains are a transitional climatic region of Tibet where arid zones, eastern humid regions, and colder regions intersect (Liu et al. 2005), making it an opportune location to examine the effects of climate change on trees. Tree-ring research has been conducted in this area since the 1970s and a number of dendrochronology studies have been published (e.g., Wu et al. 1990; Shao et al. 2005; Gou et al. 2005; Liu et al. 2006; Liang et al. 2008; Zhang et al. 2009). Several climate reconstructions have been recently conducted in the Qilian Mountains (Yang et al. (2010, 2011); Qin et al. (2010, 2011)). Similar work has been studied in the northeastern Tibetan Plateau (Gou et al. 2007). These studies have emphasized historical alterations in air temperature and rainfall as well as the frequency of climatic extremes. In addition, Shao et al. (2010) and Zhang et al. (2003) developed 3,585-year and 2,326-year chronologies, respectively, using live trees and archaeological wood in Qilian Mountains. While this past research has increased our understanding of climate change in the Qilian Mountains, only a few have considered the responses of different tree species to climate variability over time (Zhang et al. 2008; Fang et al. 2011) and the difference between sub-chronologies and the mean chronologies (Zhang and Wilmking 2010; Zhang et al. 2011).
Thus, the present study adds to the existing body of knowledge by giving further insights into the tree growth–climate response of P. tabuliformis and S. przewalskii in the Qilian Mountains. In particular, this study builds upon existing knowledge by comparing the individual tree chronology (as opposed to sub-chronologies) to the mean chronology to investigate whether interspecific differences exist. Our aim was to: (1) investigate the tree growth climate relationships by comparing individual tree chronologies to the mean chronologies; and (2) determine whether the year-to-year climate–growth responses are stationary over time. A better understanding of the tree growth–climate relationship in northwest China is important for regional tree-ring reconstructions, modeling carbon storage, and forest management.
Materials and methods
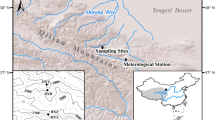
The tree-ring cores were collected from high-altitude forest areas of the eastern Qilian Mountains (Fig. 1). The height of upper tree line in this area is about 3,000–3,200 m. The TanGouZi (TGZ) study site is a mono specific P. tabuliformis forest. At low altitudes, there is an ideal environment for tree growth with thick humic soil horizons; however, as soil depth decreases with altitude, the environment for tree growth is much less favorable. S. przewalskii tree cores were sampled from the XianMiNang (XMN) site, featuring barren soil with bare rocks and shrubs under the forest cover. The distance between the sample sites and the meteorological stations ranges from 50 to 108 km. Detailed sampling information and locations of sampling sites and their surrounding meteorological stations are shown in Table 1 and Fig. 1. Samples were collected from trees at elevations of less than 100 m to maintain consistent climatic signals when sampling.
Following the standard procedures of the International Tree Ring Data Bank (ITRDB), two cores were taken on the cross-slopes sides of the trunk using increment borers at approximately 1.3 m above ground level. A total of 127 cores were acquired from 64 living trees at the P. tabuliformis site. A sum of 83 cores was harvested from 42 living trees at the S. przewalskii site (Table 1). Tree cores were processed in the laboratory using the standard procedure described by Stokes and Smiley (1968). As such, cores were air-dried, glued in prepared wooden mounts, and sanded with fine sand papers to produce a polished surface to permit the identification of all cells and cross-dating using COFECHA (Holmes 1983). Most of the tree-ring cores and the mean tree-ring series of the sample sites were from 120 to 170 years of age. We found that all the trees’ radial growth showed increases and then decreases in width over time. Both the entire chronologies and individual series were detrended using ARSTAN (Cook and Kairlukstis 1990). We chose a 60-year cubic spline function equal to 1/3-1/2 of the series length to detrend the tree’s biological growth due to the radial growth pattern and length of our series. All of the trees cores within one site produced mean standard chronologies (STD) using a bi-weight mean calculation (Cook and Holmes 1986). One or two cores of one tree that was detrended were using the standard arithmetic mean function to produce a standardized curve for the single tree chronologies (i.e., individual tree chronology).
Meteorological observations began in the early 1950s in the Qilian Mountains. In order to investigate climate–tree growth relationships in the study region, the chronologies were correlated with monthly mean air temperature and precipitation records from nearby meteorological stations (XiNing, MenYuan and WuShaoLing). General information about the three meteorological stations (XiNing, MenYuan and WuShaoLing) is given in Table 1. July is the warmest and wettest month. The distribution of temperature and precipitation throughout the year is quite similar at all three stations, which represent the same climate zone (Li 2011). The average values of the temperature and precipitation of the three stations were used as the regional mean climate data during the common period 1958–2008.
Correlation analysis was carried out between two mean tree-ring chronologies (P. tabuliformis and S. przewalskii) and observed monthly precipitation and mean air temperature records of the study area. The same correlation analysis was conducted between the individual tree chronologies and the observed climate data. Regional average values of observed meteorological data were calculated by arithmetic average, and were used as regional data in the correlation analysis.
A moving correlation analysis employs a fixed number of years progressively moved across time to compute the correlation coefficients (Biondi 1997). Considering the fact that those 30 years represent the climatic norm and that an n of 30 is held as a sufficient sample size for statistical analyses, we chose 30 years as the moving interval for our analyses. The results of the moving correlation provide a useful perspective on the evolution of tree responses to climate over time.
Results
Mean chronology response to climate change
Two different tree growth patterns are clearly visible between the two test tree species (Fig. 2). Table 2 shows the mean standard chronology (STD) at the P. tabuliformis and S. przewalskii sites. Statistical analyses of the two tree species ring width STD chronologies indicated that the P. tabuliformis had larger standard deviation (SD), mean sensitivity (MS), PC1 (%), and the signal noise ratio (SNR) values than S. przewalskii, indicating that P. tabuliformis is more sensitive to climate than S. przewalskii. The mean width and mean age of S. przewalskii were higher than P. tabuliformis (Table 2).
The correlation coefficient analysis between both species’ mean chronologies and observed mean monthly air temperature and monthly total precipitation is shown in Figs. 3 and 4. Figure 3 indicates that the tree-ring width mean chronology of P. tabuliformis at the TGZ site responded significantly and negatively to current June air temperature, but significantly and positively to current June precipitation. S. przewalskii tree-ring width mean chronology at the XMN site showed a strong and negative relationship with mean air temperature in the current May and a significant positive correlation with previous June and December air temperatures and current May precipitation (Fig. 4). The mean growth response of P. tabuliformis and S. przewalskii in May or June was significantly affected by stress induced from drought.
Individual tree chronologies response to climate change
The individual tree chronologies climate–growth responses are summarized in Figs. 5 and 6 for the P. tabuliformis and S. przewalskii sites, respectively. The importance of both mean air temperature and precipitation is illustrated in the tree growth dynamics of individual trees (Figs. 5, 6). Individual tree growth–climate correlations showed that single tree responses were different from the mean chronology response for some climatic variables but similar for others. Mean chronology has a significant relationship with the current June mean air temperature that is significantly correlated with the highest percentage of individual trees at the P. tabuliformis site (Fig. 3). The current June mean air temperature is negatively correlated with the mean chronology (r = −0.303, Fig. 3). Similarly, the largest percentages of individual trees (46.9 %) were negatively and significantly correlated with the current June air temperature at the P. tabuliformis site (Table 3). More than 37.5 % of individual trees were also significantly correlated with the current June precipitation at the P. tabuliformis site (Table 3) which is consistent with the mean chronology (Fig. 3).
Correlations between individual trees at the P. tabuliformis site with a mean monthly air temperature; and b total monthly precipitation. Each colored line represents a series of correlation coefficients of one tree with mean air temperature and precipitation from previous May to the current October; horizontal dashed lines indicate 95 % confidence level and horizontal dash-dotted lines indicate the 99 % confidence level
Correlations between individual trees at the S. przewalskii site with a mean monthly temperature; and b total monthly precipitation. Each colored line represents a series of correlation coefficients of one tree with mean air temperature and precipitation from previous May to the current October; horizontal dashed lines indicate 95 % confidence level and horizontal dash-dotted lines indicate the 99 % confidence level
The highest percentage of individual tree growth–climate correlations, however, was not consistent with the mean chronology response to climate factors. For example, more than 17 (26.7 %), 13 (20.3 %) and 14 (21.9 %) individual trees significantly responded to the previous September precipitation, December precipitation, and current January air temperature at the P. tabuliformis site, respectively (Table 3). The mean chronology was not correlated significantly with the previous September precipitation, December precipitation, or current January air temperature at the P. tabuliformis site (Fig. 3; Table 3).
Previous June and December mean air temperatures, current May mean air temperature, and current May precipitation showed a large percentage of significantly correlated individuals with radial growth at the S. przewalskii site (Table 3). The mean chronology-climate correlations are relatively high for the previous June, December and current May air temperatures (Fig. 4). More than 22 % of individual trees’ radial growth is correlated significantly with current September mean air temperature and current June precipitation, but the mean chronology was not correlated significantly with any of these climatic variables at the S. przewalskii site (Table 3; Fig. 4).
Time dependence of tree width mean chronology responses to climate
Moving correlation function analysis was carried out on the two mean chronologies and the mean air temperature and precipitation to provide key insights into the evolution of tree response to climate over time (Figs. 7, 8). The climate–growth moving relationships for prior December, current February, March, April and September air temperature and for prior October, November and December and current January, March precipitation were not significant at the 95 % confidence level (Fig. 7). This is to say that non-significant climate variables in the mean chronology, such as previous December air temperature, current February and March air temperature and previous October precipitation, have a stationary response over time at the P. tabuliformis site (p > 0.05; Fig. 7).
Moving correlation function coefficients calculated between climatic variables (a temperature; b precipitation) (from previous October to current September) and the mean chronology at the P. tabuliformis site. Period: 1958–2008; moving window: 30 years. Each point corresponds to the end of a 30-year interval. The dashed horizontal lines are the significance levels (p < 0.05)
Moving correlation function coefficients calculated between climatic variables (a temperature; b precipitation) (from previous October to current September) and the mean chronology at the S. przewalskii site. Period: 1958–2008; moving window: 30 years. Each point corresponds to the end of a 30-year interval. The dashed horizontal lines are the significance levels (p < 0.05)
In contrast, all of the climate-sensitive variables, and some non-sensitive variables in the mean chronology, such as June air temperature and June precipitation, previous October, November and current May, July and August air temperatures, produced significant transient responses, which varied markedly within the 50-year period at the P. tabuliformis site (Fig. 7). As shown in Fig. 7, the moving correlation coefficients between the P. tabuliformis tree growth and prior October air mean temperature increased, then decreased and increased over the time. The current May, June and August mean air temperatures exhibited an increased and then decreased correlation trend with tree radial growth over the time. Precipitation has changed slightly and fluctuated during past decades and there are no significant changes (p > 0.05; Fig. 7).
Most of the non-sensitive climate variables, such as June and August mean air temperature and January and February precipitation, have a stable response over time at the S. przewalskii site (Fig. 8). The mean chronology responds to previous December mean air temperature, current May air mean temperature and May precipitation significantly (Fig. 4), whereas these relationships were unstable over time at the S. przewalskii site (Fig. 8a). It is also clear that there is an increasing trend in the variability of the June precipitation at the S. przewalskii site (Fig. 8b). Previous December air temperature was a key variable, producing highly significant correlation values to radial growth for the whole time span, despite some variability at the S. przewalskii site (Fig. 8a).
Discussion
Mean and individual chronologies of P. tabuliformis and S. przewalskii were found to vary in response to climate in the eastern Qilian Mountains. This is in agreement with the results that individual trees often respond to climate differently than that predicted from mean growth–climate correlations (Ettl and Peterson 1995). Correlation analysis of the mean chronology showed that P. tabuliformis tree growth was negatively correlated with June air temperature and positively correlated with June precipitation (Fig. 3), suggesting that drought stress in June could affect the tree growth. S. przewalskii mean tree radial growth was positively correlated with the previous June and December air temperatures and current May precipitation but negatively correlated with the current May air temperature (Fig. 4), suggesting that a dry May could affect S. przewalskii tree growth. Thus, tree growth is most limited by drought stress of the growing season in the eastern Qilian Mountains. This finding is consistent with previous research (Fang et al. 2009). Temperature-induced drought stress during the growing season was found to limit tree growth. High temperature in June of the previous growing season may have a positive effect on the photosynthesis of S. przewalskii leading to increased carbohydrate reserves. These increases in photosynthesis, in combination with higher December air temperatures that prevent cold damage, can lengthen the growing season and benefit tree radial growth (Gou et al. 2008).
Statistical relationships pointed to a negative effect of current June air temperature and a positive effect of current June precipitation on tree growth at the P. tabuliformis sample site. However, tree growth responded to current May temperature negatively and May precipitation positively at the S. przewalskii site. This begs the question as to why there is a lag of 1 month in P. tabuliformis tree growth–climate response compared to S. przewalskii. We postulate that there are three reasons: (1) inter specific differences between tree species and their response to environmental influences (Carrer 2011); (2) the different altitudes that the trees inhabit; and (3) physiological differences between the two species. We have conducted ecophysiology experiments to find the physiological and ecological processes of the different tree species of the tree growth in the eastern Qilian Mountains. In reference to this last point, some initial research during 1 year has shown distinct seasonality in photosynthesis rates from S. przewalskii photosynthesis with May being significantly higher than other months in this research area. Moreover, S. przewalskii photosynthesis has a significant negative correlation with monthly mean air temperature (r = −0.392, p < 0.001).
Correlation analysis of individual trees indicates that there is a relationship between the increased percentage of individuals significantly correlated with certain climatic variables and magnitude of the mean chronology correlation with those particular climatic variables (Table 3). For example, the tree growth response of 17 trees to previous September precipitation was significant at the P. tabuliformis site; however, there was no significant response in the mean chronology (Fig. 3). Hence, knowledge acquired for researching the dynamics of the climate–growth response at individual tree level, rather than just a single value from the mean chronology, permits a better understanding of tree species and the effect of climate factors on tree growth.
Individual trees correlation analysis suggests that individual tree growth–climate relationships depend on individual tree species, tree genetics (Jelinski 1993; Ettl and Peterson 1995), and environmental factors, such as soil depth and competition from other plants. This work suggests that tree-ring researchers do not choose the tree samples randomly to capture the enhancement of climate signals when building a chronology, but should select the tree cores which responded to climate factors consistently. This is to say that a better understanding of the relationship between individual trees and climate factors would be useful when building a chronology. Such an individual tree analysis would involve: (1) determination of individual tree growth–climate relationships; and (2) a site-specific classification of trees that are clustered into different groups and sub-chronologies based on the correlation coefficients with the climate factors. If tree growth of individual tree-ring cores within a site shows different tree growth patterns (e.g., significantly or non-significantly respond to climate), the traditional methodology of building the chronology at the site level from a population of tree-ring series could lead to a reduction or loss of the climate signal. Past research has shown that a chronology based on similarly reacting single trees from a particular site increased the ability to detect climate sensitivity (Zhang et al. 2008). Thus, the stratification and temporal variability of tree growth and its response to climate change at the individual tree level are important when reconstructing the past climate change.
Moving correlation functions assess the dynamic nature of the relationships between the tree growth and climate variability in detail. There seems like a general stable response to climate factors for most of the variables affecting tree radial growth less significantly, but a major time-dependent change for the variables affecting tree radial growth more significantly, which introduce a significant and paradoxical bias in time-independent growth–climate-related quasi-linear models. Thus, in climate reconstruction, e.g., an under- or overestimation of the reconstructed climate factors could occur depending on the selected time period, which is in contrast to the uniformitarian principle.
Figure 7 showed that the influences of the June temperature and precipitation on P. tabuliformis radial tree growth are decreasing; Fig. 8 showed that the influences of May temperature and precipitation on S. przewalskii tree growth are increasing. The drought stress is more problematic for S. przewalskii than P. tabuliformis, partly because of the poor water-holding capacity at the higher altitude S. przewalskii site. The unstable relationships over time between the trees’ mean growth and climate factors might have been caused by different combination of climate factors (e.g., temperature and precipitation) (Zhang et al. 2008), but this view has yet to be substantiated. The varying relationships between tree growth and climate over time highlight the fact that there appears to be some inconsistency between our results and the principle of uniformitarianism, which could affect reconstruction of past climates and corresponding forecasts of forest succession, biomass, and carbon storage on the basis of tree rings (Briffa et al. 1998; Barber et al. 2000).
Our results showed that tree growth–climate relationships are variable over the time at P. tabuliformis and S. przewalskii sites in the eastern Qilian Mountains. Most dendrochronological studies have focused on the mean tree growth response to climate using the same period’s observational climate date for climatic reconstructions (Fritts 1976; Cook et al. 1992; Esper et al. 2002). Interpretation of growth–climate relationships based on individual tree-ring chronologies over time is limited. Thus, in order to satisfactorily reconstruct past climates at a particular site, we must understand: (1) the variation in growth among individuals and on average for representative tree species; and (2) the relationships between the trees growth and climate variables varying over the time.
Conclusion
This work has examined the temporal variability of P. tabuliformis and S. przewalskii growth and their associations with climate for individual tree-ring chronologies and mean chronologies within sites in Qilian Mountains, northwest China. The findings of this study: (1) suggest that it is necessary for dendrochronologists to investigate the individual tree growth at the tree level instead of site level and its response to climate before building a mean chronology at a particular site; (2) affirm that moving correlation analysis has documented the dynamic nature of mean chronology response to climate over time, implying a probable deviation from the uniformitarian principle applied to climate–growth relationships; and (3) individual tree responses to climate could represent a starting point for realizing that it is necessary to examine the homogeneity of the individual tree-ring series over time in one sample site prior to establishing a chronology. Even though the results of the present study are geographically limited and represent only two tree species, we contend that individual tree analysis appears to be worthwhile tool to accurately represent existing climate variables for dendroclimatology research. Our results also indicate that the time-dependent instability of climate growth relationships should be taken into account to reconstruct quality and reliability of climate signals.
References
Barber VA, Juday GP, Finney BP (2000) Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 405:668–673
Biondi F (1997) Evolutionary and moving response functions in dendroclimatology. Dendrochronologia 15:139–150
Briffa KR (1995) Interpreting high-resolution proxy climate data—the example of dendroclimatology. In: VonStorch H, Navarra A (eds) Analysis of climate variability: applications of statistical techniques. Springer, Berlin, pp 77–94
Briffa KR, Schweingruber FH, Jones PD, Osborn TJ, Shiatov SG, Vaganov EA (1998) Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 391:678–682
Carrer M (2011) Individualistic and time-varying tree-ring growth to climate sensitivity. PloSone 6:1–8
Carrer M, Urbinati C (2006) Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol 170:861–872
Cook ER, Holmes RL (1986) Users manual for program ARSTAN, Laboratory of Tree-Ring Research, University of Arizona, Tucson, USA
Cook ER, Kairlukstis LA (1990) Methods of dendrochronology. Kluwer, Dordrecht, p 394
Cook E, Bird T, Peterson M, Barbetti M, Buckley B, D'Arrigo R, Francey R (1992) Climatic change over the last millennium in Tasmania reconstructed from tree-rings. Holocene 2(3):205–217
D’Arrigo R, Wilson R, Liepert B, Cherubini P (2007) On the ‘divergence problem’ in northern forests: a review of the tree-ring evidence and possible causes. Global Planet Change 60:289–305
Esper J, Cook ER, Schweingruber F (2002) Low-frequency signals in long tree-ring chronologies for reconstructing past temperature variability. Science 295:2250–2253
Ettl GJ, Peterson DL (1995) Extreme climate and variation in tree growth: individualistic response in subalpine fir (Abies lasiocarpa). Glob Change Biol 1:231–241
Fang KY, Gou XH, Chen FH, He MS, Zhang Y, Tian QH, Peng JF (2009) Drought variation in eastern part of Northwest China over last two centuries: evidence from tree-ring. Clim Res 38:129–135
Fang KY, Gou XH, Chen FH, Li YJ, Zhang F, Kazmer M (2011) Tree growth and its association with climate between individual tree-ring series at three mountain ranges in north central China. Dendrochronologia. doi:10.1016/j.dendro.2011.02.003
Fritts HC (1976) Tree ring and climate. Academic Press, London
Gou XH, Chen FH, Yang MX, Peng JF, Li JB (2005) Climatic response of thick leaf spruce (Picea crassifolia) tree-ring width at different elevations over Qilian Mountains, northwestern China. J Arid Environ 61:513–524
Gou XH, Chen FH, Jacoby G, Cook Ed, Yang MX, Peng JF, Zhang Y (2007) Rapid tree growth with respect to the last 400 years in response to climate warming, northeastern Tibetan Plateau. Int J Climatol 27:1497–1503
Gou XH, Chen FH, Yang MX, Gordon J, Fang KY, Tian QH, Zhang Y (2008) Asymmetric variability between maximum and minimum temperatures in Northeastern Tibetan Plateau: evidence from tree rings. Sci China Ser D Earth Sci 51(1):41–55
Holmes R (1983) Computer assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:692–725
Jelinski DE (1993) Associations between environmental heterogeneity, heterozygosity, and growth rates of Populus tremuloides in a Cordilleran landscapes. Arc Antarct Alp Res 25:183–188
Li YJ (2011) Tree-ring based climate reconstruction for the Qilian Mountains. Master thesis, Lanzhou University, Lanzhou
Liang EY, Shao XM, Qin NS (2008) Tree-ring based summer temperature reconstruction for the source region of the Yangtze River on the Tibetan Plateau. Global Planet Change 61(3–4):313–320
Liu XH, Qin DH, Shao XM, Chen T, Ren JW (2005) Temperature variations recovered from tree-rings in the middle Qilian Mountain over the last millennium. Sci China Ser D 48:521–529
Liu XD, Yin ZY, Shao XM, Qing NS (2006) Temporal trends and variability of daily maximum and minimum, extreme temperature events, and growing season length over the eastern and central Tibetan Plateau during 1961–2003. J Geophys Res 111(D19):10901–10919
Lloyd A, Bunn A (2007) Responses of the circumpolar boreal forest to 20th century climate variability. Environ Res Lett 2:1–13
Mann ME, Bradley RS, Hughes MK (1998) Global-scale temperature patterns and climate forcing over the past six centuries. Nature 392:779–787
Pisaric M, Carey S, Youngbult D (2007) Anomalous 20th century tree growth, Mackenzie Delta, Northwest Territories. Canada. Geophys Res Lett 34:L05714. doi:10.1029/2006GL029139
Qin C, Yang B, Burchardt I, Hu XL, Kang XC (2010) Intensified pluvial conditions during the twentieth century in the inland Heihe River Basin in arid northwestern China over the past millennium. Global Planet Change 72(3):192–200. doi:10.1016/j.gloplacha.2010.04.005
Qin C, Yang B, Bräuning A, Sonechkin DM, Huang K (2011) Regional extreme climate events on the northeastern Tibetan Plateau since AD 1450 inferred from tree rings. Global Planet Change 5(3–4):143–154
Shao XM, Huang L, Liu HB, Liang EY, Fang XQ, Wang LL (2005) Reconstruction of precipitation variation from tree rings in recent millennial years in Delingha, Qinghai. Sci China Ser D 48:939–949
Shao XM, Xu Y, Liang EY, Zhu H, Wang S (2010) Climate implication of a 3585-year tree-ring width chronology from the northeastern Qinghai–Tibetan Plateau. Quat Sci Rev 29:2111–2122
Solberg B, Hofgaard A, Hytteborn H (2002) Shifts in radial growth responses of coastal Picea abies induced by climatic change during the 20th century, central Norway. Ecoscience 9:79–88
Stokes A, Smiley L (1968) An introduction to tree ring dating. University of Chicago Press, Chicago
Wilmking M, Juday G, Barber V, Zald H (2004) Recent climate warming forces contrasting growth responses of white spruce at tree-line in Alaska through temperature thresholds. Global Change Biol 10. doi:10.1111/j.1365-2486.2004.00826
Wilmking M, D’Arrigo R, Jacoby G, Juday G (2005) Divergent growth responses incircumpolar boreal forests. Geophys Res Lett 32. doi:10.1029/2005GLO23331
Wu XD, Zhang XG, Sun L, Cheng ZG (1990) Reconstructing middle-Tibet climate during the last 600 years by dendroclimatological method. Acta Meteorol Sinica 4:294–304 (in Chinese)
Yang B, Kang XC, Bräuning A, Liu J, Qin C (2010) A 622-year regional temperature history of southeast Tibet derived from tree rings. Holocene 20:181–190. doi:10.1177/0959683609350388
Yang B, Qin C, Bräuning A, Burchardt I, Liu JJ (2011) Rainfall history for the Hexi Corridor in the arid northwest China during the past 620 years derived from tree rings. Int J Climatol. doi:10.1002/joc.2143
Zhang YX, Wilmking M (2010) Divergent growth responses and increasing temperature limitation of Qinghai spruce growth along an elevation gradient at the northeast Tibet Plateau. Forest Ecol Manag 260:1076–1082
Zhang QB, Cheng GD, Yao TD, Kang XC, Huang JG (2003) A 2,326-year tree-ring record of climate variability on the northeastern Qinghai-Tibetan Plateau. Geophys res let 30:1739. doi:10.1029/2003GL017425
Zhang YX, Wilmking M, Gou XH (2008) Changing relationships between tree growth and climate in Northwest China. Plant Ecol. doi:10.1007/s11258-008-9478-y
Zhang Y, Gou XH, Chen FH, Tian QH, Yang ML, Peng JF, Fang KY (2009) A 1232 years tree-ring record of climate variability in the Qilian Mountains, Northwestern China. IAWA J 30(4):407–420
Zhang YX, Shao XM, Wilmking M (2011) Dynamic relationships between Picea crassifolia growth and climate at upper tree line in the Qilian Mts., Northeast Tibetan Plateau, China. Dendrochronologia 29(4):185–199
Acknowledgments
This research was supported by the National Science Foundation of China (Grant Nos. 41171039, No. 40971119 and No. 41021091), the Chinese 111 Project (Grant No. B06026), and the One Hundred Talents Program of CAS (Grant No. 29O827B11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Braeuning.
Special topic: Dendroecology in Asia.
Rights and permissions
About this article
Cite this article
Zhang, F., Gou, X., Liu, W. et al. Individual and time-varying tree-ring growth to climate sensitivity of Pinus tabuliformis Carr. and Sabina przewalskii Kom. in the eastern Qilian Mountains, China. Trees 27, 359–370 (2013). https://doi.org/10.1007/s00468-012-0772-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-012-0772-x