Abstract
Long-term data on radial increment dynamics in Mediterranean species may identify which climatic variables are the main constraints for radial growth and at which temporal scales they act. To this end, we examined stem radial fluctuations in Quercus ilex L., the dominant evergreen oak species in the Western Mediterranean Basin, over a period of 11 years (1994–2004) at a coastal site in north-eastern Spain. We used manual band dendrometers to record girth changes in trees on north- and south-facing slopes. Annual increments measured by dendrometers showed good agreement with annual tree-ring width. North-facing trees showed a lower long-term cumulative radial increment than south-facing trees. The seasonal radial increment pattern of Q. ilex was bimodal, being characterized by a greater increase in May and a lesser, more variable increase peak in September. Both phases corresponded to warm and moist climatic conditions, whereas radial increase of stems stopped in winter and occasionally in summer. Considering the whole year, mean maximum air temperature was the main factor positively affecting radial increment of Q. ilex from short- (5 days) to- long (30 days) time scales, whereas the accumulated precipitation exerted a similar effect at longer (30 days) scales, but only on south-facing trees. In summer, all trees were positively correlated with precipitation at long-time scales (30 days); however, only stem increment of south-facing trees showed a significant relation to the temperature at short-time scales (10 days). We confirmed the dominant role of temperature as the major constraint on radial increment at short time scales, despite most previous studies were mostly biased towards precipitation effects at monthly scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendroclimatology aims to establish long-term relationships between radial growth and climatic variables by examining correlations with monthly, seasonal and annual resolution (Fritts 1976). These year-to-year comparisons between monthly climatic variables and tree-ring width do not consider the radial increment dynamics at intra-annual scale. Furthermore, such analyses cannot capture delayed radial-growth responses to climate (Camarero et al. 1998). Therefore, data on radial increment dynamics is required to understand radial growth responses in relation to climatic variability at shorter time scales which may not be detected by coarser assessments based on response functions.
Most of the short-term data on stem radial increment dynamics derive from dendrometers (Drew and Downes 2009). Dendrometers provide non-destructive measurements of radial fluctuations in relation to climate, once reversible stem water increments are removed (Zweifel et al. 2001; Deslauriers et al. 2003; Bouriaud et al. 2005). Although xylem development can only be directly assessed from repeated wood-sampling analysis (pinning, micro coring), cambial dynamics can result altered, contrary to dendrometers (Deslauriers et al. 2007; Mäkinen et al. 2008). Also, band dendrometers produce estimates of radial increment for the whole stem, whereas point dendrometers and repeated wood-sampling are usually confined to smaller areas of the stem. Physiological studies based on dendrometer data have revealed that radial growth is primarily constrained by water deficit, and secondarily by tree–carbon balance (Daudet et al. 2005; Zweifel et al. 2006). Nevertheless, there are still very few long-term studies providing a more mechanistic view of radial increment dynamics as related to climatic variables, especially in drought-stressed areas as the Mediterranean Basin. Such studies should provide a functional basis for growth-climate relationships for sites with severe water deficit, i.e., a mechanistic explanation of dendrochronological correlative findings.
Evergreen trees in areas with Mediterranean climate face a double stress, drought in summer and cold in winter (Mitrakos 1980; Terradas and Savé 1992; Larcher 2000). This pattern of double climatic stress is consistent with some of the functional parameters measured in the species Quercus ilex L., which is the dominant evergreen oak tree species in the Western Mediterranean Basin (Barbero et al. 1992). First, Q. ilex presents two peaks of photosynthetic activity in spring and autumn (Gratani 2000; Corcuera et al. 2005; Gratani et al. 2008) like other Mediterranean species (Llorens et al. 2003), coinciding with periods of high precipitation and mild temperatures. Second, shoot elongation and leaf flushing in Q. ilex mainly occur in spring, and stop in summer, while a new flush can occur in late-summer or early autumn after rainfall, especially at coastal sites (Gratani 1996; Castro-Díez and Montserrat-Martí 1998; Montserrat-Martí et al. 2009). Therefore, radial increment rates of Q. ilex may be expected to follow a similar bimodal pattern, with maximum values in spring and autumn when precipitation is high and temperatures are mild. However, this pattern might be constrained to some extent by the phenology of Q. ilex, which is characterised by a major development of the crown in spring, whereas a few of its phenological phases, such as acorn maturation and possibly the thickening of primary roots, occur in autumn (Montserrat-Martí et al. 2009).
There is a lack of long-term radial increment data in drought-stressed environments such as the Mediterranean Basin, where the predicted increase in temperature and evapotranspiration might limit tree growth (IPCC 2007). Although there is an evident trend of increasing temperature, most studies have focused on the effects of simulated drought, i.e., a reduction in rainfall and in soil water availability, on Q. ilex radial growth and wood anatomy (Ogaya et al. 2003; Corcuera et al. 2004; Ogaya and Peñuelas 2007; Cotillas et al. 2009). This bias is probably related with the previous dendrochronological studies which have shown that tree-ring width in Q. ilex was mainly correlated with late-spring and early summer precipitation (Zhang and Romane 1991; Cartan-Son et al. 1992; Nabais et al. 1998–1999; Corcuera et al. 2004; Campelo et al. 2007, 2009). However, those correlative approaches based on monthly climatic data are not consistent with the strong effects of temperature on photosynthesis, respiration, and primary growth in Q. ilex found in physiological studies conducted at daily and weekly scales (Gratani et al. 2000; Gratani et al. 2008). Furthermore, some of those studies assumed that the effects of drought on primary (e.g., shoot length) and secondary (e.g., stem radial increment) growth are similar despite these two types of growth respond differently to water deficit (Montserrat-Martí et al. 2009). Long-term data on radial increment dynamics in Q. ilex provide a unique opportunity to clarify these inconsistencies and to detect the relevant temporal scales and potential lags between climatic constraints and radial increment.
We analysed an 11-year database of stem radial increment based on short-term band dendrometer recordings: (1) to describe the inter- and intra-annual patterns of stem radial increment in Q. ilex; and (2) to evaluate the role of several climatic variables (air temperature, precipitation, and radiation) as potential constraints of radial increment. We hypothesize that: (1) temperature and precipitation are the main climatic drivers of Q. ilex radial increment; and (2) that temperature effects on radial increment are more relevant at shorter time scales (weekly to biweekly) than precipitation effects (monthly scales), because temperature influences leaf gas exchange, respiration, and therefore carbon gain at shorter periods during the year. If this second hypothesis is supported by our results, we could explain: (1) why the monthly effects of temperature on radial growth have not been detected by most dendrochronological studies based on monthly climatic data; and (2) why such an approach has supported a drought-biased view of Q. ilex radial increment dynamics in the literature.
Materials and methods
Study area
The study was conducted in an interior valley of the Garraf karstic mountains (41º 20′ 26″ N, 1º 50′ 38″ E, 300 m a.s.l.) in the central Mediterranean coastal ranges belonging to the Natural Park of Garraf and Olèrdola (Catalonia, NE Spain).
The area was completely burnt in 1982, and the current vegetation cover is high and similar on both slopes: 96 and 95% at the north- and south-facing slope, respectively (results from sampling data obtained by means of 2 m point plant contact along thirty 50 m long transects, 15 transects per slope, covering the whole area of the sampled Q. ilex trees). Nevertheless, there are differences in the vegetation type and communities. Quercus coccifera maquis dominates the S-facing slope (Quercetum-lentiscetum) with some scattered Q. ilex and Pinus halepensis trees. On the N-facing slope, Q. ilex is the dominant tree species (Quercetum ilicis gallopriovinciale) (Folch 1986).
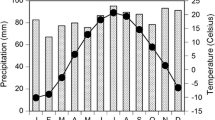
The soil, developed from lower Cretaceous limestones, is shallow and discontinuous and shows a low water-holding capacity. Based on climatic data from the nearest meteorological station at Begues (ca. 8 km from the study site), the climate in the study area is Mediterranean (“Appendix”, Fig. 6). The mean annual temperature for the period 1974–2004 was 13.4°C with a maximum mean monthly temperature of 21.1°C (July) and minimum of 5.1°C (January). The mean annual precipitation was 659.9 mm (period 1974–2004), most of which occurs in autumn (33% was recorded between September and November) and spring (25% was recorded between March and May). Daily and monthly evapotranspiration were estimated using a modified Thornthwaite water-budget procedure based on the temperature and precipitation data (Willmott et al. 1985). According to these estimates, the water deficit period lasts ca. 3 months, from June to August.
Dendrometer measurements and radial increment data
Changes in stem radius of Q. ilex were monitored using stainless-steel band dendrometers (Agricultural Electronics Co., Tucson, USA). In July 1993, we installed band dendrometers on the largest stem of ten multi-stemmed trees of Q. ilex at a height of 77 ± 39 cm (mean ± SD), after removing the outer layer of dead bark. Five of the selected trees were located on a north slope (N), whereas the other five were located at a similar elevation on the opposite south slope (S). At the beginning of the study period, trees did not have significantly different mean diameters at 1.3 m (diameter at the breast height, dbh) related to exposure (N, 8.8 ± 4.0 cm; S, 9.3 ± 2.9 cm; one-way ANOVA, F = 0.05, P = 0.83). Band dendrometers were read to the nearest 0.01 mm from August 1993 to January 2005. Mean sampling frequency was 28 ± 1 days (mean ± SE), but mean sampling frequency was greater in spring and autumn (17 ± 1 days), the presumed seasons of greater radial increment, than in winter and summer (39 ± 2 days). According to Keeland and Sharitz (1993), measurements obtained in the first 3 months after installation are within the adjustment period of the band dendrometers and should be discarded. Therefore, 1,993 measurements were discarded, increasing the initial adjustment period to 5 months. The measurements were corrected for temperature effects taking into account the band thermal expansion factor provided by the manufacturer (11.2 × 10−6 mm−1 °C−1).
The cumulative perimeter data provided by the dendrometers were converted to cumulative radial increment data assuming that the stem was cylindrical and dividing the girth data by 2π. The long-term cumulative radial increment was calculated for N- and S-facing trees to evaluate long-term growth trends as a function of contrasting aspect. For each tree, daily radial increment was calculated by dividing the radial increment by the number of days between two consecutive observation dates. Monthly radial increment was obtained as a sum of daily radial increments. Monthly radial increments for all trees (n = 10) were used to represent the seasonal dynamics of radial increment for the 11-year study period. The month of initiation and cessation of radial increment was determined when 5 and 95% of the annual radial increment had occurred, respectively (Fraser 1962).
Since dendrometers detect girth changes unrelated to wood formation, such as swelling and shrinking of bark and xylem, the radial data were first carefully analyzed to discard anomalous measurements. We considered anomalous measurements those corresponding to: (1) sudden changes (increase or decrease) higher than 15% of the total annual increment, and (2) unusual radial-changes observed in one tree, but not in the others. The main causes of dendrometer malfunction were hits by animals and persons, and insects blocking the pieces of the dendrometers. Twenty-two measurements were considered anomalous and discarded from a total of 1,274 measurements corresponding to 121 sampling dates distributed among the 10 sampled trees. Finally, to evaluate the accuracy of the dendrometer measurements in estimating radial growth, the second largest stem of each multi-stemmed tree was cut and cross-sections were obtained at a height of 60 cm. Cross-sections were used to identify annual tree-rings which were visually cross-dated. Tree-ring widths were measured along the longest radii to the nearest 0.01 mm using a LINTAB measuring device (Rinntech, Heidelberg, Germany). Two out of the ten cross-sections were discarded, because the stems were too small. Mean annual tree-ring width was compared with the corresponding annual radial increment derived from dendrometers.
Statistical analyses
Differences in cumulative radial increment as a function of sampling date (time) and slope (N vs. S) of trees were assessed in a linear mixed model using individual log-transformed data. We considered tree size (initial dbh) as covariate to remove possible size effects on radial increment. Mixed models allowed us to evaluate the effects of fixed factors (time, orientation) on the same trees (random factor) at different sampling dates as in similar repeated-measurement analyses (Littell et al. 2006).
The relationships between mean daily radial increment rate (mm day−1) of N- and S-facing trees and daily climatic data were assessed using the Pearson correlation coefficient (r). Climatic data included mean minimum and maximum air temperature, radiation, evapotranspiration, and summed rainfall for the 5, 10, 15, and 30 days before each sampling date. These intervals were chosen to detect delayed responses of radial growth to changes of climate. We performed these correlations for the whole year and for the period of water deficit, i.e., from June to August. To account for the effects of temporal autocorrelation in correlation analyses, we estimated the 95% confidence intervals for the correlation coefficients by bootstrapping with an average block length proportional to the estimated data autocorrelation (Mudelsee 2003).
Results
The mean tree-ring width did not differ significantly from the corresponding mean annual radial increment for the study period (1994–2004) indicating that the dendrometer captured the overall trends of radial growth in Q. ilex (Fig. 1). Indeed, dendrometer data explained a significant proportion of tree-ring width variance (R 2 = 0.75, P < 0.001). The individual cumulative radial increment for the period 1994–2004 ranged between 11.4 and 24.7 mm, with a mean value of 16.2 mm (Fig. 2). Orientation had a significant effect on tree growth; there are statistically significant differences between N- and S-facing trees (Table 1). When comparing the mean cumulative values of N- and S-facing trees, the difference of radial increment was more apparent after 2001. From that year on, N-facing trees grew less than the S-facing trees (Fig. 2). The annual radial increment data derived from dendrometer measurements showed a mean value of 1.47 mm, with the maximum in 1996 (2.58 mm) and the minimum in 1997 (0.79 mm) (Fig. 3a).
Comparison between annual tree-ring widths measured on cross-sections (squares) and from dendrometer records (full circles). For each year, annual radial increment (mean ± SD) of Quercus ilex are presented; the dendrometer data were obtain from the largest stem (n = 10) and tree-ring widths from the second largest stem (n = 8). Total annual precipitation is represented by the grey line
Cumulative (a) and monthly (b) radial increment patterns of Quercus ilex compared with climatic conditions (c) during the 11-year study period (1994–2004). Cumulative radial increment data correspond to individual trees (n = 10, five on a north slope, black lines, and five on a south slope, dashed lines), and to north (empty circles) and south (full circles) mean values. Radial increment data are monthly means (±SD) for the north-facing trees (empty circles) and south-facing trees (full circles). Climatic variables are monthly total precipitation (thin line) and mean temperature (thick line), and the grey areas correspond to dry periods
The monthly radial increment dynamics of Q. ilex showed a bimodal pattern with two major peaks in May and September. The mean spring maximum radial increment rate was higher than the autumn peak: on average ca. 0.4 and 0.15 mm month−1, respectively (Figs. 3b, 4). Radial increment started around the beginning of April and the highest radial increment rate was reached in May. From May on, radial increment rates fell to the lowest values in July or August. After the second peak in autumn, there was no any significant radial increment from the end of November until the beginning of the next spring (Fig. 3b).
Mean cumulative (a) and monthly (b) radial increment patterns in absolute values of Quercus ilex compared with 1995 patterns for trees growing on the north- (N 1995, empty circles) and south- (S 1995, full circles) facing slopes. For each month, diamonds indicate the means (±SD) of 10 trees for the 11-year study period (1994–2004). Empty and full circles represent the 1995 mean tree growth for trees on the north and south slopes. In both graphs, the grey area corresponds to the inferred radial increment period, and the striped zone represents the most frequent period of occasional cessation of stem growth in summer
The highest monthly radial increments during spring were observed in May 1994 and June 1996 (ca. 0.8 mm month−1) (Fig. 3b), whereas, the highest radial increment rates during autumn were observed in September 1995, October 1996 (only for S-facing trees) and September 1999 (Figs. 3b, 4). In general, S-facing trees showed higher monthly radial increments than N-facing trees, especially during the autumn (Figs. 3b, 4). Finally, a noticeable stem shrinkage (ca. −0.01 mm month−1 on average) was also observed during extreme dry summers, such as in 1994 and 2003 (Fig. 3b).
From 1994 to 2004, annual tree-ring width followed, approximately, the pattern of total annual precipitation, the relationship being linear and significant (R 2 = 0.44, P = 0.03, N = 11) (Fig. 1). In contrast, the relationship with mean annual temperature is not significant, indicating that water availability is the major limiting factor for growth as expected under a Mediterranean climate. However, the distribution pattern of precipitation throughout the year is important for Q. ilex growth. In the study, area 25% of precipitation falls between March and May, and 33% between September and November; the peaks of maximum radial increments of Q. ilex were attained during these two periods. However, this pattern varied in certain years. For example, considering total annual precipitation, 1997 was an average year with 766 mm (compared to 660 mm for the period 1974–2004). Nevertheless, during this year about 300 mm of precipitation fell in January and December, when trees do not grow. Also, the amount of precipitation in March (4 mm) and September (22 mm) was very low, and radial growth during the year 1997 was the lowest (Fig. 3).
The highest positive correlations between daily radial increment rates and lagged climatic variables were found for mean maximum temperatures averaged for the 10 and 15 days before the sampling date (Table 2). These correlations were always higher for the mean growth rate of N-facing trees compared to S-facing trees. Similar results were found for mean minimum temperatures, although correlation coefficients were lower than in the case of maximum temperatures, and for evapotranspiration (results not shown). In the case of radiation, similar positive correlations with radial increment rates were found with climatic values averaged for 15 days before the sampling date, in both N- and S-facing trees (r = 0.30–0.35). In contrast, we only found a positive correlation (r = 0.22, P < 0.05) between the summed precipitation for 30 days before sampling and daily radial increment rate in S-facing trees, whereas this relationship was not significant in N-facing trees (r = 0.14, P = 0.13). Considering only the summer period, daily radial increment rates were inversely related to temperature variables. The strongest negative correlations were found between daily summer radial increment rates of S-facing trees and 30 days lagged maximum temperatures, whereas a positive correlation was found with 30 days lagged precipitation for both N- and S-facing trees.
Discussion
Quercus ilex is a slow growing Mediterranean species with small annual tree-rings, which grows mainly in spring and autumn. Stem radial increments started at the beginning of April, when temperatures are high enough for vegetative growth. Vegetative activity in Q. ilex begins when an average daily minimum air temperature is ca. 6.9°C (Gratani et al. 2000). In our study area, this temperature is reached at the end of March or at the beginning of April (data not shown), 1 month earlier than at the site studied by Gratani et al. (op. cit.) in North Italy. At the end of November or beginning of December, Q. ilex trees stopped growing and cambium remained inactive until next spring. In summer, trees grew slowly or not at all as a result of drought and high temperatures (Gratani et al. 2008). In winter, the cambium of Q. ilex remained inactive due to low temperatures and also probably due to the short photoperiod, since this had been mostly observed in more continental areas (Cherubini et al. 2003; Corcuera et al. 2004). Air temperature and radiation control leaf flushing of Q. ilex in similar coastal areas (Ogaya and Peñuelas 2007). However, our coastal study site has mild conditions, which indicates that photoperiod may be more relevant than temperature in the spring reactivation of the cambium.
Consequently, Q. ilex showed a bimodal radial pattern of increments throughout the year with two radial increment peaks in May and in September when water availability and warm temperatures allow high photosynthetic rates (Gratani et al. 2008), and promote primary and secondary growth (Gratani 1996; Montserrat-Martí et al. 2009). This pattern of xylem production is responsible for the frequent formation of intra-annual density fluctuations observed in this species (Cherubini et al. 2003; Campelo et al. 2007). A similar pattern of radial increment in two different seasons has also been recorded in other Mediterranean and tropical tree species (Liphschitz and Lev-Yadun 1986; Venugopal and Krishnamurthy 1987). Such recurrent bimodal radial-growth pattern is consistent with the presumed sub-tropical origin of many evergreen species of the Mediterranean flora such as Q. ilex (Palamarev 1989).
The Q. ilex bimodal radial increment was asymmetrical. The maximum radial increment rate in May was usually higher than the autumn peak, and most of the annual growth occurred in spring (ca. 70% of the annual radial increment). This bimodal pattern also showed high-plasticity in response to climatic conditions during the growing period (Figs. 3, 4), which may be an advantage in areas with a Mediterranean climate where the annual rainfall pattern is variable. For example, in 1997, the year of the lowest annual radial increment, the second growth peak was very small and took place in November and ca. 80% of the increment occurred in spring before a severe drought (Campelo et al. 2007); besides, in response to drought S-facing trees stopped growing in June, July, September and October, while N-facing trees stopped growing in the same months, except for June. The autumn growth peak was quite prominent in 1995 and 1999, and it was even higher than the peak recorded in spring of these years (Figs. 3b, 4). Such late-summer growth enhancement seems to be a plastic response to precipitation since S-facing trees showed a higher growth and numerous new shoots were also formed by several Q. ilex trees after the abundant rainfall in August and September 1995 (Gutiérrez pers. obs.). In addition, precipitation in the study area is usually more variable after the summer than before it (mean SD for spring (fall) months was 48 (58) mm, period 1974–2004), so the late-summer radial increment peak may be more episodic than the more regular spring peak.
In summer, the radial increment rates of Q. ilex always decreased and stems even shrank in response to extreme summer drought and the reduction of soil water availability (Zhang and Romane 1991; Zweifel et al. 2001). These results are consistent with the interruption of primary growth in this species in response to summer water deficit (Gratani 1996; Montserrat-Martí et al. 2009). Similar results were also found in tropical tree species showing cambial dormancy during dry periods (Dünisch et al. 2002). Nevertheless, summer reactivation of Q. ilex radial increment in response to rainfall events was observed in some years (e.g., 2001), and this may be attributed to the positive influence of summer rainfall on the primary growth of the species in drought-stressed areas (Gratani et al. 2000; Corcuera et al. 2004).
Radial increment appeared to be limited by low temperatures during the growing periods of spring and autumn. Also, this is an interesting and unexpected result (Table 2). Usually, it is assumed that high temperature is one of the most limiting factors for trees growth in the Mediterranean Basin, which is true in summer. In support of our results, Llorens et al. (2003) demonstrated that photosynthetic rates in two Mediterranean shrubs species are also limited by low temperatures in spring and autumn in the same study area. Therefore, we expect that the late-summer and autumn response in radial increment may have been lower or even null in colder continental areas, because low-temperature stress in autumn would be greater (Corcuera et al. 2004).
We found that temperature and precipitation influenced the stem radial increment at different temporal scales and in a site-dependent way. In general, S-facing trees showed higher radial increment rates than N-facing trees and this difference became more evident after 2001. In the northern hemisphere, N-facing slopes have lower temperatures and higher moisture compared to S-facing slopes. In our study area, the mean of 5-day to 30-day maximum temperatures were positively correlated with radial increment rates in both slopes, but this effect was stronger in N- than in S-facing trees (Table 2). Although S-facing slopes have a longer photoperiod, which can be an advantage for plants, evapotranspiration is also higher, which increases drought stress in summer (Gratani et al. 2008). Therefore, S-facing trees were more sensitive to summer temperatures and precipitation (Table 2). Higher precipitation during the summer enhanced radial increment of Q. ilex trees, this effect being more evident for S-facing trees. For example, in 1995 approximately 50% of the stem radial increment occurred in late-summer, after the rainfall in August. In this year, double rings were formed and were more frequent on S-facing trees (Campelo et al. 2007). This site-dependent response together with the possibly higher competition between Q. ilex trees on the N-facing slope could explain why N-facing trees have lower radial increments (Figs. 2, 3).
Previous dendrochronological results of Q. ilex in Mediterranean areas found that precipitation (evapotranspiration) in spring and early summer was the main positive (negative) factor for tree-ring growth (see references in Fig. 5). Furthermore, field experiments inducing a reduction in soil water availability reported similar decreases in radial growth of Q. ilex (Ogaya et al. 2003), which agrees with physiological studies showing a decrease of photosynthesis in response to water deficit (Gratani et al. 2008). Nevertheless, the dendrochronological results were based on total tree-ring width and monthly climatic variables (Fig. 5). Hence, they lacked growth data regarding the seasonal radial increment dynamics in response to climatic variability at lower intra-annual time scales. According to our results, total annual precipitation integrates long-term effects on tree-growth. But at the same time, temperature exerts its influence at shorter (and also at long) time scales. For example, the significant correlations between radial increment rates of Q. ilex and mean maximum and minimum temperatures with a time lag of 5, 10 and 15 days cannot be detected using total tree-ring width and monthly climatic variables. Interestingly, the correlations with precipitation were only significant with a time lag of 30 days in S-facing trees, supporting the hypothesis that these trees are under more drought stress than N-facing trees (Table 2). This also means that in general, precipitation has a long-term effect on radial growth than temperature which influences leaf gas exchange, respiration, or photosynthesis at shorter scales (Filella et al. 1998; Gratani et al. 2008).This could explain why dendrochronological studies based on tree-ring width and monthly climatic data correlate more often with precipitation than with temperature (Fig. 5).
A summary of growth-climate relationships for Quercus ilex based on published studies. The frequency data correspond to the number of significant (P < 0.05) correlations found between monthly climatic data (T, mean temperature; P, total precipitation) and radial-growth index. Frequencies of positive and negative relationships are indicated as bars pointing upwards or downwards, respectively. Months abbreviated by lower- and uppercase letters correspond to the previous and current (tree-ring formation) years, respectively. Data are based on the following studies: Zhang and Romane (1991), Cartan-Son et al. (1992), Nabais et al. (1998–1999), Corcuera et al. (2004), Campelo et al. (2007, 2009), and Gea-Izquierdo et al. (2009)
During the study period, an increasing trend in minimum temperatures was observed in the study area, especially in January, September, October and November (data not shown). Although, precipitation in Mediterranean climates shows two peaks from March to May and from September to November, the pattern and the amount of precipitation are more irregular from year-to-year, with no clear trend during the study period (data not shown). Thus, growth plasticity is the best strategy to balance a more predictable variable (temperature) with a more unpredictable variable (precipitation).
In conclusion, the 11-year dataset of dendrometer measurements provided an appropriate record of the intra- and inter-annual radial increment and growth dynamics. Trees on the N-facing slope grew less than S-facing trees, which is consistent with a positive effect of temperature on radial increment. Q. ilex showed a bimodal radial increment pattern with a major and more regular increment peak in May and a minor and more unpredictable peak in September. Both phases of pronounced radial increment in spring and autumn corresponded to warm and humid conditions, and were separated by low or null radial increment rates in summer and winter. We found a marked plasticity in the bimodal radial increment pattern of Q. ilex, since years with high late-summer increment were characterized by high precipitation in the same season. During the periods of most active growth, spring and autumn, air temperature was the main limiting factor on radial increment of Q. ilex from short- (5 days) to- long (30 days) time scales, whereas on south-facing trees precipitation exerted also a positive effect on growth at longer (30 days) scales.
References
Barbero M, Loisel R, Quézel P (1992) Biogeography, ecology and history of Mediterranean Quercus ilex ecosystems. Vegetatio 99–100:19–34
Bouriaud O, Leban JM, Bert D, Deleuze C (2005) Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol 25:651–660
Camarero JJ, Guerrero-Campo J, Gutiérrez E (1998) Tree-ring growth and structure of Pinus uncinata and Pinus sylvestris in the Central Spanish Pyrenees. Arctic Alpine Res 30:1–10
Campelo F, Gutiérrez E, Ribas M, Nabais C, Freitas H (2007) Relationships between climate and double rings in Quercus ilex from northeast Spain. Can J For Res 37:1915–1923
Campelo F, Nabais C, García-González I, Cherubini P, Gutiérrez E, Freitas H (2009) Dendrochronology of Quercus ilex L. and its potential use for climate reconstruction in the Mediterranean region. Can J For Res 39:2486–2493. doi:10.1139/X09-163
Cartan-Son M, Floret C, Galan MJ, Grandjanny M, Le Floc’h E, Maistre M, Perret P, Romane F (1992) Factors affecting radial growth of Quercus ilex L. in a coppice stand in southern France. Vegetatio 99–100:61–68
Castro-Díez P, Montserrat-Martí G (1998) Phenological pattern of fifteen Mediterranean phanaerophytes from Quercus ilex communities of NE Spain. Plant Ecol 139:103–112
Cherubini P, Gartner BL, Tognetti R, Bräker OU, Schoch W, Innes JL (2003) Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol Rev 78:119–148
Corcuera L, Camarero JJ, Gil-Pelegrin E (2004) Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees 18:83–92
Corcuera L, Morales F, Abadia A, Gil-Pelegrin E (2005) Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. Tree Physiol 25:599–608
Cotillas M, Sabaté S, Gracia C, Espelta JM (2009) Growth response of mixed mediterranean oak coppices to rainfall reduction. Could selective thinning have any influence on it? For Ecol Manag 258:1677–1683
Daudet FA, Ameglio T, Cochard H, Archilla O, Lacointe A (2005) Experimental analysis of the role of water and carbon in tree stem diameter variations. J Exp Bot 56:135–144
Deslauriers A, Morin H, Urbinati C, Carrer M (2003) Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Quebec (Canada). Trees 17:477–484
Deslauriers A, Rossi S, Anfodillo T (2007) Dendrometer and intra-annual tree growth: what kind of information can be inferred? Dendrochronologia 25:113–124
Drew DM, Downes GM (2009) The use of precision dendrometers in research on daily stem size and wood property variation: a review. Dendrochronologia 27:159–172
Dünisch O, Bauch J, Gasparotto L (2002) Formation of increment zones and intraannual growth dynamics in the xylem of Swietenia macrophylla, Carapa guianensis, and Cedrela odorata (Meliaceae). IAWA Bull 23:101–119
Filella I, Llusìa J, Piñol J, Peñuelas J (1998) Leaf gas exchange and fluorescence of Phillyrea latifolia and Quercus ilex samplings in severe drought and high temperature conditions. Environ Exp Bot 39:213–220
Folch R (1986) La vegetació dels Països Catalans. Ed. Ketres, Barcelona
Fraser DA (1962) Tree growth in relation to soil moisture. In: Kozlowski TT (ed) Tree growth. The Ronald Press, New York, pp 183–204
Fritts HC (1976) Tree rings and climate. Academic Press, New York
Gea-Izquierdo G, Martín-Benito D, Cherubini P, Cañellas I (2009) Climate-growth variability in Quercus ilex L. west Iberian open woodlands of different stand density. Ann For Sci 66:802. doi:10.1051/forest/2009080
Gratani L (1996) Leaf and shoot growth dynamics of Quercus ilex L. Acta Oecol 17:17–27
Gratani L (2000) Leaf temperature effects on gas exchange in Quercus ilex L. growing under field conditions. Plant Biosyst 134:19–24
Gratani L, Pesoli P, Crescente MF, Aichner K, Larcher W (2000) Photosynthesis as a temperature indicator in Quercus ilex L. Glob Planet Change 24:153–163
Gratani L, Varone L, Catoni R (2008) Relationship between net photosynthesis and leaf respiration in Mediterranean evergreen species. Photosynthetica 46:567–573
IPCC (2007) Climate change 2007: impacts, adaptation and vulnerability. Cambridge University Press, Cambridge
Keeland BD, Sharitz RR (1993) Accuracy of tree growth measurements using dendrometer bands. Can J For Res 23:2454–2457
Larcher W (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst 134:279–295
Liphschitz N, Lev-Yadun S (1986) Cambial activity of evergreen and seasonal dimorphics around the Mediterranean. IAWA Bull 7:145–153
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS system for mixed models. SAS Institute, Cary
Llorens L, Peñuelas J, Filella I (2003) Diurnal and seasonal variations in the photosynthetic performance and water relations of two co-occurring Mediterranean shrubs, Erica multiflora and Globularia alypum. Physiol Plant 8:84–95
Mäkinen H, Seo J-W, Nöjd P, Schmitt U, Jalkanen R (2008) Seasonal dynamics of wood formation: a comparison between pinning, microcoring and dendrometer measurements. Eur J For Res 127:235–245
Mitrakos K (1980) A theory for Mediterranean plant life. Acta Oecol 1:245–252
Montserrat-Martí G, Camarero JJ, Palacio S, Pérez-Rontomé C, Milla R, Albuixech J, Maestro M (2009) Summer-drought constraints the phenology and growth of two coexisting Mediterranean oaks with contrasting leaf habit: implications for their persistence and reproduction. Trees 23:787–799
Mudelsee M (2003) Estimating Pearson’s correlation coefficient with bootstrap confidence interval from serial dependent time series. Math Geol 35:651–665
Nabais C, Freitas H, Hagemeyer J (1998–1999) Tree-rings to climate relationships of Quercus ilex L. in the NE-Portugal. Dendrochronologia 16–17: 37–44
Ogaya R, Peñuelas J (2007) Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecol 189:291–299
Ogaya R, Peñuelas J, Martinez-Vilalta J, Mangiron M (2003) Effect of drought on diameter increment of Quercus ilex, Phillyrea latifolia, and Arbutus unedo in a holm oak forest of NE Spain. For Ecol Manag 180:175–184
Palamarev E (1989) Paleobotanical evidences of the Tertiary history and origin of the Mediterranean sclerophyll dendroflora. Plant Syst Evol 162:93–107
Terradas J, Savé R (1992) The influence of summer and winter stress and water relationships on the distribution of Quercus ilex L. Vegetatio 99–100:137–145
Venugopal N, Krishnamurthy KV (1987) Seasonal production of secondary xylem in the twigs of certain tropical trees. IAWA Bull 8:31–40
Willmott CJ, Rowe CM, Mintz Y (1985) Climatology of the terrestrial seasonal water cycle. J Climatol 5:589–606
Zhang SH, Romane F (1991) Diameter growth of Quercus ilex L. and the interannual variability of climatic characteristics. Ann Sci For 48:225–234
Zweifel R, Item H, Hasler R (2001) Link between diurnal stem radius changes and tree water relations. Tree Physiol 21:869–877
Zweifel R, Zeugin F, Zimmermann L, Newbery DM (2006) Intra-annual radial growth and water relations of trees -implications towards a growth mechanism. J Exp Bot 57:1445–1459
Acknowledgments
This study was initiated thanks to the Spanish project (Ref. FOR91-0689) directed by Dr. Gabriel Montserrat. It was also partially funded by the EU project FORMAT (Ref. ENV4-CT97-0641). We thank the Unidad de Sanidad Forestal (Gob. Aragón) and the Parc Nat. Garraf (Dip. Barcelona) for their support. We are very grateful to A. Bernat and M. Abril (Dept. d’Ecologia, Univ. Barcelona) who helped during fieldwork. We thank Robin Rycroft for revising English, and two anonymous referees whose criticisms and comments helped us to improve the manuscript. FC was funded by FCT (Portuguese Ministry for Science and Technology) through a PhD fellowship (SFRH/BD/10677/2002). JJC acknowledges the financial and collaborative support of project CGL2008-04847-C02-01/BOS (CICyT, FEDER,) ARAID and Globimed.
Conflict of interest
The authors declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Braeuning.
Appendix
Appendix
See Fig. 6.
Rights and permissions
About this article
Cite this article
Gutiérrez, E., Campelo, F., Camarero, J.J. et al. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees 25, 637–646 (2011). https://doi.org/10.1007/s00468-011-0540-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-011-0540-3