Abstract
Embryogenic cell lines initiated from immature zygotic embryos of Pinus nigra Arn. ssp. Austriaca were characterized in terms of macromorphological traits (colour, bipolar structures formation, germination ability) and their embryogenic potential was defined as high, medium or null. Quantification of global genomic DNA methylation revealed the existence of specific DNA methylation levels for the determinated embryogenic potentials. The line considered as effectively embryogenic, i.e., with the ability of develop the whole embryogenic program and producing plants, showed the lowest methylation levels. There was also proved the existence of an inverse relationship between total contents of free PAs and embryogenic potential, being the highest contents of free putrescine and spermidine in the non-embryogenic line and the lowest in the effectively embryogenic one. Relationships among DNA methylation levels, profiles of free individual polyamine contents and embryogenic potentials based on the ability to produce well-formed somatic embryos with effective plant conversion are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis is an alternative propagation technique to traditional procedures, presenting numerous applications in plant breeding (Von Arnold et al. 2002), but problems like induction window, lost of embryogenic potential, variable ability of plant conversion, lost of competence along subcultures and cryopreservation limit the production that this technique offers. Somatic embryogenesis in Pinus nigra Arn. has been successfully achieved and the embryogenic tissues are being maintained in long-term cultures (Salajova and Salaj 1992; Jasik et al. 1995; Salajova et al. 1999; Salajova and Salaj 2005; Salajova et al. 2005).
The embryogenic tissues of this species are characterised by the presence of numerous somatic embryos capable of maturation and subsequent plantlet regeneration (Salajova et al. 1999) although the maturation has been cell line dependent. Structural investigations have revealed different cytological and morphological features of somatic embryos in different cell lines (Salajova and Salaj 2001). The morphology of somatic embryos profoundly affected the maturation capacity (Salajova and Salaj 2005). Analytical methods have shown that the cell lines differ in the extracellular proteins released to the liquid medium in suspension cultures (Petrovska et al. 2004).
Production of embryos under in vitro conditions is a key experimental system to study reactivation and cell division, tissue organization, morphogenic competence or rejuvenation (Dudits et al. 1991) and offers an in vitro experimental system to study embryogenesis (Berros et al. 2005), cell differentiation and totipotency (Gaj 2004; Quiroz-Figueroa et al. 2006). Somatic embryogenesis induction and development have been investigated (Dodeman et al. 1997) in model systems, but remains unclear for most of the agroforesty important species.
Cell differentiation and development are controlled through temporal and spatial activation and silencing of specific genes. In contrast to animal development, plant development is plastic and strongly influenced by biotic and abiotic factors. Therefore, plants require specific interaction between developmental programs and the signalling pathways that are triggered from external stimuli. This interaction is coordinated epigenetically, i.e., at the level of chromatin organization (Causevic et al. 2006). In the last few years, methylation of genomic DNA has become a centre of scientific attraction due to its importance in understanding animal and plant development (Razin and Riggs 1980). Specific changes in total DNA methylation in plants were proved to be related to the developmental phase (juvenile and mature-vegetative versus mature-reproductive) (Fraga et al. 2002b) being also possible to relate the specific methylation status to the further in vitro morphogenic ability (Valledor et al. 2007).
Furthermore, it was confirmed that the regain of morphogenic ability by reinvigoration techniques was linked to the recovery of specific DNA methylation status (Fraga et al. 2002a). DNA methylation is essential for both morphogenesis and proliferation during somatic embryogenesis (Lo Schiavo et al. 1989; Santos and Fevereiro 2002; Causevic et al. 2005). The existence in carrot nuclear extracts of protein factors exhibiting specific affinities for conventional and non-conventional methylation acceptor sites were also described (Pitto et al. 2000) showing that their activities are modulated during vegetative cell growth and somatic embryo development. In this context, a question arises whether the maturation capacity of Pinus nigra embryogenic cell lines is dependent on the methylation status of tissues.
Mainly due to their polycationic character, the polyamines (PAs), and specially the long chain ones, have been implicated in a wide range of biological processes including cell division and differentiation, somatic embryogenesis, senescence and environmental stress (Kaur-Sawhney et al. 2003; Fraga et al. 2004; Alcázar et al. 2006; Kusano et al. 2007). There is a possible relationship between PAs and DNA methylation, since their biosynthetic pathways share the enzyme decarboxylated S-adenosylmethionine (dSAM) as a common substrate (Fraga et al. 2002a).
In the case of Pinus radiata, a decrease in putrescine (Put) with respect to the long chain polyamines spermidine (Spd) and spermine (Spm), Put/(Spd + Spm) ratio during ageing could lead to a concomitant fall of dSAM levels (Fraga et al. 2002c), and consequently to a higher activation of the DNA methyltransferase, which would partially explain the usual gene silencing (Rey et al. 1994) observed during maturation.
The loss of embryogenic competence associated with differential expression of age-depending genes constitutes a limiting factor for a successful clonal propagation of adults. The knowledge of competence bases in plants, where morphogenesis is stable but not static, is based on information about differentiation plasticity. On this matter, epigenetic mechanisms are fundamental to understand in a short time the plant developmental paradigm from a practical and basic point of view. Taking this into account, the aim of this work is to set if genomic DNA global methylation allows the characterization of different potential embryogenic lines. Furthermore, DNA methylation can be related with PAs contents through dSAM levels. Thus, the quantification of free PAs as a complementary indicator of somatic embryogenesis process is proposed.
Materials and methods
Plant material
Embryogenic cell lines were initiated from immature zygotic embryos of Pinus nigra Arn. ssp. Austriaca. Green cones were collected from open-pollinated trees growing in natural stands (Drazovce, Nitra, Slovak Republic). The collected cones were stored in plastic bags for 5–10 days at 4°C. Afterwards, the immature seeds were excised and surface sterilised with 10% (v/v) hydrogen peroxide for 10 min and rinsed three to four times with sterile distilled water. The megagametophytes containing immature embryos were excised under sterile conditions and eight of them were placed per Petri plate.
Embryogenic induction and establishment
For embryogenic cultures initiation, medium DCR (Gupta and Durzan 1985), supplemented with 2,4-dichlorophenoxyacetic acid (2 mg/l), N 6-benzyladenine (0.5 mg/l), casein hydrolysate (500 mg/l), glutamine (50 mg/l) and sucrose (20 g/l), was used. Before autoclaving, the pH of the medium was adjusted to 5.7, and 3 g/l Gelrite (Duchefa) were added to solidify. The cultures were kept in darkness at 25°C along all the culture phases.
Maintenance and multiplication of embryogenic tissues
For long-term culture, the induced cell lines were maintained on DCR medium as previously described for the initiation. Transfers to fresh media were regularly done at 2–3 weeks intervals during 6 months. Afterwards, the embryogenic competence of each single line was tested according to the following traits:
-
Colour and consistence of tissues.
-
Presence of conform bipolar structures, observed by the light microscopy.
-
Cellular organization of embryonal mass and suspensor: cellular configuration was studied with an Axioplan 2 (Carl Zeiss, Germany) microscope using two different cytological techniques: (a) Martoja and Martoja staining (Martoja and Martoja 1967), according to which selected pieces were fixed with 45% acetic acid during 2 s and then stained with 1% (w/v) orceine in acetic acid for 5 min; (b) small pieces of tissues were stained with 2% (w/v) acetocarmine, and then squashed and covered by cover slip.
-
Production, after maturation phase, of well-formed somatic embryos per gram of fresh mass.
-
Embryo germination ability (as a percentage of germinating embryos).
-
Embryogenic potential, determined on the basis of the ability of different lines to organize, produce embryos and germinate plants.
Somatic embryo maturation
Maturation of embryos occurred on DCR basal medium supplemented with 95 μM abscisic acid and 9% maltose, finally gelified with Gelrite (0.4%) according to Salajova et al. (1999).
Somatic embryo germination
Mature cotyledonary somatic embryos were desiccated for 2–3 weeks (Salajova and Salaj 2005) and then germinated in an hormone-free medium containing 30 g/l maltose (Duchefa) and 1% (w/v) activated charcoal.
Genomic DNA methylation analysis
During the maintenance of embryogenic tissues, samples for different lines were collected after 8 days of transference to fresh DCR medium, frozen in liquid nitrogen and then stored at −80°C until use. Genomic DNA methylation analyses were done using Fraga et al. (2002d) protocol.
DNA extraction
Genomic DNA extraction was carried out with a plant genomic DNA extraction kit (DNeasy Plant MiniKit, Qiagen) following the instructions of the supplier with some modifications to obtain a RNA-free DNA (Hasbún et al. 2008).
Genomic DNA hydrolysis
DNA samples were denatured and then 1 μl of 10 mM ZnSO4 and 2 μl of nuclease P1 (Sigma S.A., 200 units/ml in 30 mM C2H3O2Na) were added, mixture was incubated overnight at 37°C; 1.20 μl of Tris (0.5 M, pH 8.3) and 0.20 μl alkaline phosphatase (357 units/ml and 2.5 M (NH4)2SO4, Sigma) were then added and the mixture was incubated for additional 2 h at 37°C. Samples were centrifuged at 15,000g for 20 min and stored at 4°C.
HPCE analysis
An uncoated fused silica capillary (Waters Chromatography, S.A. 600 × 0.075 mm2 I.D. effective length 570 mm) was used in a capillary electrophoresis system (Capillary Ion Analyzer, Waters Chromatography S.A.) connected to a Millennium data processing station (Waters Chromatography S.A.). The running buffer was 48 mM NaHCO3, pH 9.6 containing 60 mM SDS (Fraga et al. 2002b). Running temperature was 20°C and operating voltage 17.5 kV. Absorbance was monitored at 254 nm on-column. Before each run, the capillary was conditioned by washing first with 1 M NaOH for 2 min, and then with 1 mM NaOH for 1 min. Finally, it was filled with running buffer for 3 min. Buffer and washing solutions were filtered through 0.22 μm pore size filters. Hydrolyzed samples were injected hydrostatically for 15 s at a height of 9.8 cm upon the cathode.
All samples were analysed twice and six replicates per line were used. Quantification of the relative methylation of each DNA sample was performed as the percentage of 5-methyldeoxicytidine (5-mdC) of respect with the total deoxicytidines (dC), methylated and unmethylated: 5-mdC peak area × 100/(dC peak area + 5-mdC peak area).
Polyamine analysis
Samples from the embryogenic tissues were frozen in liquid nitrogen, lyophilised for 48 h and stored at −80°C until used. Extraction, purification and quantifying of free (not covalently bound to other molecules) PAs, including the long chain PAs degradation product 1,3-diaminopropane (DAP), were carried out according to the methodology described by Fraga et al. (2003).
Polyamine extraction
Twenty-five milligrams of lyophilised material were homogenized in perchloric acid 5% (v/v) and centrifuged. The supernatant contained the free fraction of PAs.
Dansilation of polyamine
Aliquots of 200 μl of the supernatant were mixed with 200 μl of a saturated sodium carbonated solution and 400 μl of dansyl chloride (5 mg/ml acetone). The mixture was incubated for 12 h in darkness. After that, the excess of dansyl chloride was removed with 100 μl of proline solution (100 mg/ml in Milli-Q® water) and the mixture was incubated in darkness during 30 min. Then, dansylated polyamines were extracted with toluene.
HPLC analysis
Separation and quantification of dansylated polyamines were carried out by reverse-phase high performance liquid chromatography (HPLC) according to the method described by Marcé et al. (1995) with some modifications. The HPLC system consisted of a pump (Model 600E), a microprocessor controller (Model 600), a fluorescence detector (Model 474) and an auto-sampler (Model 717 Plus) all from Waters Chromatography S.A., connected to a processing data station Millenium 2010 (Waters Chromatography S.A.). A Kromasil 5 μm (250 × 4 mm2) reverse-phase column was used.
Aliquots of 20 μl of samples, previously filtered through a 0.45 μm filter (Millipore S.A.) were injected. The mobile phase was acetonitrile 190 UV/gradient quality (Romil S.A.) and water and the polyamines were separated through the following elution gradient: 68% acetonitrile for 4 min, then 1 min linear gradient to until reach 100% acetonitrile, which was kept constant for 4 min, and finally 1 min linear gradient until reach 68% acetonitrile (initial conditions). The flow rate was constant at 1.5 ml/min. Eluted polyamines were excited at 350 nm and fluorescence emission was monitored at 500 nm. All samples were analysed by duplicate and three analytical replicates by sample were taken. Results are given as mean ± SE. Put, Spd, Spm and 1,7-diamino heptane (HTD) used as standards were purchased from Sigma S.A.
Losses in the process
In order to determine the losses along the extraction and dansylation, 0.925 KBq of 14C-Spm (Amersham España S.A.) were added to initial extracts. Aliquots of 25 μl were taken at each step and sample radioactivity was quantified in a Vallac 1409 scintillation counter. Results were corrected taking into account loses measured for each sample.
Statistical Analysis
Statistical processing of genomic methylation and polyamine analyses were performed with SigmaStat 3.1 for Windows (Systat Software Inc., San José, CA). Normality and variance homogeneity were checked by Kolmogorov–Smirnov and Bartlett tests, respectively. Quantitative factors were contrasted by one-way analysis of variance (ANOVA) and post hoc Holm-Sidak test (P ≤ 0.001).
Results and discussion
Characterization of cell lines
The embryogenic lines obtained showed different degrees of embryogenic competence.
Based on the previously defined traits and after 6 months of culture, it was possible to discriminate among lines according to their morphology and embryogenic potential (Table 1). Only the line E196, renamed as LE2, showed considerable somatic embryo development. Consequently, this line was considered as effectively embryogenic with high embryogenic potential, developing the whole embryogenic program and finally plants.
Line E177, renamed as LE1, developed a limited number of precotyledonary embryos (10 not conformed embryos produced/grams of fresh weight, g.fw) without germination ability. Therefore, because only first events of embryogenic program were developed, this line was considered as low embryogenic potential. Line E181, renamed as LNE, was considered as null embryogenic potential line with absence (0 mature somatic embryos produced/g.fw) of any embryogenic development.
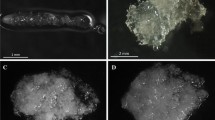
Line LE2 was morphologically characterized by white and mucilaginous tissue (Fig. 1a) and its cultures were plenty of bipolar structures (80 mature somatic embryos produced/g.fw), i.e., somatic embryos showing an embryonal mass (Fig. 1b) formed by tightly packed meristematic cells and a suspensor formed by long, vacuolised cells organized into bundles. Somatic embryos generation and development followed the conifer morphogenetic pattern (Van Zyl et al. 2002), i.e., starting from precotyledonary stages (Fig. 1c), which are followed by mature cotyledonary somatic embryo differentiation (Fig. 1d) and, finally, germination and plant conversion (Fig. 1e) with a germination percentage over 30%.
Macro- and Micromorphological aspects and cell organisation of embryogenic lines derived from immature zygotic embryos of Pinus nigra Arn. a Embryogenic tissue containing bipolar structures from cell line LE2. b Well-formed somatic embryo from cell line LE2. c Precotyledonary somatic embryo from cell line LE2. d Cotyledonary somatic embryos from cell line LE2. e Plantlets regenerated from somatic embryos from LE2 cell line. f Non-embryogenic tissue from cell line LNE. g Meristematic cells intermingled with long vacuolised cells from cell line LNE. h Embryogenic tissue with low regeneration potential from cell line LE1. i Somatic embryos from tissue with low regeneration potential. The embryonal mass is composed of loosely aggregated mass of meristematic cells. The suspensor cells are attached to the embryonal mass without organisation into bundles
Nevertheless, the null embryogenic potential line (LNE) is constituted by yellowish calluses with very soft consistence (Fig. 1f) and composed of unorganised cells without potential to develop cotyledonary somatic embryos and, according to results of Jasik et al. (1995), with no presence of neither bipolar structures or suspensor organisation; occasionally meristematic cells appeared (Fig. 1g).
The remaining line (LE1) showed intermediate characteristics, i.e., embryogenic masses with mucilaginous consistence and white colour (Fig. 1h) with the presence of less bipolar structures than LE2. Somatic embryos of this line showed a partial differentiation of embryonal masses (Fig. 1i) and suspensors. In spite of the existence of differentiation process in their tissues, this line do not express effective plant conversion ability.
Characterization of studied embryogenic lines has allowed the definition of, at least, two stages in embryo development: cell proliferation (guarantees tissue permanence and the generation of cell populations with embryogenic competence), and differentiation and development of embryonic structures (embryonal masses and suspensors) which reveal the polyembryo phenomenon typical of Gymnosperms.
Pinus nigra early somatic embryos were defined as bipolar structures with an embryonal mass and a secondary suspensor (Jasik et al. 1995). Generally, somatic embryos deriving from these structures have germination ability and give plant regeneration. Only the line with higher embryogenic potential (LE2) develops effective somatic embryogenesis including plant conversion.
Methylation levels of cell lines
The quantification of global DNA methylation by the use of different methods during somatic embryogenesis was done by several groups (Santos and Fevereiro 2002; Chakrabarty et al. 2003; Baurens et al. 2004; Leljak-Levanic et al. 2004; Tchorbadjieva and Pantchev 2004; Yamamoto et al. 2005), being results in conifers scarce (Van Zyl et al. 2002; Klimaszewska et al. 2009).
The quantification of global genomic DNA methylation in Pinus nigra, shows an inverse relationship between DNA methylation levels and embryogenic potential (Fig. 2). Thus, higher methylation levels (30% of 5mdC) corresponded to the line without embryogenic capacity (LNE) and gradually decreased with the increase of the embryogenic potential (LE1 and LE2, with 22% and 18% of 5mdC, respectively).
Percentage of methylated cytosines in genomic DNA in the indicated cell lines. Different letters in columns indicate significant differences among means (P < 0.001, ANOVA and post hoc Holm-Sidak tests). LNE: Cell line without embryogenic potential. LE1: Medium embryogenic potential line. LE2: High embryogenic potential line
Only LE2 line showed well-conformed bipolar structures which developed complete plants. Cell line LE1 developed pre-cotyledonary and cotyledonary somatic embryos. We can state that in Pinus nigra tissues, low levels of methylation are coincident with total somatic embryo development and conversion. Low rate of developed bipolar structures and nule plant conversion ability seems to be, in this species, determined by higher DNA methylation status.
Lower DNA methylation levels could be explained on the basis of (a) DNA methylation involves generally gene silencing and (b) embryogenic program is a multiprocess where gene regulation must be properly regulated. Regarding this subject, cDNA array analysis of 373 genes during Picea abies somatic embryogenesis showed the existence of a phase-dependent gene regulation (Van Zyl et al. 2002). At least 35 genes changed their expression during somatic embryogenic development, from which 22 were directly associated with embryo pattern formation. Consequently, we could suppose that the line with effective somatic embryogenesis (LE2) would express whole embryogenic program development genes in addition to maintenance genes. Contrarily, line LNE would express supporting genes exclusively and it would have somatic embryogenesis program genes silenced, which agree with results obtained in leaf-derived non-embryogenic calli of Eleutherococcus senticosus Maxim (Chakrabarty et al. 2003).
Although recently, in an attempt to study the biological characterization of young and aged embryogenic cultures of Pinus pinaster Ait. Klimaszewska and collaborators (2009) showed, in the culture conditions tested, both in the presence or absence of demethylation drug 5-aza C, inconsistent changes on DNA methylation along culture; the need of specific hypomethylated status for somatic embryogenesis development was published dealing with Dactylis glomerata embryogenic and non-embryogenic calli (Tchorbadjieva and Pantchev 2004) by the use of isoschizomers DNA restriction. Most authors (Finnegan et al. 1996; Van Zyl et al. 2002; Chakrabarty et al. 2003; Tchorbadjieva and Pantchev 2004) underline that DNA methylation percentage is related with the correct differentiation and developmental processes. Results obtained in Pinus nigra indicate that epigenetic regulation system could permit bipolar structures formation when 5-mdC level is not higher than 18%.
Levels of free polyamines in cell lines
There was an inverse relationship between total contents of free PAs (Fig. 3) and embryogenic potential. Consequently, free contents of each individual PA were the highest in non-embryogenic line (LNE), which was characterized as callogenic and without embryogenic ability. Contrarily, LE2 line contained the lowest levels of total free PAs, due to the lowest Put contents of all the analysed lines. Furthermore, in the lines with embryogenic ability (LE1 and LE2), free Spd contents are the lowest without significant differences between them. Free Spm was the less abundant free polyamine and DAP contents are approximately zero in all the lines.
Contents of individual and global free polyamines and DAP analysis. Different letters indicate significant differences among means into the same colour columns (P < 0.001, ANOVA and post hoc Holm-Sidak tests). LNE: cell line without embryogenic potential; LE1: medium embryogenic potential line; LE2: high embryogenic potential line, Put: putrescine; Spd: spermidine; Spm: spermine; PAs-t: titer of free fraction polyamines
These results agree with the decrease in Put contents observed in calli derived from leaves of germinated seeds of the angiosperm eggplant after embryogenic potential acquisition (Yadav and Rajam 1998).
Due to PAs policationic nature, they have got polianions binding ability (Kaur-Sawhney et al. 2003), especially in the case of the tetraamine (Spm). There has been demonstrated that most of Spm could be join to chromatin (Hirasawa and Suzuki 1985). This fact could explain, at least partially, the low levels of free Spm, especially in embryogenic lines. The contents of Spm and Spd were similar in embryogenic lines (LE1, LE2), being Spd the majoritary polyamine in the most embryogenic line, which possessed also the lowest Put levels. Non-embryogenic line showed a different situation because Put and Spd had similar contents being the highest of all the lines. These facts could be related with PAs functions attributed by diverse authors in researches made in angiosperms (Berros et al. 1997), and gymnosperms (Minocha et al. 1999). If it is admitted that Put favours cell division (Schons and Brasil 1995), its high levels in LNE fit in the callogenic character without embryogenic feature of this line.
According to the results, Put and Spd contents (Spm had low and constant levels) can be used as criteria for the physiological characterization of somatic embryogenesis in Pinus nigra Arn., where low levels of Put and Spd were concomitant with the development of the embryogenic program reaching the highest embryogenic potential when Spd levels were higher than Put.
Despite the hormonal and PAs profiles published (Klimaszewska et al. 2009) dealing with young and aged cultures of Pinus pinaster in somatic embryogenesis, it is well known that endogenous and exogenous plant growth regulators contents affect correct induction and development of responses mediated by DNA methylation events or other processes as was reported by Lo Schiavo et al. (1989), Minocha et al. (1999) and Leljak-Levanic et al. (2009). With this respect, it is worth to say that dSAM is involved in long chain PAs (Spd and Spm) biosynthesis and DNA methylation competitive inhibition because it is an aminopropyl donator during long chain PAs biosynthesis and, at the same time, it acts as a competitive inhibitor of the DNA methyl transferase. According to such a scheme higher contents of long chain PAs would have some association with higher methylation levels. In fact, the results suggest a relationship among DNA methylation levels, long chain PAs contents and embryogenic potential. The concomitancy between effective embryogenic potential and higher DNA methylation could be explained on the basis of the gene regulation hypothesis through specific methylation of gene promoters (Valledor et al. 2007). According to the results, it could also exist a molecular base to explain indirectly, by means of the connexion between PAs biosynthesis and DNA methylation, the concomitancy between higher contents of free long chain PAs and embryogenic potential.
Abbreviations
- dSAM:
-
S-adenosyl methionine decarboxilase
- PAs:
-
Polyamines
- DAP:
-
1,3-diaminopropane
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
References
Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876
Baurens FC, Nicolleau J, Legrave T, Verdeil JL, Monteuuis O (2004) Genomic DNA methylation of juvenile and mature Acacia mangium micropropagated in vitro with reference to leaf morphology as a phase change marker. Tree Physiol 24:401–407
Berros B, Álvarez C, Rodríguez R (1997) Effect of putrescine synthesis inhibitors on somatic embryogenesis in hazelnut. J Appl Bot 71:69–138
Berros B, Hasbún R, Radojevic L, Salajova T, Cañal MJ, Rodríguez R (2005) Protocol for hazelnut somatic embryogenesis. In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants, Springer, Dordrecht, pp 413–426
Causevic A, Delaunay A, Ounnar S, Righezza M, Delmotte F, Brignolas F, Hagège D, Maury S (2005) DNA methylating and demethylating treatments modify phenotype and cell wall differentiation state in sugarbeet cell lines. Plant Physiol Biochem 43:681–691
Causevic A, Gentil MV, Delaunay A, Abu El-Saud W, García Z, Pannetier C, Brignolas F, Hagège D, Maury S (2006) Relationship between DNA methylation and histone acetylation levels, cell redox and cell differentiation states in sugarbeet lines. Planta 224:812–827
Chakrabarty D, Yu KW, Paek KY (2003) Detection of DNA methylation changes during somatic embryogenesis of Siberian ginseng (Eleuterococcus senticosus). Plant Sci 165:61–68
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Dudits D, Bögre L, Gyögyey J (1991) Molecular and cellular approaches to the analysis of plant embryo development from somatic cells in vitro. J Cell Sci 99:475–484
Finnegan E, Peacock W, Dennis E (1996) Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci USA 93:8449–8454
Fraga M, Cañal MJ, Rodríguez R (2002a) In vitro morphogenetic potential of differently aged Pinus radiata trees correlates with polyamines and DNA methylation levels. Plant Cell Tissue Organ Cult 70:139–145
Fraga M, Rodríguez R, Cañal MJ (2002b) Genomic DNA methylation-demethylation during aging and revigoration of Pinus radiata. Tree Physiol 22:813–816
Fraga M, Rodríguez R, Cañal MJ (2002c) Phase-change related epigenetic and physiological changes in Pinus radiata D. Don. Planta 215:672–678
Fraga M, Uriol E, Diego LB, Berdasco M, Esteller M, Cañal MJ, Rodríguez R (2002d) High-performance capillary electrophoretic method for the quantification of 5-methyl 2′-deoxycytidine in genomic DNA: application to plant, animal and human cancer tissues. Electrophoresis 23:1677–1681
Fraga M, Rodríguez R, Cañal MJ (2003) Reinvigoration of Pinus radiata is associated with partial recovery of juvenile-like polyamine concentrations. Tree Physiol 23:205–209
Fraga M, Berdasco M, Diego LB, Rodríguez R, Cañal MJ (2004) Changes in polyamine concentration associated with aging in Pinus radiata and Prunus persica. Tree Physiol 24:1221–1226
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regulat 43:27–47
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179
Hasbún R, Valledor L, Rodríguez JL, Santamaría E, Ríos D, Sánchez M, Cañal MJ, Rodríguez R (2008) HPCE quantification of 5-methyl-2′-deoxycytidine in genomic DNA: methodological optimization for chesnut and other woody species. Plant Physiol Biochem 46:815–822
Hirasawa E, Suzuki Y (1985) Occurrence of spermine in chromatin of Zea mays. Plant Growth Regul 3:239–245
Jasik J, Salajova T, Salaj J (1995) Developmental anatomy and ultrastructure of early somatic embryos in European black pine (Pinus nigra Arn.). Protoplasma 185:205–211
Kaur-Sawhney R, Altabella T, Tiburcio AF, Galston AW (2003) Polyamines in plants: an overview. J Cell Mol Biol 2:1–12
Klimaszewska K, Noceda C, Pelletier G, Label P, Rodríguez R, Lelu-Walter MA (2009) Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). In Vitro Cell Dev Biol Plant 45:20–33
Kusano T, Yamaguchi K, Berberich T, Takhashi Y (2007) Advances in polyamines research in 2007. J Plant Res 120:345–350
Leljak-Levanic D, Bauer N, Mihaljevic S, Jelaska S (2004) Changes in DNA methylation during somatic embryogenesis in Curcubita pepo L. Plant Cell Rep 23:120–127
Leljak-Levanic D, Mihaljevic S, Jelaska S (2009) Variations in DNA methylation in Picea Omorika (Panc) Purk. embryogenic tissue and the ability for embryo maturation. Propagat Ornamen Plant 9(1):3–9
Lo Schiavo F, Pitto L, Giuliano G, Torti G, Nuti-Ronchi V, Marazziti D, Vergara R, Orselli S, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Marcé M, Brown D, Capell T, Figueras X, Tiburcio A (1995) Rapid high-performance liquid chromatographic method for the quantification of polyamines as their dansyl derivates: application to plant and animal tissues. J Chromatogr B Biomed Appl 666:329–335
Martoja R, Martoja M (1967) Initiation aux techniques de l’histologie animal. Masson et Cie, Paris
Minocha R, Smith D, Reeves C, Steele K, Minocha S (1999) Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiol Plant 105:155–164
Petrovska B, Moravcíková J, Obert B, Salaj T, Petrova A, Salaj J (2004) Suspension cultures of flax-extracellular proteins and morphogenic response. In: Biotechnology, as theory and practice in horticulture, Book of abstracts and programme, Fifth IVCHB symposium, Debrece, Hungary, p 110
Pitto L, Cernilogar F, Evangelista M, Lombarda L, Miarelli C, Rocci P (2000) Characterization of carrot nuclear proteins that exhibit specific binding affinity towards conventional and non-conventional DNA methylation. Plant Mol Biol 44:659–673
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tiss Organ Cult 86:285–301
Razin A, Riggs A (1980) DNA methylation and gene function. Science 210:604–610
Rey M, Díaz-Sala C, Rodríguez R (1994) Polyamines as markers for juvenility in filbert. Acta Hort 351:233–237
Salajova T, Salaj J (1992) Somatic embryogenesis in European black pine (Pinus nigra Arn.). Biol Plant 36:213–218
Salajova T, Salaj J (2001) Somatic embryogenesis and plantlet regeneration from cotyledon explants isolated from embling and seedlings of hybrid firs. J Plant Physiol 158:747–755
Salajova T, Salaj J (2005) Somatic embryogenesis in Pinus nigra: embryogenic tissue initiation, maturation and regeneration ability of established cell lines. Biol Plant 49:333–339
Salajova T, Salaj J, Kormutak A (1999) Initiation of embryogenic tissues and plantlet regeneration from somatic embryos of Pinus nigra Arn. Plant Sci 145:33–40
Salajova T, Rodríguez R, Cañal MJ, Diego LB, Berdasco M, Radojevic L, Salaj J (2005) Protocol of somatic embryogenesis of Pinus nigra Arn. In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants. Springer, Dordrecht, pp 81–93
Santos D, Fevereiro P (2002) Loss of DNA methylation affects somatic embryogenesis in Medicago truncatula. Plant Cell Tissue Organ Cult 70:155–161
Schons J, Brasil O (1995) Poliaminas na embriogênese somatic em cenoura (Daucus carota L.). Sci Agric 52:534–536
Tchorbadjieva M, Pantchev I (2004) DNA methylation and somatic embryogenesis of orchardgrass (Dactylis glomerata L.). Bulg J Plant Physiol 30:3–13
Valledor L, Hasbún R, Meijón M, Rodríguez JL, Santamaría E, Viejo M, Berdasco M, Feito I, Fraga M, Cañal MJ, Rodríguez R (2007) Involvement of DNA methylation in tree development and micropropagation. Plant Cell Tissue Organ Cult 91:75–86
Van Zyl L, Bozhkov P, Clapham D, Sederoff R, Von Arnold S (2002) Up, down and up again is a signature global gene expression pattern at the beginning of gymnosperm embryogenesis. Gene Expr Patterns 3:83–91
Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Yadav J, Rajam M (1998) Temporal regulation of somatic embryogenesis by adjusting cellular polyamine content in eggplant. Plant Physiol 116:617–625
Yamamoto N, Kobayashi H, Togashi T, Mori Y, Kikuchi K, Kuriyama K, Tokuji Y (2005) Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor. J Plant Physiol 162:47–54
Acknowledgments
The project was supported by Spanish national projects AGL-2004-00810; CIT-010000-2007-5; the Slovak Grant Agency VEGA, Proj. No. 2-5022-25; and COST 843-STSM at Oviedo University to T. Salajova. FICYT foundation supported the fellowship of the young researchers M. Pérez and M. Viejo.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Lüttge.
Rights and permissions
About this article
Cite this article
Noceda, C., Salaj, T., Pérez, M. et al. DNA demethylation and decrease on free polyamines is associated with the embryogenic capacity of Pinus nigra Arn. cell culture. Trees 23, 1285–1293 (2009). https://doi.org/10.1007/s00468-009-0370-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-009-0370-8