Abstract
Seeds of Sesbania virgata (Cav.) Pers. (Leguminosae) contain galactomannan as a cell wall storage polysaccharide in the endosperm. After germination, it is hydrolysed by three enzymes: α-galactosidase (EC 3.2.1.22), endo-β-mannanase (EC 3.2.1.78) and β-mannosidase (EC 3.2.1.25). This work aimed at studying the role of the testa (seed coat) on galactomannan degradation during and after germination. Seeds were imbibed in water, with and without the testa, and used to evaluate the effect of this tissue on storage mobilisation, as well as its possible role in the galactomannan hydrolases activities. Immunocytochemistry and immunodotblots were used to follow biochemical events by detecting and localising endo-β-mannanase in different tissues of the seed. Endo-β-mannanase and α-galactosidase activities were found in the testa and latter in the endosperm during galactomannan degradation. The former enzyme was immunologically detected in the testa, mainly during germination. The absence of the testa during imbibition led to the anticipation of protein mobilisation and increased of the α-galactosidase activity and galactomannan degradation. Thus, the testa appears to play a role during storage mobilisation in the legume seed of S. virgata probably by participating in the control of the production, modification and/or storage of the hydrolases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Angiosperms display different strategies for establishment that include accumulation of different cell wall storage polysaccharides in their seeds (Buckeridge et al. 2000c). These polymers are mobilised during seedling development and their products are used for several purposes such as energy generation and production of raw material for building cells and tissues (Mayer and Poljakoff-Mayber 1975).
The seeds of many legumes are known to accumulate galactomannan in their endospermic cell walls, but they also occur in seeds of species of other plants families such as Annonaceae and Convolvulaceae (Tookey et al. 1962; Dea and Morrison 1975; Guzmán and Hernandez 1982; Buckeridge and Dietrich 1996; Buckeridge et al. 2000b). Sesbania virgata Cav. (Pers.) (previously published by our group under the synonym of Sesbania marginata Benth.) is a small legume tree that occurs mainly in the gallery forests in Neotropical regions and is associated with early stages of ecological succession (Potomati and Buckeridge 2002).
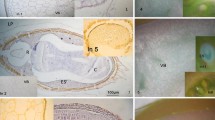
Transversal sections of seeds of S. virgata. The seeds of this species have a resistant testa (t) and a massive endosperm (e) inside which the embryo (em) is immersed a. Between the testa, with exotesta (ex), mesotesta (me), endotesta (en) and the endosperm (e), with protein bodies (→) and thick walls, an aleurone layer (*) b. Scale bar=84 μm. The diameter of the cross section of one seed is approximately 0.5 mm
Besides playing a role as a post-germinative reserve, galactomannans have also been demonstrated to perform other functions, such as modulation of the mechanical strength of the endosperm of seeds to facilitate radicle protrusion (Groot and Karssen 1987) and to control water imbibition in early stages of germination (Reid and Bewley 1979). It takes up proportionally high amounts of water and distributes it around the embryo, protecting it against loss of water (Reid and Bewley 1979).
Galactomannans are composed of a linear backbone of β-(1→4)-linked D-mannose residues to which single units of D-galactose residues are attached by α-(1→6)-linkages, providing polymers with different mannose:galactose ratios (Dea and Morrison 1975; Reid 1985). Three hydrolytic enzymes are known to be involved in galactomannan degradation: α-galactosidase (EC 3.2.1.22), endo-β-mannanase (EC 3.2.1.78) and β-mannosidase (EC 3.2.1.25) (Reid and Meier 1972; Mc Cleary and Mathenson 1976; Buckeridge and Dietrich 1996).
In seeds of Cyamopsis tetragonolobus (guar), the aleurone layer is thought to be responsible for the production of these hydrolytic enzymes (Reid 1985), as has been demonstrated for seeds of Trigonella foenum-graecum (fenugreek), Trifolium incarnatum and Medicago sativa (Reid and Meier 1972). However, in seeds of Ceratonia siliqua (carob), the enzymes are probably produced and secreted into the cell walls by living endospermic cells (Seiler 1977). Differently from carob, in seeds of S. virgata, an aleurone layer is clearly seen between testa (seed coat) and endosperm (Fig. 1), but at the same time the endosperm cells are living (Buckeridge and Dietrich 1996; Potomati and Buckeridge 2002). On the basis of this observation, Buckeridge et al. (2000a) suggested that in seeds of S. virgata, the hydrolytic enzymes might be produced both in the aleurone and endosperm cells.
Besides the endosperm, the testa can prevent water imbibition, provide mechanic resistance, interfere with gaseous exchanges and prevent or promote the release of inhibitors that are related with establishing/breaking seed dormancy respectively (Bewley and Black 1994). Kontos and Spyropoulos (1996) suggested that the testa of C. tetragonolobus controls the production of α-galactosidase and endo-β-mannanase in the endosperm of developing carob seeds. The inhibitory effect of the testa or the seed enclosing tissues on endo-β-mannanase production has also been shown in mature and developing tomato seeds (Kontos and Spyropoulos 1996).
In this paper, we present evidence that the testa is related to the storage mobilisation and galactomannan-hydrolysing enzyme activities, possibly through the production, modification and/or storage of these enzymes that are present in the tissue. From experiments in which the testa was detached during germination and early seedling growth, a cause–effect relationship was observed between testa and storage mobilisation.
Material and methods
Plant material, seed germination and sampling
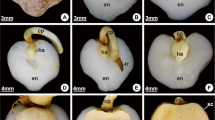
Seeds of S. virgata were collected from trees found in the urban region of São Paulo, Brazil. Seeds were scarified mechanically by abrasion of the testa, placed on one layer of filter paper in 5 or 9 cm Petri dishes (5 and 10 or 30 seeds per plate respectively) and then incubated in 0.5 ml of water per seed at 25°C under a 12 h photoperiod for 6 days. Germination percentage was recorded daily up to the second day, when 100% of the seeds showed radicle protrusion (Fig. 2).
Detection of enzyme activities
Seeds incubated in water were harvested from the second to the sixth day after imbibition (five seeds per plate) and dissected in order to separate endotesta and endosperm. These tissues were crushed with 50 mM sodium acetate buffer pH 5.0, containing 100 mM NaCl and centrifuged (10,000×g, 5°C). Aliquots of the supernatants were assayed for α-galactosidase and β-mannosidase activities using 50 mM ρ-nitrophenyl-α-D-galactopyranoside and ρ-nitrophenyl-β-D-mannopyranoside respectively as substrates (Reid and Meier 1973; Buckeridge and Dietrich 1996). For endo-β-mannanase assays, aliquots of the supernatants were assayed using a 1% solution of S. virgata galactomannan as substrate. Viscometric measurements were obtained by recording the flow times at regular intervals of the mixtures through a 0.2 ml glass pipette that has been used as a viscometer. The specific viscosity (ηsp) at each time was calculated as follows: [ηsp=(t−t 0)/t 0], where t 0 is the viscometer flow time (s) for the enzyme plus buffer and t is the flow time for this solution with galactomannan. The logarithmic function of ηsp was plotted against the elapsed time, giving a straight line which permitted the calculation of the number of arbitrary units of endo-β-mannanase activity (viscometric units) as 10 times the reciprocal of the number or minutes required for the ηsp to reach half of its initial value (adapted from Reid and Davies 1977).
Endo-β-mannanase
Western immunoblotting
The purified endo-β-mannanase, obtained as described by Lisboa et al. (2006), after separation by SDS-PAGE (Laemmli 1970, Hames 1990), was transferred to a nitrocelulose membrane, for 2 h in 0.4 M glycine–20% methanol buffer using a constant current of 400 mA, which was subsequently incubated for 1 h in 100 mM potassium ferricyanide, to inhibit peroxidase seed activity (Guéra and Sabater 2002). The membrane was blocked for 3 h in a solution containing 0.1% gelatine, 1% BSA in 0.1 M phosphate buffer saline (PBS) pH 7.0, with some drops of Tween 20. The membrane was incubated overnight in a solution containing anti-endo-β-mannanase antibody diluted 1:200 in PBS, prepared in rabbits against endo-β-mannanase purified from coffee seeds, provided by Prof. Dr. J. Giorgini from the Department of Biology of University of São Paulo, Brazil. The membrane was washed with 50 mM Tris–HCl pH 7.4 and subsequently incubated for 1 h in a solution containing goat anti-rabbit IgG conjugated to peroxidase diluted 1:200 in PBS. The membrane was washed again and the reaction was visualised by the addition of diaminobenzidine (DAB) solution (50 mg in 100 ml 50 mM Tris–HCl pH 7.4) followed by 0.03% hydrogen peroxide (adapted from Buckeridge and Reid 1994).
Dot Western immunoblotting
Seeds incubated in water were harvested from the first to sixth day after imbibition (30 seeds per plate) and dissected in order to separate testa, endosperm, cotyledons and radicle. These tissues were crushed with 50 mM Tris–HCl pH 7.8, filtered and centrifuged (13,000×g, 10 min, 5°C). The aliquots of the supernatants were assayed for protein (Bradford 1976). For each extract, a volume corresponding to 100 μg of proteins was separated, freeze dried and resuspended in 50 mM Tris–HCl pH 7.4. Volumes corresponding to approximately 2 μg of protein were applied on the surfaces of nitrocellulose membranes, which were submitted to technique described above for Western immunoblotting. The reaction, characterised by the dark granulation in the extract, indicated the presence of endo-β-mannanase in the tissue.
For quantification of endo-β-mannanase, the membranes were subjected to densitometric analysis, which was performed with KDS 1D image analysis software (Kodak Digital Science 1D 3.0.1). The relative protein quantity of each tissue, from the first to sixth day after imbibition, was expressed in percentage and calculated from their respective intensity against the total intensity in each tissue for all the period of imbibition.
Immunofluorescence
Seeds incubated in water were harvested on the third day after imbibition (10 seeds per plate) and fixed in a solution of Karnovisky (paraformaldehyde and glutaraldehyde in sodium cacodylate buffer), according to Karnovisky (1965), for 48 h. After hydration in an ethanol series, the seeds were incubated at 60°C in 20% polyethylene glycol (PEG), being embedded in pure PEG when the concentrations reached 100% (Freund 1970; Richter 1981). Transversal sections with approximately 15–25 μm thick slices were obtained with the help of a slip microtome (Richter 1981), and submitted to immunofluorescence labelling (adapted from Buckeridge and Reid 1994; Orfila and Knox 2000; Willats et al. 2001). This technique was similar to the technique described earlier for Western immunoblotting, although the sections were incubated in a solution containing goat anti-rabbit IgG conjugated to diluted 1:320 in PBS, without addition of diaminobenzidine solution. As a control, sections were submitted to the same process, but without any antibody, to evaluate autofluorescence of the tissues, and other sections were blocked and incubated only in the solution containing goat anti-IgG conjugated to fluorescein isothiocyanate diluted 1:320 in PBS to detect possible non-specific reactions of the second antibody (data not shown). The sections were observed in a fluorescence microscope (Axioplan 2-MC80DX) and photographed. The reaction, characterised by green fluorescence in the tissue, indicated the presence of endo-β-mannanase.
Quantification of proteins, α-galactosidase activity and reducing sugars
Seeds incubated in water, with and without testa, were harvested from the first to fourth day after imbibition (five seeds per plate) and dissected in order to separate endosperm and testa. These tissues were crushed with 20 mM Tris–HCl pH 7.8, filtered and centrifuged (13,000×g, 5 min). Aliquots of the supernatants were assayed for proteins (Bradford 1976), α-galactosidase activity, as described earlier (Reid and Meier 1973; Buckeridge and Dietrich 1996), and reducing sugars (Somogyi 1945).
Results
The seeds of S. virgata present a thick endosperm, with approximately 20% of its dry weight being galactomannan (Buckeridge and Dietrich 1996). Figure 1 shows transversal sections of this seed as a general view (Fig. 1a) and a closer view of the testa and endosperm (Fig. 1b). The latter shows the endosperm, in which storage cell walls (unstained regions in the endosperm) and the cytoplasm containing protein bodies (arrow in Fig. 1b) can be clearly seen.
Changes in galactomannan hydrolases
Seed germination starts on the first day, reaching 100% on the second day after imbibition (Fig. 2). After radicle protrusion, storage mobilisation takes place, flowing up to the fourth and fifth days after imbibition (Buckeridge and Dietrich 1996; Tonini et al. 2006). According to Buckeridge and Dietrich (1996), the mobilisation of storage cell wall polysaccharide galactomannan in seeds of S. virgata takes place, thanks to the raise and fall of the activities of hydrolases in the endosperm. In the present work, we investigated these activities both in endosperm and testa tissues. Figure 3 shows a comparison between the changes in activity of the three galactomannan hydrolases (α-galactosidase, endo-β-mannanase and β-mannosidase) in the endotesta and the endosperm of seeds of S. virgata. The activities of α-galactosidase and β-mannosidase increased together in the endotesta and in the endosperm (Fig. 3A and C). The activities were highest on the third day for α-galactosidase and β-mannosidase (Fig. 3A and C) in both tissues, while a delay was observed in the appearance of endo-β-mannanase in the endosperm in relation to the endotesta, with peaks of activity on the fourth and fifth days after imbibition respectively (Fig. 3B). Also, for α-galactosidase, a higher level of activity was observed up to the fifth day in the endosperm, whereas it decreased steadily in the endotesta from the third to the sixth day after imbibition (Fig. 3A).
Endo-β-mannanase
Immunodetection of endo-β-mannanase by dot Western immunoblotting, and subsequent quantification by densitometry, was used to follow the specific production of enzyme protein in all tissues of the seed. This enzyme could be localized, since the antibody against endo-β-mannanase from coffee seeds demonstrated specificity towards the purified enzyme from S. virgata (Fig. 4). The detection of endo-β-mannanase in the cotyledons occurred during all the experimental period, presenting an increase from the third day after imbibition (Fig. 5, Table 1). These changes were followed by the changes observed in the radicle, although the radicle presented a decrease on the third day of imbibition, followed by a increase after the third day (Fig. 5, Table 1). In the endosperm, the enzyme was present principally on the third and fourth days after imbibition, presenting also an increase on the fifth day (Fig. 5, Table 1). In the testa, endo-β-mannanase was detected with higher intensity on the first (around 40% germination) and second days after imbibition (100% germination), presenting a decrease from the third to the fifth day after imbibition, with a minor increase on the sixth day (Fig. 5, Table 1).
In order to endo-β-mannanase in the testa, an immunofluorescence technique was utilised. Although the detection of this enzyme in the endosperm and aleurone layer was not possible due to the presence of strong autofluorescence related to the protein bodies (Fig. 6a and b), endo-β-mannanase could be detected in all layers of the testa (exotesta, mesotesta and endotesta). It was principally associated to the cell walls of the osteosclereids in the mesotesta (Fig. 6c and d).
Immunofluorescent labelling of transversal sections of seeds of S. virgata, localising endo-β-mannanase on the third day after imbibition in water. White light a, c. Fluorescence b, d. Control without any antibody a, b. Sections treated with primary and second antibodies c, d. Scale bars = 87 μm (pb, protein bodies; e, endosperm; en, endotesta; ex, exotesta; me, mesotesta; *, aleurone layer; →, the green fluorescence shows the reaction that characterises the presence of endo-β-mannanase
The role of the testa on storage mobilisation in the endosperm
To directly test the role of the testa on storage mobilisation in the endosperms of S. virgata, an experiment was performed in which the testa was dissected out and storage mobilisation was followed by measurement of the contents of soluble proteins and galactomannan degradation, which was estimated by measuring the levels of α-galactosidase activity and the content of reducing sugars in the endosperm.
The presence of the testa significantly controlled protein mobilisation, since protein degradation was faster in seeds imbibed without testa in comparison with seeds imbibed in the presence of this tissue. On the third day after imbibition, the seeds, which had their testa detached, presented half of the level of proteins as compared to seeds imbibed with the testa (Table 2).
The effect of the testa on galactomannan degradation was indirectly evaluated through the observation of the changes in activity of α-galactosidase in the testa and endosperm. We found that enzyme activity increased less in seeds in which the testa was maintained during all the experimental period (Table 2). Another form to estimate the pace of galactomannan degradation is through the production of reducing sugars in the endosperm during galactomannan degradation. A comparable trend was observed for the two parameters, i.e. the levels of reducing sugars increased less in intact seeds than in seeds in which the testa had been detached (Table 2).
As a control, the levels of proteins, α-galactosidase and reducing sugars were followed in isolated testas (Table 2). Whereas the levels of reducing sugars were similar throughout the period of germination and seedling development, proteins and α-galactosidase increased approximately twofold from the first (around 40% germination) to the fourth day (100% germination). This with the results shown in Figs. 3, 5 and 6 that show the presence of enzymes in the testa during and after germination.
Discussion
Galactomannan hydrolases activities and localisation of endo-β-mannanase
The three enzymes known to be responsible for the post-germinative galactomannan breakdown (α-galactosidase, endo-β-mannanase and β-mannosidase) were detected in the endotesta of S. virgata (Fig. 3). The facts that the maximum activity of α-galactosidase and β-mannosidase occurred on the third day after imbibition in the endotesta and endosperm and the activity of endo-β-mannanase reached its maximum around the fourth and fifth days in these tissues (Fig. 3), confirm previous suggestions that these enzymes are involved in degradation of galactomannan, acting in concert in the endosperm after germination. According to McCleary (1983), Buckeridge and Dietrich (1996) and Buckeridge et al. (2000b), the mobilisation of the galactomannan is a post-germinative phenomenon that starts only after radicle protrusion. In seeds of S. virgata, the radicle emergence occurs in all seeds on the second day after imbibition (Fig. 2) (Potomati and Buckeridge 2002).
According to Lisboa et al. (2006), the action of the α-galactosidase from S. virgata on galactomannan is a necessary condition to grant access of endo-β-mannanase to the main chain of galactomannan polymer in seeds of S. virgata. This explains the appearance of α-galactosidase activity prior to endo-β-mannanase (Fig. 3A and B; see also Buckeridge and Dietrich 1996). This also underlines the importance of the previous debranching of the polymer and probably explains the particularly high level of α-galactosidase activity found during galactomannan mobilisation in vivo in species containing highly substituted polymers (McCleary and Mathenson 1975; Buckeridge et al. 2000a; Lisboa et al. 2006).
The presence of the three galactomannan hydrolases activities in the endotesta and endosperm (Fig. 3) suggests the possibility that the syntheses of these enzymes occur in the endosperm and/or in the testa. In seeds of C. siliqua, the enzymes are probably produced and liberated into the cell walls by the endospermic cells (Seiler 1977), while in seeds of T. incarnatum, M. sativa, T. foenum-graecum and C. tetragonolobus, the aleurone layer is thought to be the cell layer responsible for the production of galactomannan hydrolases (Reid and Meier 1972; Reid 1985). In seeds of S. virgata, it has been proposed that galactomannan hydrolytic enzymes might be produced both in the aleurone and also by endosperm cells (Buckeridge et al. 2000a). Although our results cannot define precisely whether the enzymes are produced in the aleurone layer and/or in the rest of the endosperm, they suggest the participation of the testa in the production, modification and/or storage of these enzymes.
Since the endo-β-mannanase was immunodetected in the testa (Figs. 5 and 6d, Table 1), and the cells of endotesta are living (Tonini et al. 2006), it is possible that this tissue participates in the production, modification and/or storage of this enzyme. Kontos and Spyropoulos (1996) suggested that the testa controls the production of the endo-β-mannanase in the endosperm of developing carob seeds. The inhibitory effect of the testa on endo-β-mannanase production has also been shown in mature and developing tomato seeds (Kontos and Spyropoulos 1996).
The immunodetection (Fig. 5, Table 1) as well as activity (Fig. 3b) of the endo-β-mannanase in the endosperm was found principally after germination, coinciding with the period of galactomannan degradation that occurs during the post-germinative process of seeds of S. virgata (Buckeridge and Dietrich 1996). The higher levels of detection of the endo-β-mannanase in the endosperm on the third and fourth days after imbibition, preceding its maximum detectable activity on the fifth day, suggests the presence of this enzyme in the endosperm immediately after germination for the consequent galactomannan degradation (Figs. 3B and 5, Table 1).
A possible explanation for the presence of the endo-β-mannanase in the radicle during germination (Fig. 5, Table 1) might be related to the occurrence of endosperm weakening around this tissue, which is necessary to complete the radicle emergence and germination (Groot et al. 1988; Leubner-Metzger et al. 1995; Welbaum et al. 1995). In fact, it can be observed that the enzyme decreased after germination on the third day after imbibition (Fig. 5 and Table 1). Also, endo-β-mannanase has been detected in the radicle during this process in the same species (Lisboa et al. 2006).
However, the presence of this enzyme in the radicle and cotyledons after germination (Fig. 5, Table 1) can be related to galactomannan degradation in the endosperm and/or to the hydrolysis of manno-oligosaccharides that are produced during galactomannan degradation and supposed to be translocated to the embryo in seeds of S. virgata (Buckeridge and Dietrich 1996). Activities of α-galactosidase and β-mannosidase were also detected in the embryo of seeds of S. virgata (Buckeridge and Dietrich 1996) and lettuce (Bewley and Black 1994).
The role of the testa
According to Garciarrubio et al. (1997), abscisic acid (ABA) prevents the degradation of storage proteins in seeds of Arabidopsis thaliana. In the present work, we observed that the testa of S. virgata, which has high concentrations of ABA (Tonini et al. 2006), apparently provoked a similar effect, since detachment of the testa accelerated the decrease in the levels of storage proteins after germination (Table 2). Thus, it is possible to speculate that testa probably also modulates the utilisation of enzymatic and structural proteins by the seed through ABA.
A metabolic control of cell wall storage mobilisation by the testa can be suggested on the basis of the changes observed in activity of α-galactosidase during and after germination. The activity of this enzyme was higher in the absence of the testa during imbibition (Table 2). According to Kontos and Spyropoulos (1996), developing seeds of C. siliqua displayed inhibitory action by the testa in the production of α-galactosidase. Furthermore, seeds of the same species and also of T. foenum-graecum showed the production of α-galactosidase regulated by inhibitory substances in the endosperm and/or testa.
Regarding galactomannan degradation, we observed that the production of reducing sugars was higher in the absence of the testa during imbibition (Table 2). As reducing sugars (glucose and fructose) are the main products of galactomannan degradation in the endosperm (Buckeridge and Dietrich 1996), it can be suggested that the testa of S. virgata participates in the control of storage mobilisation. Furthermore, the testa probably interferes in the galactomannan degradation, through the production, modification and/or storage of in the tissue, consequently interfering in the action and/or the dynamic distribution of these enzymes in the endosperm.
The testa's control of galactomannan degradation can be related to the presence of ABA in the tissue (Tonini et al. 2006). Indeed, ABA has been shown to act as a potent inhibitor of galactomannan degradation in seeds of fenugreek (Reid and Meier 1973), carob (Seiler 1977), lettuce (Halmer and Bewley 1979), tomato (Nomaguchi et al. 1995) and S. virgata (Potomati and Buckeridge 2002), interfering in the activity of galactomannan-hydrolysing enzymes (Dulson et al. 1988; Nomaguchi et al. 1995; Giorgini and Comoli 1996; Nonogaki and Morohashi 1996; Toorop et al. 1999, 2000; Potomati and Buckeridge 2002).
In the present work, we present evidence that the testa participates in the control of storage protein mobilisation and galactomannan degradation. We suggest that the testa interferes in the storage mobilisation by controlling the production, modification and/or storage of the hydrolases, so that embryo growth would be synchronised with storage mobilisation. This is likely to confer higher efficiency for intercommunication among the three seed tissues (testa, endosperm and embryo), possibly improving ecophysiological performance of the seedlings in their natural environment.
References
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buckeridge MS, Reid JSG (1994) Purification and properties of a novel β-galactosidase or exo-(1→4)-β-galactanase galactanase from the cotyledons of germinated Lupinus angustifolius L. seeds. Planta 192:502–511
Buckeridge MS, Dietrich SMC (1996) Mobilisation of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth (Leguminosae-Faboideae). Plant Sci 117:33–43
Buckeridge MS, Dietrich SMC, Lima DU (2000a) Galactomannans as the reserve carbohydrate in legume seeds. In: Gupta AK, Kaur N (eds) Carbohydrate reserves in plants–synthesis and regulation. Elsevier Science, Paris, pp 283–317
Buckeridge MS, Santos HP, Tiné MAS (2000b) Mobilisation of storage cell wall polysaccharides in seeds. Plant Physiol Biochem 38:141–156
Buckeridge MS, Tiné MAS, Santos HP, Lima DU (2000c) Polissacarídeos de reserva de parede celular em sementes. Estrutura, metabolismo, funções e aspectos ecológicos. Revista Brasileira de Fisiologia Vegetal, Edition Especial, pp 137–162
Dea ICM, Morrison A (1975) Chemistry and interactions of seed galactomannan. Adv Carbohydr Chem Biochem 31:241–312
Dulson J, Bewley JD, Johnson RH (1988) Abscisic acid is an endogenous inhibitor in the regulation of mannanase production by isolated lettuce endosperms. Plant Physiol 87:660–665
Freund H (1970) Handbuch der mikroskopie in der tecknik, Band V, Tell 1. Frankfurt
Garciarrubio A, Legaria JP, Covarrubias AA (1997) Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 203:182–187
Giorgini JF, Comoli E (1996) Effect of embryo and exogenous GA3 on endospermic endo-β-mannanase activity of Coffea arabica L. during germination and early seedling growth. Revista Brasileira Fisiologia Vegetal 8:43–49
Groot SPC, Karssen CM (1987) Gibberellins regulate seed-germination in tomato by endosperm weakening–a study with gibberellin-deficient mutants. Planta 171:525–531
Groot SPC, Kieliszewska-Rokickia B, Vermeer E, Karssen CM (1988) Gibberellins-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle emergence. Planta 174:500–504
Guéra A, Sabater B 2002 Changes in the protein and activity levels of the plastid NADH–plastoquinone–oxidoreductase complex during fruit development. Plant Physiol Biochem 40:423–429
Guzmán JM, Hernandez GL (1982) Anatomía de la semilla y germinación de Turbina corumbosa (L.) Raf., Convolvulaceae. Phyton 42:1–8
Halmer P, Bewley JD (1979) Mannanase production by the lettuce endosperm: control by the embryo. Planta 144:333–340
Hames BD (1990) One-dimensional polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of proteins: a practical approach, 2nd edn. Oxford University Press, New York, pp 1–147
Karnovisky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolatily for use in electron microscopy. J Cell Biol 27:137–13
Kontos F, Spyropoulos CG (1996) Seed coat inhibits the production of α-galactosidase and endo-β-mannanase in the endosperm of developing carob seeds. Plant Physiol Biochem 34:787–793
Laemmli UK (1970) Cleavage of the structural protein during the assembly of the head of bacteriophage T4. Nature 223:680–685
Lisboa CGS, Tonini PP, Tiné MAS, Buckeridge MS (2006) Endo-mannanase from the endosperm of seeds of Sesbania virgata (Cav.) Pers. (Leguminosae): purification, characterisation and its dual role in germination and early seedling growth. Brazil J Plant Physiol 18
Leubner-Metzger G, Frundt C, Vogeli-Lange R, Meins F Jr (1995) Class I β-(1→3) glucanases in the endosperm of tabacco during germination. Plant Physiol 109:751–759
Mayer AM, Poljakoff-Mayber A (1975) The germination of seeds, 2nd edn. Pergamon Press, Oxford
McCleary BV (1983) Enzymic interaction in the hydrolysis of galactomannan in germinating guar: the role of exo-β-mannanase. Phytochemistry 22:649–658
McCleary BV, Matheson NK (1975) Galactomannan structure and β-mannanase and β-mannosidase activity in germinating legume seeds. Phytochemistry 14:1187–1194
McCleary BV, Matheson NK (1976) Galactomannan utilization in germinating legume seeds. Phytochem 15:43–47
Nomaguchi M, Nonogaki H, Morohashi Y (1995) Development of galactomannan-hydrolysing activity in the micropylar endosperm tip of tomato seed prior to germination. Physiol Plant 94:105–109
Nonogaki H, Morohashi Y (1996) An endo-β-mannanase develops exclusively in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol 110:555–559
Orfila C, Knox JP (2000) Spatial regulation of pectic polysaccharides in relation to pit fields in cell walls of tomato fruit pericarp. Plant Physiol 122:775–782
Potomati A, Buckeridge MS (2002) Effect of abscisic acid on the mobilisation of galactomannan and embryo development of Sesbania virgata (Cav.) Pers. (Leguminosae-Faboideae). Rev Brasil Bot 25:303–310
Reid JSG (1985) Structure and function in legume-seed polysaccharides. In: Brett C, Hilman JR (eds) Biochemistry of plant cell walls. Cambridge University Press, Cambridge, pp 259–268
Reid JSG, Bewley JD (1979) A dual role for the endosperm and its galactomannan reserve in the germinative physiology of fenugreek (Trigonella foenum-graecum L.) an endospermic leguminous seed. Planta 147:145–150
Reid JSG, Davies C (1977) Endo-β-mannanase, the leguminous aleurone layer and storage galactomannan in germinating seeds of Trigonella foenum-graecum L. Planta 133:219–222
Reid JSG, Meier H (1972) The function of the aleurone layer during galactomannan mobilisation in germination seeds of fenugreek (Trigonella foenum-graecum L.), crimson clover (Trifolium incarnatum L.) and lucerne (Medicago sativa L.): a correlative biochemical and ultrastructural study. Planta 106:44–60
Reid JSG, Meier H (1973) Enzymic activities and galactomannan mobilisation in germination seeds of fenugreek (Trigonella foenum-graecum L. Leguminosae). Secretion of α-galactosidases and β-mannosidase by aleurone layer. Planta 112:301–308
Richter HG (1981) Wood and bark anatomy of Lauraceae. I. Aniba Aublet. IAWA Bull 2:79–87
Seiler A (1977) Glaktomannanabbau in keimenden Johanisbrotsamen (Ceratonia siliqua L.). Planta 134:209–221
Somogyi M (1945) A new reagent for the determination of sugars. J Biol Chem 160:61–63
Tonini PP, Lisboa CGS, Freschi L, Mercier H, Mazzoni-Viveiros SC, Buckeridge MS (2006) Effect of abscisic acid on galactomannan degradation and endo-β-mannanase activity in seeds of Sesbania virgata (Cav.) Pers. (Leguminosae)
Tookey HL, Lohmar RL, Wolff IA (1962) New sources of seed mucilages. J Agric Food Chem 10:131–133
Toorop PE, Bewley JD, Abrams SR, Hilhorst HWM (1999) Structure–activity studies with ABA analogs on germination and endo-β-mannanase activity in tomato and lettuce seeds. J Plant Physiol 154:679–685
Toorop PE, Van AAC, Hilhorst HWM (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51:1371–1379
Welbaum GE, Muthui WJ, Wilson JH, Grayson RI, Fell RD (1995) Weakening of muskmelon perisperm envelope tissue during germination. J Exp Bot 46:391–400
Willats WGT, McCartney L, Knox JP (2001) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213:37–44
Acknowledgements
Authors wish to acknowledge financial support by FAPESP (BIOTA-98/05124-8). PPT and COS were also granted with fellowships from CAPES and CNPq, respectively. MSB acknowledges a personal grant from CNPq. We thank Professor Dr. Jarbas Giorgini for the kindly gifting anti-endo-β-mannanase antibody and Dra. Marie-Anne Van Sluys for allowing the use of the densitometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Treutter
Rights and permissions
About this article
Cite this article
Tonini, P.P., Lisboa, C.G.S., Silva, C.O. et al. Testa is involved in the control of storage mobilisation in seeds of Sesbania virgata (Cav.) Pers., a tropical legume tree from of the Atlantic Forest. Trees 21, 13–21 (2007). https://doi.org/10.1007/s00468-006-0091-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-006-0091-1