Abstract
Morphological plasticity was studied for advanced regeneration trees in different light environments of the mountainous, mixed-species forests in the Carpathian Mountains of Romania. The primary species in these mixtures were very shade tolerant silver fir (Abies alba Mill.) and European beech (Fagus sylvatica L.), and midtolerant Norway spruce (Picea abies (L.) Karst). Seedlings/saplings of these species were selected for measurements in different stands from two different geographical locations. Various morphological traits (specific leaf area, live crown ratio, crown width to length ratio, terminal to lateral ratio, number of internodal shoots, number of shoots in terminal whorl, stem symmetry, stem orientation, stem forking) for each regenerating tree were measured during summers of 2001 and 2002. Percentage of above canopy light and stand basal area measures were used to assess the available growing space for each seedling/sapling. Regression relationships were developed for the different morphological indicators as a function of these two variables. All species adapted their morphology along the gradient in light and basal area. Spruce seemed to be less adapted to low light conditions than both fir and beech. However, no significant differences in terms of shade tolerance were detected using the above indicators. In really dense stand conditions (less than 20% above canopy light and stand basal area above 36 m2 ha−1), probability for stem forking in beech increased. In open, all three species adapted their morphology for vigorous growth. Under such conditions, spruce was better adapted than fir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regeneration in multiaged stands is subject to different levels of canopy cover and competition for ground resources. To survive in such environments, species adapt to different growing conditions by changing their phenotype (Schlichting 1986). These morphological changes are usually an expression of physiological adaptations to the surrounding environment (Barnes et al. 1997). Species able to survive in a low resource environment are considered tolerant. Barnes et al. (1997), proposed the term “understory tolerant” for those species that are able to survive and prosper under a forest canopy. However, for trees growing in a forest understory, very often light is the dominant limiting factor and therefore, shade tolerance can be used as a substitute for understory tolerance (Barnes et al. 1997). Givnish (1988) argued that the magnitude of morphological and physiological adaptability is related to the degree of shade tolerance. Therefore, shade tolerant species should show higher capacity to adapt their architecture and physiology and survive in light-limiting environments in the understory compared to less shade tolerant species (Chen et al. 1996; Williams et al. 1999).
Morphological indicators could be used to quantify the phenotypic plasticity of regeneration and compare among species (Klinka et al. 1992; Beaudet and Messier 1998; Ruel et al. 2000; Duchesneau et al. 2001; Claveau et al. 2002). Reviewing several studies on this topic, Messier et al. (1999) proposed that shade tolerant conifer species would persist longer in deep shade by being able to alter their growth and form to favor better light interception and to minimize respiration. Specific alterations would be: reducing height growth and favoring more lateral growth, reducing the live crown length, reducing number of shoots in terminal whorl and on top internode (the top internode represents the stem length between the first two subsequent whorls at the top of the tree), increasing the horizontal display of needles and branches, and adapt leaf morphology to optimize light capture in shade. Additionally, the most shade tolerant species would almost stop height growth to avoid any increase in respiration costs not rewarded by an adequate increase in available light (Messier et al. 1999; Messier and Nikinmaa 2000). Alternatively, less shade tolerant species, although able to reduce to a certain extent their height growth in shade, would not adapt their architecture and therefore would be eliminated. They concluded that Abies spp. tend to be more plastic than Picea spp. and these differences could be used by management to manipulate species composition. However, they acknowledged this would be a challenging task as these genera are not very different in their abilities to tolerate shade.
The mixed-species forests of this study are temperate ecosystems found between 800 and 1300 m altitude in Romania's mountain region. These forests are dominated by mixtures of silver fir (Abies alba Mill.), Norway spruce (Picea abies (L.) Karst), and European beech (Fagus sylvatica L.). Among these, fir and beech are considered extremely shade tolerant while spruce is considered intermediate (Stanescu et al. 1997). Each of these species has different requirements and abilities in terms of regeneration establishment and growth. For both silver fir and European beech, establishment and early survival is dependent on overstory protection, as early or late frosts and strong insolation apparently limit their regeneration in more open areas (Negulescu and Ciumac 1959; Haralamb 1967; Evans 1984; Savill 1991; Stanescu et al. 1997; Padraic et al. 1998). Alternatively, seedlings of midtolerant Norway spruce establish and grow better in more open environments with just lateral shade (Haralamb 1967; Stanescu et al. 1997).
These differences in shade tolerance and establishment ability imply different adaptations for spruce compared to fir and beech. Furthermore, the contrasting crown architecture, decurrent for beech and excurrent for fir, may produce different responses for the two shade tolerant species to the surrounding environment. Differences among species in photosynthetic abilities and allocation of growth could lead to differences in tree architecture, providing competitive advantages to certain species in a given environment. As a result of these competitive gains, diverse patterns of stand development could occur (Oliver and Larson 1996).
The main objectives of our study were to: (i) determine if different morphological traits are good estimators for species adaptability to changing environments; (ii) assess the gradients in these morphological indicators as a function of the growing space and compare among species; and (iii) describe possible patterns in stand composition and provide recommendations for mixed-species management.
Materials and methods
Study area
The study was located in the Carpathian Mountains of Romania, within the natural range of the temperate, mixed beech-conifer forests. Study sites were established on state forest managed by the National Forest Administration at two different locations: Poiana Rusca Mountains (Rusca Montana Forest District at 45°39′22′′ N and 22°22′30′′ E) and Retezat Mountains (Retezat National Park and Retezat Forest District at 45°19′05′′ N 22°45′14′′ E). General data for sampled regeneration and overstory trees are presented in Table 1. Although the range in height, diameter, and age was rather large at each location (Table 1), the overstory of sampled stands included one or two cohorts. A few very old (244–350 years) remnant trees were found at both Rusca Montana and Retezat but were not considered to represent a separate cohort (age class). Often, for sampled overstory trees, large differences in diameter at breast height (DBH) and/or total height did not reflect the presence of two separate age classes but only different size classes.
Information about site, climate, stand structure, and management history was obtained from the management plans of the two forest districts. Geologic substrates are slightly different between the two locations: at Rusca Montana is metamorphic (marble, paragneiss, and schist) while at Retezat the substrate is mostly igneous (volcanic) with some metamorphic components (schist). Soil depth was greater at Rusca Montana with ground vegetation represented mostly by blackberry (Rubus hirtus Waldst. and Kit.) (i.e. lower acidity). At Retezat, wood sorrel (Oxalis acetosella L.) on shallow soils with rocks at surface covered by green mosses show potential for higher acidity.
Altitude ranged from around 800 to 1300 m (the natural range for these mixed forests). The climate is temperate-continental with wet and cool summers and cold winters with high precipitation (most of it being snow). Annual average temperature is approximately 8–9°C and annual average precipitation around 700–1400 mm. A large range of slopes (2–45%), aspects (N, N-E, S, S-E, S-W, E, W), and crown cover (from open conditions to completely closed stands) were represented in the study.
Growing space measurements
The light that penetrates the overstory and reaches a regenerating tree is very often the most important limiting factor for growth. However, light represents only one resource and is rather difficult to estimate or measure. Alternatively, stand basal area is often used to assess stand density and competition. At the tree level, this measure is less precise than light but it represents effects of past competition related to both above-ground and below-ground factors. Both percentage of the above canopy light and overstory basal area were used as surrogates for the available and occupied growing space of sampled regenerating trees.
For light measurements we followed a procedure developed by Parent and Messier (1996) and verified for mixed-species stands with irregular canopies (Messier and Parent 1997; Gendron et al. 1998). This method requires completely overcast and homogenous sky conditions with the solar disk invisible. Under such weather conditions light is isotropic and a single instantaneous point measurement of light is sufficient to estimate the mean daily percentage of the photosynthetic photon flux density (PPFD) for that microsite.
In our study, light environment was computed as percentage of above canopy light (PACL) that reaches the tip of a seedling/sapling. Light in this context means only the photosynthetic active radiation (PAR) measured as PPFD in μmol m−2 s−1. PACL represents the ratio of two measurements: one below the canopy and one above the canopy.
Within the study areas, large openings (around 0.5 ha at Rusca and 0.3–1.0 ha at Retezat) were used to measure the light regime as a surrogate for the above the canopy reading. For these measurements, an AccuPAR ceptometer (Model PAR-80, Decagon Devices, Inc., Washington, USA) having a probe with 80 light sensors was used. To measure below canopy light, at the tip of each regeneration tree for tree morphology measures or at shoot level for leaf morphology measures, a Li-250 light meter with a Li-190SA quantum sensor attached (LI-COR, Inc., Nebraska, USA) was used. For small trees the sensor was placed on a metal ruler, while for taller trees the sensor was attached to a height pole. In both cases a leveling fixture was used to ensure a horizontal sensor and accurate readings. Exact time (h min s) was recorded for each individual measurement (i.e., tree).
To ensure the compatibility of the readings taken by the sensors of the different instruments in open and under canopy, an external quantum sensor (Li-190SA, LI-COR) was attached to the ceptometer and placed next to the middle of the probe during measurements taken during summer of 2002. Simultaneous readings taken by both sensors (AccuPAR probe and Li-190SA) in the same light environment were compared. The Accupar readings were almost always greater. However, although differences were between 0 and 11.69%, the average was 3.7% while the median was only 2.5%. The larger differences could be due to the fact that AccuPAR readings represent an average of 80 sensors while the Li-Cor is a single sensor reading. The readings of Li-Cor also tended to drift away if the sensor was not repeatedly matched to the AccuPAR sensors. Therefore, above canopy readings data collected with the ceptometer were used. Finally, after matching the two clocks, we were able to compare light measurements (below and above canopy) and to compute PACL values.
Basal area of overstory trees in the surrounding stand was determined for each regenerating tree using point sampling. A prism with a 20 basal area factor (BAF in ft2 ac−1) was used because it provided the recommended 5–12 trees per sampling point (Avery and Burkhart 2002). Values were converted to metric units (m2 ha−1).
Leaf/needle morphology
The specific leaf area (SLA) computed as the ratio of projected leaf area to dry mass was used to assess changes in leaf and needle morphology. Samples were collected in 2001 at Rusca Montana Forest District from 125 random individual trees (45 beech trees, 40 spruce trees, and 40 fir trees) growing in different light environments. All sampled trees for beech and fir were naturally regenerated, while spruce trees had either natural or planted origin. For simplicity, we will use the term “leaf” to refer to both deciduous leaves and conifer needles.
After light readings for each sample were recorded, the subject shoot with leaves was removed from the tree for further analysis. For conifers, only current year foliage on shoots in the terminal whorl was considered for analysis. To avoid carbon loss through respiration the photosynthetic material was placed on ice in the field and later was refrigerated. Before analysis, leaves were separated from the shoot, placed on transparent overheads, and photocopies were made using a Minolta EP1054 copier. The sample for each individual tree included between 10 and 35 leaves for beech, 79–308 needles for fir, and 186–374 for spruce. Leaf copies were scanned and analyzed for projected area with image analysis software (WinFOLIA, version 2001a for beech leaves and WinSEEDLE, version 2001a, for conifer needles, Regent Instruments, Inc.). After copies of each sample were taken, the photosynthetic material from each sample shoot was placed in separate paper bags and dried to constant weight at 105°C for 24 h. The dry weight was recorded in g (±0.01) using an electronic scale. Only leaf blades were considered for area analysis for beech, and therefore, petioles were removed and not included in either dry weight or leaf area data sets.
To verify the accuracy of the copier and to account for possible errors, a small piece of black paper was used as a calibration object and was placed on each overhead when making copies. The area of the calibration object was later determined with the same scanner used for measuring leaf area. For each paper copy with leaves, a ratio between the area of the original object and the area from the subject copy was used to develop a correction factor. Even though, this ratio was small (ranging from 0.99 to 1.03), it was used to adjust all leaf area measurements. Finally, the specific leaf area (SLA) was computed as the ratio of projected area for all leaves from the subject shoot to their dry weight (mm2 g−1).
Understory tree morphology
Various traits were used to measure morphological changes in regeneration trees of subject species as a function of their growth environment (Ruel et al. 2000; Claveau et al. 2002). Ratio of average crown width to crown length (CWLR), and live crown ratio (LCR, computed as length of living crown per total height of tree) were used for all three species. Additionally, for the conifers that have excurrent crowns characterized by a single terminal leader, the ratio of terminal leader length to average length of lateral shoots in the terminal whorl (terminal to lateral ratio, TLR), the number of shoots in the terminal whorl, and the number of internodal shoots (i.e., lateral shoots on the top internode) were also used. To account for particularities in morphology of the decurrent beech, we used the following indicators: stem vertical orientation (orthotropic/plagiotropic) (Nicolini 2000); crown symmetry (bilateral/radial) (Nicolini 2000); and the presence or absence of a unique terminal leader (forked/unforked) (Nicolini and Caraglio 1994).
During the summer of 2002, a different set of 145 naturally regenerated seedlings/saplings of the subject species were sampled in eight different stands with mixed regeneration and diverse canopy cover conditions (Table 1). For each regenerating tree, shoot length and internodal length (the length between terminal whorl and previous year's whorl) were measured using a tape (±1 mm). Total height and height to crown base were measured with a height pole or a tape for small seedlings (±1 cm). The base of the live crown was considered where the lowest living branch occurred. Crown width was determined as the average radius along the four cardinal directions. On each direction, the distance from the stem to the furthest point reached by the crown was measured to the nearest cm using a tape. For beech trees, forking, orientation, and symmetry of the stem were noted. Stems were considered to grow orthotropic (vertical) if the slope delimited by their main axis and the zenith was less than 10% or plagiotropic (leaning stems) otherwise. Here, the slope was assessed as the departure of the main axis from vertical direction (e.g., a 10 cm departure for 1 m height equals 10% slope). Total height for leaning stems was determined on a vertical direction and not along the stem. According to Nicolini (2000) stem symmetry was bilateral for trees with relative flat crowns having lateral branches placed generally in the same vertical plane and radial for trees with lateral branches (especially branches on the upper portion of the stem) spread out in more than one plane. Furthermore, if two upgrowing terminal shoots of similar size were present the tree was considered forked, and unforked otherwise (Nicolini and Caraglio 1994).
Analysis
Data analysis was performed using S-Plus 6.0 statistical software package. Linear and nonlinear models were used to analyze changes in morphology as a function of light and stand basal area. For number of shoots in terminal whorl and on top internode, height growth of previous year (i.e., length of the top internode) was used as an explanatory variable. To detect differences among species a 95% confidence level was used. Nonoverlapping between confidence intervals of two different species would imply a statistically significant difference. In the case of beech, logistic regression was used to assess the effects of same explanatory variables on stem forking, symmetry, and orientation.
Results
Leaf morphology
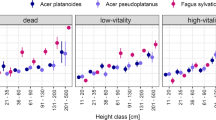
For all three species, SLA decreased in a nonlinear fashion with increasing PACL that reached the leaves on a subject shoot (Fig. 1). The shape of the relationship was characterized by an initial sharp decrease in SLA values at relatively low light levels (between 0 and 20 PACL), followed by a less pronounced reduction with further increase in light levels. To model this trend, logarithmic equations were used for each species (Table 2). The model used for beech had a greater amplitude and covered a larger range of data that did not overlap with that of conifers (Fig. 1). Models used for fir and spruce encompassed a narrower range of SLA values and were not statistically different across the entire light range (Fig. 1d, confidence intervals not shown for clarity purposes).
Tree morphology
All three species showed similar trends for changes in LCR, CWLR, and TLR (Figs. 2–4). Nonlinear equations best fitted the changes in these variables in relationship to light (PACL) (Table 2). With increasing light, both LCR and TLR increased following a logarithmic trend, while a decreasing logarithmic function was used in the case of CWLR. When basal area was the explanatory variable, increasing power models were used for CWLR and linear decreasing models for both LCR and TLR (Table 2, Figs. 2–4).
When all species were compared with regard to these traits, few differences among species were found (Fig. 5, confidence intervals not shown for clarity purposes). For the regressions between LCR and light (PACL), no significant differences among species were detected. For basal area, conifers had similar trends, while the beech tended to have a significantly higher LCR in high density stands (BA>60 m2 ha−1) but only compared to spruce. For both growing space surrogates (light and basal area), beech had significantly lower CWLR values compared to the fir only in environments with PACL < 50% and BA > 40 m2 ha−1, except a very narrow range at high densities (PACL < 3%; BA > 90 m2 ha−1). However, for such a small range in growing space the lack of a difference may be due to an artifact in our data. Differences between the two conifers for this variable were not significant for the entire range of growing space conditions. Comparing only the conifers for TLR, the trends are parallel, the spruce having greater ratios across the entire range of growing space. However, significant differences were found only in environments having PACL > 5% and BA < 65–70 m2 ha−1. A ratio less than 1 (when the lateral growth is greater than terminal growth) was achieved for spruce at PACL ≈10–12% and BA > 55 m2 ha−1 and for fir at PACL ≈4–5% and BA >80 m2 ha−1.
The number of shoots in the terminal whorl and internodal shoots increased with increasing light and decreasing basal area for both spruce and fir (data not shown). The amplitude of the change along the range of growing space is larger for spruce than for fir. For both species, the scatter in data points in more open environments is considerably greater compared to that of dense stands. When these two variables were compared to the height growth of the previous year, a better correlation was found. Both variables increased with increasing internodal length, and power equations were used to fit the relationships (Fig. 6, Table 2).
For the morphological traits of beech, a summary of results for logistic regressions is presented in Table 3 and Fig. 7. The parameter for the independent variable represents the slope and shows the degree of fit of the logistic model. A steeper slope implies a stronger relationship. For each trait, slopes had similar absolute values for both explanatory variables. In all cases, a larger scatter in the data was observed when basal area was used instead of light to represent growing space. A weak correlation between the occurrence of stem forking and both light and basal area levels was found. Chances for a forked stem varied between 10 and 60% along the entire range of light and between 15 and 75% for basal area. However, both explanatory variables had a much stronger influence on stem orientation (slope –0.22 for PACL and 0.22 for BA, respectively). The probability of finding a stem with plagiotropic growth increased sharply (from 0 to above 90%) when PACL was below 30% and basal area was more than 40 m2 ha−1. Although the influence of growing space on spatial crown form was not as strong as in the case of stem orientation, the probability for a bilateral symmetry increased considerably for PACL less than 60% (from 10 to 90%) and basal area more than 20 m2 ha−1 (from 15 to 100%).
Discussion
Leaf morphology
The changes in SLA along the light gradient found in this study agree with previous findings for these species. Bartelink (1997) found that SLA for European beech trees decreased sharply from the top to the bottom of the crown. The light gradient within a forest canopy was assumed to cause this variability but no regression model was developed to describe the existing trend. The SLA values Bartelink reported (8000–34000 mm2 g−1) are relatively close to our results for this species (12500–41000 mm2 g−1). The differences may be due to different light ranges covered in the two studies. The higher values in our study may also be explained by a possible underestimation of leaf dry weight and overestimation in SLA. This could have happened because the leaf drying procedure was performed at 105°C (compared to 70°C in the above mentioned study), and some volatile leaf components may have evaporated during the process.
For Norway spruce, Ninemets and Kull (1995) showed that the ratio of leaf dry mass per area (the inverse of our SLA) increased with increasing diffuse solar radiation. More recently, using the same ratio for both Norway spruce and silver fir, Grassi and Bagnaresi (2001) also found an increasing trend in leaf dry mass per area with increasing relative irradiance. Although these findings agree with our results, in both studies, linear models were used to describe the trends.
If shade tolerant species are able to adapt better to the understory conditions compared to more intolerant species (Givnish 1988; Williams et al. 1999), the difference in shade tolerance between fir and spruce should be reflected by changes in needle morphology along the light gradient. Such a difference should be most apparent in the light-limiting environments where fir would be better adapted for growth and survival. However, our results did not support this assumption thereby implying that morphology at the leaf level does not correspond to the differences in shade tolerance between the two conifers (Fig. 1d). The lack of a significant difference in needle morphology between these species with changing light was also reported by Grassi and Bagnaresi (2001). They showed that, even though morphological changes for these two species were similar along the light gradient, needle physiology was different. As a result, fir's foliage was better suited to low light environments but in high light its adaptation was largely morphological and not necessarily physiological. On the other hand, photosynthetic capacity of spruce needles was better adapted to high intensity light regimes by increasing N allocation to Rubisco and bioenergetics (process not found in the case of fir).
For all three species, to insure trends found were due to changing light and not height of the tree, we compared SLA values among trees from a different data set (Stancioiu 2003) having similar heights but growing in different light conditions. As these trees had different SLAs we concluded that light and not height is the driving factor in changing leaf morphology.
Our analysis used projected leaf area only. The differences in leaf morphology among the species imply different expansion factors to all-sided leaf area. Compared to beech leaves, the rhomboid shape of conifer needle cross section would result in a higher increase of leaf surface when projected area is expanded to all-sided. The ratio of total leaf area to projected leaf area also increases with light at least for Picea spp. (Ninemets and Kull 1995). Therefore, while the trend in SLA for beech would not change much, for conifers the trend could be less steep than those shown in Fig. 1.
Tree morphology
All three species adapted their morphology to the available growing space. The opposite trends observed when using light and basal area to represent growing space are not surprising as the two variables are inversely correlated (Brunner 1993; Stancioiu and O'Hara 2005). However, the shape of this relationship would vary for different canopy structures and composition (Barnes et al. 1997)
Live crown ratio
In general, live crown length increases with increasing canopy dominance (Kozlowski et al. 1991). Our results support this theory as the live crown ratio (LCR) decreased with decreasing available resources for all three species (Fig. 5). Although shade tolerant species tend to have lower crown ratio in deep shade (Messier et al. 1999), in the same light environment, intolerant species should have even lower crown ratios (Smith et al. 1996) as their lower branches are less able to efficiently use the light and will die. Therefore, when comparing species with different degrees of shade tolerance, we expect the reduction in live crown ratio to be considerably smaller for tolerant than for less tolerant species. In our study, the midtolerant spruce tended to have a lower LCR than beech and fir in crowded conditions. However, our results did not confirm our expectation as differences were not significant except at basal areas higher than 60 m2 ha−1 when compared to beech only (Fig. 5a and d).
Crown width to length ratio
For trees able to survive and grow in a limiting environment, lateral growth should be favored over height growth (Messier et al. 1999). As a result, a lower crown width to length ratio should characterize trees in open conditions compared to overtopped trees. All three species conformed to the expected trend, as CWLR decreased with increasing light and decreasing basal area (Fig. 5b and e). Although, with increasing competition, the midtolerant spruce showed a tendency for a lower CWLR compared to fir, no significant differences were detected. For beech, although it is very shade tolerant and its behavior should be similar to that of fir, the CWLR tended to be lower compared to both conifers for the entire range in growing space. However, differences were significant only when compared to fir for PACL ≈3–50% and BA ≈40–90 m2 ha−1.
Terminal to lateral ratio
The decrease in TLR with decreasing available resources shown in Fig. 5 suggests that both conifers in our study were able to reduce height growth in favor of lateral growth at low light levels. Furthermore, with decreasing light, the fir tends to get a ratio below 1 at higher light levels than spruce. However, in these environments, differences between species were not significant, and therefore, the assumption that fir could better adapt to low light (Messier et al. 1999) is not supported.
Changes in beech morphology as a function of light (a—forking, b—stem orientation, c—stem symmetry) and basal area (d—forking, e—stem orientation, f— stem symmetry). (PACL, percentage of above canopy level; BA, basal area in m2 ha−1; Plagiotropic = 1, Orthotropic = 0, Forked = 1, Unforked = 0, Bilateral = 1, Radial = 0)
On Fig. 5, silver fir showed greater LCR and CWLR than spruce. Although differences were not significant, this implies that fir may compensate the loss in foliage due to reducing LCR by an increase in crown width (i.e., increase in CWLR). Therefore, fir would not only shed the nonproductive foliage at the bottom of the crown but also would add new productive foliage in lateral positions for better light harvest. Maintaining a balanced photosynthetic to respiring tissue ratio could explain why this species can persist in shade for long periods of time (Florescu and Nicolescu 1998) and also why firs could respond faster than spruces to a sudden release (Messier et al. 1999).
Alternatively, for spruce, the reduction in crown length by shedding the lower branches is probably not compensated by an increase in crown width. Height growth is not expected to compensate this loss without an adequate increase in light and therefore, at the tree level, the photosynthetic capacity decreases while respiration and maintenance costs remain constant or even increase. Additionally, the less efficient photosynthesis of spruce needles in deep shade (Grassi and Bagnaresi 2001) and possibly a shallower root system compared to beech and fir (Stanescu et al. 1997) make this species less able to compete for below ground resources with the surrounding stand. All these reasons may explain why its growth is significantly impaired in such environments when compared to that of beech and fir (Stancioiu and O'Hara 2005). In time all these may result in a negative net photosynthesis of the entire crown, which would lead eventually to tree death.
Number of nodal and internodal shoots
For conifers, both the number of internodal shoots and shoots in the terminal whorl decreased with decreasing available resources supporting the assumptions of Messier et al. (1999). However, a large scatter with increasing growing space implied that other factors may be responsible for the dynamics of these traits. Indeed, both variables seemed to be better correlated to the internodal length (i.e., the height growth of the previous year) (Fig. 6) agreeing with findings of Cannell (1974) for lodgepole pine (Pinus contorta Dougl. ex Loud.) and Sitka spruce (Picea sitchensis (Bong.) Carr).
Height growth of previous year should be more related to these variables as, for these species with preformed growth, the number of terminal and internodal buds would be set at the end of the previous growing season. This stronger relationship could also be explained by the fact that height growth, although directly influenced by both light and basal area, expresses the combined effects of both and also additional effects of site quality and climate. Additionally, measurement precision for height growth of small trees is much better than for light and basal area.
Our results for both species showed a sharp increase in the number of internodal shoots when length of the internode was greater than 0.4 m (Fig. 6). The number of spruce shoots was significantly higher than for fir above this threshold. Also, for number of shoots in terminal whorl, except for very short internodes (<0.05 m), the number of shoots for fir was significantly smaller than for spruce. Therefore, the spruce may adapt better than fir to increasing light environments by adding more new photosynthetic material on the internodes and also in the terminal whorl.
Stem forking, orientation, and symmetry
The previous studies that proposed indicators for beech morphology did not provide a physical measurement of growing space (Nicolini and Caraglio 1994; Nicolini 2000). Our results found these indicators to be useful in quantifying morphological changes along the gradients of light and basal area.
In our study, beech trees with a forked top occurred in all growing conditions, ranging from open to the densest stands. This confirmed the conclusions of Nicolini and Caraglio (1994) who linked the presence/absence of a fork in beech trees to both deep shaded and open environments. However, they proposed that causes of fork formation would be different in each case. In deep shade, the terminal leader (main axis) grows more horizontally (i.e., plagiotropic), becomes practically a branch, and one of the upper lateral branches catches up and may even outgrow the leader. On the other hand, in high light environments, two different processes could cause forking of fast growing trees. Sometimes a vigorous upper lateral branch with an upward orientation can catch up with the leader or even outgrow it. Another cause for fork formation in open conditions is the death of the terminal meristem, the leader being replaced by two lateral branches immediately below the injury. This is not something uncommon in these open environments, where growth tends to be polycyclic (Nicolini and Caraglio 1994) and the chance of the green terminal leader to be damaged by early frosts is greater than for trees under closed canopies. Under forest cover, this type of damage is not as prevalent due to a milder environment compared to open-grown conditions and because growth in such conditions is mostly monocyclic (Nicolini 1997) and shoots are already hardened at the time of the first frost. This is supported by our study (data not shown) as 11 out of the 12 trees that showed polycyclic growth were growing in environments with PACL≥45%. However, because we did not record if leader was damaged or not, we cannot state the precise cause of fork formation.
As stem forking could occur in both open and closed stand conditions (Nicolini and Caraglio 1994), it is not surprising that the logistic regression did not provide a very clear conclusion when the entire range of growing space was used. However, our data showed that a fork was formed more often in shaded conditions (Fig. 7a and d). Although data points were more scattered when basal area was the explanatory variable (Fig. 7d), results were not very different. Most of the forked trees (75% = 15 out of 20) occurred in environments with PACL≤20% and BA≥36 m2 ha−1. However, they represented 62.5% of all the trees in the case of light and only 55.5% of all the trees in the case of basal area.
In terms of stem orientation, our study shows that the highest probability to find trees with plagiotropic growth is at light levels below 30% (PACL) or basal area more than 40 m2 ha−1 (Fig. 7b and e). The slope of the model is steeper than for the other two traits indicating a strong relationship. In such environments both height and stem volume growth are considerably less than for more open environments (Stancioiu and O'Hara 2005). Therefore, these results support the findings of Nicolini (2000) that beech trees with a vertical (i.e., orthotropic) main stem are more vigorous than those with leaning stems (i.e., plagiotropic growth).
Related to stem and crown symmetry (Fig. 7c and f), radial crowns tended to form mostly when stand conditions were open (PACL≥40%, BA≤30 m2 ha−1) matching vigorous growth rates reported for this species by Stancioiu and O'Hara (2005). The slope of the logistic regression implies a weaker relationship than in the case of stem orientation but much stronger compared to stem forking. Although a physical measure of light or other resources was not provided, Nicolini (2000) also found that trees with radial symmetry would occur in more open conditions, while those with flat crowns (i.e., bilateral symmetry) will occur in dense stands with closed canopies. Also, trees in the former category would grow more vigorously than those in the latter. To explain this, Nicolini showed that even though meristems inside the buds are positioned along two parallel straight lines close to one side of the stem (“plagiotropic distic” pattern) the architecture of the future axis is not always flat (i.e., bilateral). If growing conditions are good and therefore growth in height is vigorous, the shoot would twist during its elongation and the resulting structure has a rather radial symmetry with leaves or shoots in more than one plane. This would help the tree take advantage of the better growing conditions, and efficiently fill surrounding growing space.
Overall, the peculiarities in beech growth had a great influence on its stem and crown architecture. Compared to conifers, which usually have a round crown profile, beech tree crowns varied from flat to round. As a result, measurements of the crown width in four cardinal directions could be drastically different. Furthermore, due to the presence of leaning and forked stems in this species, measuring crown length was more difficult compared to the conifers. These facts could explain some of the variability in data points for this species in low light environments for both crown width to length and live crown ratios. The major influence of light on beech morphology could also explain the larger scatter when basal area is used for growing space. Also, the ability to die back and re-grow (Florescu and Nicolescu 1998), should help beech adapt much easier to its respiration requirements and withstand shaded conditions for long periods of time. Therefore, the adaptability of crown form for this species is more dynamic than that of the conifers. Overall, these particularities could also imply a different adaptation mechanism in beech.
Conclusions
Although spruce showed a slightly lower ability to adapt its architecture in order to tolerate very shaded environments, these differences were not significant when compared to fir and beech. Thus, the morphological indicators used in this study were not able to separate these shade tolerant species. Physiological traits and other morphological indicators (e.g., below ground growth) are more likely to explain the contrasting behavior of these species in shaded environments.
If very dense stand conditions are maintained, spruce will likely be eliminated since it is less able to keep a positive carbon balance than fir and beech. However, in such conditions, chances for stem forking of beech are high. Therefore, when beech with good-form (unforked, orthotropic, having radial symmetry) are desired, PACL should be more than 30% or basal area should be below 30–35 m2 ha−1. For already established regeneration, all three species adapt their architecture for vigorous growth in open conditions. With increasing height growth, spruce seems to adapt better than fir. However, as growth rates are similar for all species (Stancioiu and O'Hara 2005), we could conclude that in open conditions advance regeneration of all three species can coexist without having a significant competitive advantage over the others.
Stand management for more than one species is often a challenging task. Compared to even-aged stands, structural and functional complexity increases considerably in stands with multiple age classes. When regeneration of more than one species is desired, silviculturists need to create growing conditions that match resource requirements for each species. Although in our example, all three species showed relatively similar phenotypic plasticity, differences in morphology and physiology exist and this information could help managers favor certain species or maintain a balanced mixture of the natural regeneration.
References
Avery TE, Burkhart HE (2002) Forest measurements, 5th edn. McGraw-Hill, Boston
Barnes VB, Zak DR, Denton SR, Spurr SH (1997) Forest ecology. Wiley, New York
Bartelink HH (1997) Allometric relationships for biomass and leaf area of beech (Fagus sylvatica L). Ann For Sci 54:39–50
Beaudet M, Messier C (1998) Growth and morphological responses of yellow birch, sugar maple, and beech seedlings growing under a natural light gradient. Can J For Res 28:1007–10015
Brunner A (1993) Die Entwicklung von Bergmischwaldkulturen in den Chiemgauer Alpen und eine Methodenstudie zur ökologischen Lichtmessung im Wald. Forstliche Forschungsberichte München 128
Cannell MGR (1974) Production of branches and foliage by young trees of Pinus contorta and Picea sitchensis: Provenance differences and their simulation. J Appl Ecol 11:1091–1115
Chen HYH, Klinka K, Kayahara GJ (1996) Effects of light on growth, crown architecture, and specific leaf area for naturally established Pinus contorta var. latifolia and Pseudotsuga menziesii var. glauca saplings. Can J For Res 26:1149–1157
Claveau Y, Messier C, Comeau PG, Coates KD (2002) Growth and crown morphological responses of boreal conifer seedlings and saplings with contrasting shade tolerance to a gradient of light and height. Can J For Res 32:458–468
Duchesneau R, Lesage I, Messier C, Morin H (2001) Effects of light and intraspecific competition on growth and crown morphology of two size classes of understory balsam fir saplings. For Ecol Manage 140:215–225
Evans J (1984) Silviculture of broadleaved woodland. Forestry Commission Bulletin 62, UK
Florescu II, Nicolescu NV (1998) Silvicultura, vol II—Silvotehnica. Editura Universitatii Transilvania din Brasov, Brasov
Gendron F, Messier C, Comeau PG (1998) Comparison of various methods for estimating the mean growing season percent photosythetic photon flux density in forests. Agric For Meteorol 92:55–70
Givnish T (1988) Adaptation to sun and shade: a whole plant perspective. Aust J Plant Physiol 15:63–92
Grassi G, Bagnaresi U (2001) Foliar morphological and physiological plasticity in Picea abies and Abies alba saplings along a natural light gradient. Tree Physiol 21:959–967
Haralamb A (1967) Cultura speciilor forestiere, Editia a 3 a. Editura Agro-Silvica, Bucuresti
Klinka K, Wang Q, Kayahara K (1992) Light-growth response relationships in Pacific silver fir (Abies amabilis) and subalpine fir (Abies lasiocarpa). Can J Bot 70:1919–1930
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic, London
Messier C, Nikinmaa E (2000) Effects of light availability and sapling size on the growth, biomass allocation, and crown morphology of understory sugar maple, yellow birch, and beech. Ecoscience 7(3):345–356
Messier C, Parent S (1997) Reply—The effects of direct-beam light on overcast-day estimates of light availability: on the accuracy of the instantaneous one-point overcast-sky conditions method to estimate mean daily PPFD under heterogeneous overstory canopy conditions. Can J For Res 27:274–275
Messier C, Doucet R, Ruel JC, Claveau Y, Kelly C, Lechowicz MJ (1999) Functional ecology of advance regeneration in relation to light in boreal forests. Can J For Res 29:812–823
Negulescu EG, Ciumac G (1959) Silvicultura. Ministerul agriculturii si silviculturii— Editura agro-silvica de stat, Bucuresti
Nicolini É (1997) Approche morphologique du développement du hêtre (Fagus sylvatica L.). PhD thesis, University of Montpelier II
Nicolini É (2000) New observations on the morphology of beech (Fagus sylvatica L.) growth units. Symmetry of the shoots reflects the vigor of the trees. Can J Bot 78:77–87
Nicolini É, Caraglio Y (1994) L'influence de divers caractères architecturaux sur l'apparition de la fourche chez le Fagus sylvatica, en fonction de l'absence ou de la présence d'un couvert. Can J Bot 72:1723–1734
Ninemets U, Kull O (1995) Effects of light availability and tree size on the architecture of assimilative surface in the canopy of Picea abies: Variation in needle morphology. Tree Physiol 15:307–315
Oliver CD, Larson BC (1996) Forest stand dynamics. Wiley, New York
Padraic MJ, Huss J, McCarthy R, Pfeifer A, Hendrick E (1998) Growing broadleaves: Silvicultural guidelines for ash, sycamore, wild cherry, beech, and oak in Ireland. COFORD-National Council for Forest Research and Development, National University of Ireland, Belfield, Dublin
Parent S, Messier C (1996) A simple and efficient method to estimate light availability under a forest canopy. Can J For Res 26:151–154
Ruel J-C, Messier C, Doucet R, Claveau Y, Comeau P (2000) Morphological indicators of growth response of coniferous advance regeneration to overstory removal in the boreal forest. For Chron 76:633–642
Savill PS (1991) The silviculture of trees used in British forestry. CAB International, Wallingford, UK
Schlichting CD (1986) The evolution of phenotypic plasticity in plants. Ann Rev Ecol Syst 17:667–693
Smith DM, Larson BC, Kelty MJ, Ashton PMS (1996) The practice of silviculture: applied forest ecology, 9th edn. Wiley, New York
Stancioiu PT (2003) Regeneration dynamics in mixed-species, multiaged, mountainous forests of Romania. PhD thesis, University of California at Berkeley
Stancioiu PT, O'Hara KL (2005) Regeneration growth in different light environments of mixed-species, multiaged, mountainous forests of Romania. Eur J Forest Res (in press)
Stanescu V, Sofletea N, Popescu O (1997) Flora forestiera lemnoasa a Romaniei. Editura Ceres, Bucuresti
Williams H, Messier C, Kneeshaw D (1999) Effects of light availability and sapling size on the growth and crown morphology of understory Douglas-fir and lodgepole pine. Can J For Res 29:222–231
Acknowledgements
The National Forest Administration and Retezat National Park granted access to field sites and inventory data. Access to laboratory supplies and technology was provided by Faculty of Biology and Geology (“Babes-Bolyai” University, Cluj, Romania) and by Traian Cadariu (Orastie, Romania). Part of the fieldwork during 2001 was funded by Adonis group—Belgium. Assistance provided by Daniel Vasiu with field and laboratory work is greatly appreciated
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stancioiu, P.T., O'Hara, K.L. Morphological plasticity of regeneration subject to different levels of canopy cover in mixed-species, multiaged forests of the Romanian Carpathians. Trees 20, 196–209 (2006). https://doi.org/10.1007/s00468-005-0026-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-005-0026-2