Abstract
Sulfate transport processes and its regulation were studied in roots of poplar trees (Populus tremula x P. alba). From the exponential increase in sulfate uptake with temperature an activation energy (Ea) of 9.0±0.8 kJ mol−1 was calculated. In the concentration range 0.005–10 mM sulfate uptake showed biphasic Michaelis-Menten kinetics with a Km of 3.2±3.4 μM and a Vmax of 49±11 nmol SO42− g−1 FW h−1 for the high-affinity uptake system (phase 1) and a Km of 1.33±0.41 mM and a Vmax of 255±25 nmol SO42− g−1 FW h−1 for the low-affinity system (phase 2). Xylem loading decreased linearly with temperature and remained unchanged within the sulfate concentration range studied. Regulation of sulfate uptake and xylem loading by O-acetyl serine (OAS), Cys, reduced glutathione (GSH), Met and S-methylmethionine (SMM) were tested by perfusion into the xylem sap with the pressure probe and by addition to the incubation medium. When added directly to the transport medium, Cys and GSH repressed, and OAS stimulated sulfate uptake; xylem loading was stimulated by Cys, repressed by GSH and only slightly affected by OAS. When perfused into the xylem, none of the compounds tested affected sulfate uptake of excised roots, but xylem loading was stimulated by SMM and OAS and repressed by Met. Apparently, the site of application strongly determined the effect of regulatory compounds of sulfate transport processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur is the fifth or sixth most abundant element in plants (Herschbach and Rennenberg 2001). It is present in essential compounds of the plant’s primary metabolism like amino acids (Cys, Met), sulfolipids and the molybdenum cofactor, as well as in secondary metabolites like DMSP, alliin, glucosinolates and isothiocyanates (Benning 1998; Kocsis et al. 1998; Mendel and Schwarz 1999; Manabe et al. 2000; Tabe and Droux 2000; Fahey et al. 2001). Sulfur is mainly needed in its reduced form (e.g. as Cys, Met), but is almost exclusively taken up in its oxidized form as sulfate by the roots (Leustek et al. 2000), although uptake of reduced sulfur has been observed under controlled conditions (Seegmüller and Rennenberg 2002). The uptake (and transport) of sulfate is facilitated by sulfate transporter proteins and driven by a proton gradient generated by ATPases (e.g. Nissen 1971; Hawkesford et al. 1993; Smith et al. 1995, 1997). It is expected that uptake is dependent on temperature, pH and sulfate concentration. Part of the sulfate taken up is stored and/or metabolized in the roots (Sunarpi and Anderson 1996), the other part is loaded into the xylem and stored in the stem or transported to the shoot (Herschbach and Rennenberg 1997), where it is reduced and incorporated into carbohydrate skeletons (Hell 2003; Herschbach 2003). Despite its significance in sulfur nutrition, information about the characteristics and regulation of xylem loading of sulfate is scarce (Herschbach and Rennenberg 1991; Kreuzwieser et al. 1996; Seegmüller et al. 1996; Kreuzwieser and Rennenberg 1998). Most studies on the characterization and regulation of sulfate uptake and xylem loading by plant roots have been focused on herbal plants and external ion concentration (e.g. Clarkson et al. 1983; Herschbach and Rennenberg 1991; Nissen 1991; Hawkesford et al. 1993; Smith et al. 1995, 1997; Kreuzwieser and Rennenberg 1998; Herschbach et al. 2002). But xylem loading of sulfate may also be affected by the composition of the xylem sap. Reduced sulfur compounds which play a role in the regulation of sulfate uptake and xylem loading have been found in the xylem sap (e.g. Schneider et al. 1994; Herschbach et al. 2000). In addition, research in the 1990s showed that the characteristics and regulation of sulfate uptake and xylem loading of trees can differ from those of herbs (Herschbach and Rennenberg 1997), probably because trees intensively store and remobilize sulfur compounds in the stem and/or roots (Herschbach and Rennenberg 1995, 1996, 1997; Hartmann et al. 2000).
Experiments with externally applied or phloem fed compounds revealed that Cys, Met and glutathione regulate sulfate uptake and adapt it to the plant’s demands (e.g. Lappartient and Touraine 1996; Bolchi et al. 1999; Herschbach and Rennenberg 2001). SMM is a reduced sulfur compound in the phloem (Bourgis et al. 1999) with so far unknown effects on the regulation of sulfate uptake and xylem loading. OAS is the metabolic precursor that provides the carbohydrate skeleton in Cys synthesis and is thought to be involved in the metabolic cross-talk between sulfur and nitrogen assimilation (Hell 2003). It is also therefore a good candidate for a crosstalk between nitrogen metabolism and sulfate transport processes. In this study, we investigated the characteristics and regulation of sulfate uptake and xylem loading of poplar roots (Populus tremula x P. alba). For this purpose, we optimized sulfate uptake and xylem loading for incubation time, plant age and pH and measured the dependency of sulfate uptake and xylem loading on temperature and external sulfate with excised roots. To study the effect of regulatory compounds we either exposed the excised roots to these compounds in the incubation medium or perfused the xylem with these compounds using a pressure probe (Steudle 1993).
Materials and methods
Plant material and culture conditions
The experiments were performed with hybrid poplar trees (Populus tremula x P. alba) clone INRA 717 1-B4, (Strohm et al. 1995). Poplars were micropropagated and cultivated on a 0.5 MS medium under sterile conditions for 4 weeks as previously described. Explants were grown for 6–8 weeks at a 16 h light/8 h dark period in a greenhouse on a 1-l mixture of one part silica sand (particle size 0.06–0.2 mm), one part sterilized commercial soil and two parts Perlite (Agriperl, Perlite-Dämmstoff, Dortmund, Germany). Plants were fertilized every 2 weeks with a commercial fertilizer (Hakaphos blau, COMPO, Münster, Germany) at 3 g/l, 200 ml/plant (for contents of the fertilizer: see Herschbach et al. 2000). Plants grown for 6–8 weeks on this substrate showed no significant difference in sulfate uptake and xylem loading rates (data not shown) and were used for the experiments.
Uptake and xylem loading of sulfate
Sulfate uptake and xylem loading were measured with excised roots in an incubation chamber as previously described (Herschbach and Rennenberg 1991). Briefly, poplar roots were washed with tap water and root ends were cut in transport medium (standard conditions: 5 mM bisTRIS, 0.5 mM CaCl2 and 0.5 mM K2SO4 at pH 7.0). A total of six excised root ends were placed horizontally in the chamber. After 2 h preincubation transport medium at the root tips was exchanged and radioactivity (H225SO4 in H2O, carrier-free; 1.85 105 kBq/charge; ICN, Irvine, USA; 150–300 μl, resulting in a radioactivity between 8.7 and 17.4 kBq ml−1) was added. Sulfate uptake and xylem loading of sulfate were determined at 19°C under standard conditions from the radioactivity in the roots and the radioactivity exuded as previously described (Herschbach and Rennenberg 1991). Both uptake processes, i.e. sulfate uptake and xylem loading were linear with time after 1 h up to 6 h of incubation and were measured for 4 h under standard conditions. Since addition of sucrose did not affect the transport processes, energy limitation of the cut roots can be excluded.
Calculation of activation energy
Activation energy was calculated with the following equation (Steinkamp 1984):
with UPT: sulfate uptake (nmol SO42− g−1 FW h−1), Ea: activation energy (J mol−1), R: gas constant (8.3143 J K−1 mol−1), T: temperature (K).
Synthesis of SMM
Because SMM is commercially available only as the toxic iodide salt (SMMI), this compound was converted into its non-toxic chloride by ion-exchange chromatography on a AG2-X8-Cl resin (100–200 mesh; Bio-Rad, Hercules, Canada, ion exchange capacity 1.2 mmol ml−1). To remove water, SMMI (Sigma, St. Louis, Canada) was first freeze-dried for 6 days; 3.278 g of SMMI was then dissolved in 50 ml distilled water and 33.737 g of the ion exchange resin was added. The mixture was stirred for 1 h and then filtered, to remove the resin. During this procedure, the color of the solution turned from yellow to colorless. The filtrate was frozen in 2-ml portions in liquid nitrogen.
Solutions of regulatory compounds
The following compounds were applied as regulatory substances: 1 mM OAS (Sigma, St. Louis, USA), 1 mM Cys (min. 99%, Merck, Darmstadt), 10 mM GSH (Boehringer Mannheim), 1 mM Met (minimum 99%, Sigma Aldrich Chemie, Steinheim) and 1 mM SMM (made from SMMI: Sigma, St. Louis, USA). Solutions were made freshly before each experiment. Regulators were dissolved at the concentrations mentioned in transport medium and added to the root incubation chamber. In xylem perfusion experiments, compounds were dissolved in distilled water to a concentration resulting in a calculated concentration of 1 or 10 mmol kg−1 root FW. The pH was adjusted to 6.0, which corresponds to the pH of the xylem sap (data not shown).
Perfusion of the root xylem
A pressure probe (Steudle 1993) was used for applying regulatory solutions to the root xylem (Fig. 1). For this purpose the shoots of the poplar trees were cut 5–10 cm above the soil with a razor blade on the node above the first internode (counted from the shoot base). A minimum of 2 cm shoot length was required for putting the pressure probe over the cut end. The leaf on this node was removed, leaves below the cut node, which are close to the soil, were left intact. The pressure probe was installed over the cut end and was sealed by a flexible ring, 0.2–0.6 cm in diameter (Fig. 1e). This ring was made of Xantropren L blue paste made flexible with Xantropren Activator NF Optosil liquid (Dormagen). Using a 100 μl syringe (Type 1710N, Hamilton, Bonadur, Switzerland) a defined volume of the solution was drawn into the capillary (Fig. 1b,,). The solution was injected into the root xylem by applying an over pressure of 2.0±0.1 bar N2 (Nitrogen 5.0, Sauerstoffwerk, Friedrichshafen) for 2 h (Fig. 1a–c). The pressure was measured continuously during injection (Type 230149-D1, Span Instruments, Plano, Texas, USA). Guttation out of the leaves during injection was unavoidable, but only a few drops were lost. Pilot experiments with 1% (m/v) eosin scarlet dye solution (91.1%, Fluka), which contrasted with the color of the roots and substrate, showed that 2 h incubation was sufficient to fill the xylem of the entire root with the solution (data not shown). At the end of the injection period, the remaining solution in the capillary was removed and the volume was measured for calculation of the theoretical regulator concentration in the roots (Table 2). Poplar roots took up 0.870±0.329 ml solution during a 2 h perfusion period with the pressure probe under the conditions mentioned above. Sulfate transport processes of the perfused roots were determined with excised roots as described above.
Thiol analysis
Preparation of the samples was performed with a modification of the method reported by Strohm et al. (1995). Fine roots were homogenized in liquid nitrogen and aliquots of 100 mg root powder were added to pre-cooled 1 ml 0.1 M HCl containing 100 mg cleaned insoluble PVPP. The samples were shaken and then centrifuged at 4°C and 14,000 g for 30 min. An aliquot of 240 μl of each sample was mixed with 260 μl 0.2 M CHES (Sigma Chemie, Deisenhoven) (pH 9.3); 30 μl 6 mM DTT (Sigma Chemie, Deisenhoven) were added and the mixture was incubated for 1 h to reduce oxidized thiols. For derivatization 10 μl 30 mM of the fluorescent dye monobromobimane (mBBr, Thiolyte MB, Calbiochem-Behring, Frankfurt) in acetonitrile (Merck, 99.8%) were added to the samples and incubated for 15 min in the dark. Thiol derivatives were stabilized by acidification with acetic acid (80 μl 30% v/v; Merck, 99.8%) and stored at 4°C until analysis. Samples were centrifuged at 4°C and 14,000 g for 20 min to remove fine particles. The supernatants were taken for thiol analysis.
Thiol content was measured by reverse-phase HPLC (Beckman HPLC-Gold, Beckman Instruments, Bioindustrial Business Unit, Fullerton, USA) by a modified protocol after Schupp and Rennenberg (1988). Aliquots of 200 μl per sample were injected. Identification and quantification of the peaks were achieved with a standard containing 0.1 mM Cys (min. 99%, Merck, Darmstadt) and 1 mM reduced GSH (Boehringer Mannheim). Recovery was 76±34% for Cys and 79±26% for GSH (n=60 and 61, respectively).
Results
Sulfate uptake and xylem loading are temperature dependent
Sulfate uptake and xylem loading showed an optimum at pH 6.0–7.0 and were dependent on temperature in the range from 9°C to 29°C (Fig. 2). From 9°C to 21.5°C sulfate uptake increased exponentially. An Ea for sulfate uptake of 9.0±0.8 kJ mol−1 was calculated in this range. Between 21.5°C and 29°C sulfate uptake showed a broad temperature optimum. Xylem loading of sulfate declined linearly from 9°C to 29°C (relative xylem loading = −0.29T+10.36, R2=0.40, P<0.0001).
Temperature-dependent sulfate uptake (a), exudation (b) and xylem loading (c). Sulfate concentration in the transport medium was 0.5 mM. For experimental details see Materials and methods. Statistics were performed with ORIGIN5.0 using non-linear (sulfate uptake: Boltzmann formula, sulfate exudation: Gaussian formula) and linear (xylem loading of sulfate) regression with single values. Data shown are means ± SD from experiments with 3–4 plants with 2–3 replicates each (n=6–18)
Sulfate uptake shows biphasic Michaelis-Menten kinetics
Sulfate uptake showed biphasic Michaelis-Menten kinetics between 0.005 mM and 10 mM sulfate (Fig. 3). Affinity (Km) and maximum uptake velocity (Vmax) were calculated from Lineweaver-Burk, Eadie-Hofstee and Hanes plots (Bisswanger 1979). The Km of the high-affinity uptake system (phase 1) amounted to 3.2±3.4 μM and the Vmax to 49±11 nmol SO42− g−1 FW h−1; the Km of the low-affinity system (phase 2) amounted to 1.33±0.41 mM and the Vmax 255±25 nmol SO42− g−1 FW h−1. The transition from phase 1 to 2 was found at 0.302±0.048 mM SO42− (Table 1). Xylem loading of sulfate remained unchanged in the concentration range of 0.005–10 mM sulfate (Fig. 4).
Sulfate uptake as a function of sulfate concentration [high affinity uptake system (insert): 0.005–0.250 mM; low affinity uptake system: 0.5–10.0 mM] in the transport medium. Incubation temperature was 19°C. For experimental details see Materials and methods. Statistics were performed with ORIGIN5.0 using non-linear (Michaelis-Menten formula) regression with single values. Data shown are means ± SD from experiments with three plants with two replicates each (n=6)
The pressure probe is a suitable means to inject regulatory solutions into the root xylem
Perfusion of poplar roots with distilled water overnight caused a significant reduction in sulfate uptake and absolute xylem loading of sulfate (data not shown). Therefore, perfusion was reduced to 2 h. To compare xylem and medium treatments with regulatory compounds, poplar trees used for the medium treatments of excised roots were also decapitated 2 h before starting sulfate uptake experiments. The amount of regulatory solution used for perfusion was independent from plant age and treatment (data not shown). Statistical analysis indicated a weak linear correlation of perfused distilled water with shoot height (R2=0.40) and root + stump FW (R2=0.52). Compared to xylem sap concentrations of Cys, GSH and Met, perfusion resulted in an enrichment of about 2–3 orders of magnitude based on root fresh weight (Table 2).
Xylem loading but not sulfate uptake is regulated by compounds perfused into the xylem
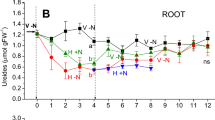
If Met was perfused into the xylem, a slight repression of absolute xylem loading (P<0.14) and percentile xylem loading (P<0.09) was observed (Fig. 5). Xylem loading kinetics of sulfate in the presence of Met were significantly repressed (Fig. 6). In contrast, SMM stimulated absolute and percentile xylem loading (Fig. 5). Due to the high variation, P-values amounted to 0.08 for absolute and to 0.12 for percentile xylem loading, respectively. Xylem loading kinetics were significantly stimulated by SMM (Fig. 6). The difference in the amount of stimulation of xylem loading by SMM in Figs. 5 (percentage of control) and 6 (absolute values) is due to different calculation procedures of the raw data. Between different sets of experiments high difference in absolute values for xylem loading (0.9±0.6 nmol SO42− g−1 FW h−1 and 1.9±1.3 nmol SO42− g−1 FW h−1) of controls was observed (Fig. 6). Similarly to SMM, OAS caused a slight stimulation of absolute xylem loading of sulfate (P<0.10) and percentile xylem loading (P<0.15). Although effects of xylem-applied compounds were detectable, the Cys and glutathione contents of the roots perfused with Met, SMM and OAS remained unchanged (data not shown). Xylem loading kinetics of sulfate were linear and lag phases did not exceed 0.4 h (data not shown). Met, SMM and OAS applied to the xylem did not alter sulfate uptake; Cys and GSH applied to the xylem did not have any effect on sulfate transport processes (Fig. 5).
Regulation of sulfate uptake, absolute and percentile xylem loading by organic compounds in the root xylem. Excised roots were incubated in 35S-sulfate for 2.5 h at pH=6.0. For each experimental day, the control treatment was set at 100%. Data shown are means ± SD from experiments with n=10 plants. Horizontal line 100±17% (control)
Influence of 1 mM methionine (Met) and S-methylmethionine (SMM) in the root xylem on the xylem loading kinetics of sulfate. The results of Met and SMM presented in Figs. 5 and 6 are from the same experiments. Perfusion of the root xylem was performed as described in Materials and methods. Excised roots were incubated at pH=6.0. Statistics were performed with SPSS 9.0 using linear regression with single values. Comparison of two straight lines was performed with EXCEL 6.0 (Mead et al. 1993). Significant differences at the 0.05-level are indicated by different letters. Data shown are means ± SD from experiments with 9–10 replicate plants (n=9–10). Open symbols controls; filled symbols Met, SMM
Sulfate uptake and xylem loading are regulated by compounds in the transport medium
Cys added to the transport medium significantly repressed sulfate uptake, but stimulated percentile xylem loading (Fig. 7). GSH repressed sulfate uptake slightly (P<0.08) and absolute and percentile xylem loading significantly (Fig. 7). Met, SMM and OAS in the transport medium had no effect on sulfate uptake and xylem loading (Fig. 7). Xylem loading kinetics of sulfate were linear and lag phases did not exceed 0.4 h (data not shown).
Regulation of sulfate uptake, absolute and percentile xylem loading by organic compounds in the transport medium. For experimental conditions, calculations and statistics see Fig. 5. Significant differences at the 0.05-level are indicated with asterisks. Data shown are means ± SD from experiments with n=9–10 plants. * P<0.05; horizontal line 100±15% (control)
Discussion
Characteristics of sulfate uptake and xylem loading
The present experiments provide the first data for the activation energy Ea of sulfate uptake by tree roots. Although the value may be inaccurate because of the complex nature of the sulfate transport process (Bassirirad 2000), the Ea obtained is comparable to the activation energy found for algae and herbs (Holmern et al. 1974; Biedlingmaier and Schmidt 1989; Bassirirad 2000).
Because different transformations of the Michaelis-Menten formula (Eadie-Hofstee, Lineweaver-Burk and Hanes) with each having particular weaknesses (Bisswanger 1979), Km and Vmax were calculated as means of each of these transformations. Sulfate uptake of poplar roots showed for each transformation biphasic Michaelis-Menten kinetics (Table 1). Mono- and multiphasic sulfate uptake kinetics were found in fungi (Cuppoletti and Segel 1974, 1975; Yildiz et al. 1994; Cherest et al. 1997), bacteria (Menon and Varma 1982; Ritchie 1996), and several lower (Coughlan 1977; Matsuda and Colman 1995; Mimura et al. 1998) and higher plant species (e.g. Clarkson et al. 1983; Hawkesford et al. 1993; Kreuzwieser et al. 1996; Seegmüller et al. 1996). The number of phases, as well as Km and Vmax values were dependent on plant species and varieties (e.g. Jones and Smith 1981; Rennenberg et al. 1988). Beech trees showed triphasic sulfate uptake kinetics (Kreuzwieser et al. 1996), but oak trees only one phase (Seegmüller et al. 1996). Km of the high affinity uptake system of poplar is comparable to oak trees, but much lower than that of beech trees. Whether multiphasic uptake kinetics are due to different conformations of one individual sulfate transporter or due to the action of several transporters is a matter of debate (Nissen 1971, 1991; Borstlap 1983). Indications that sulfate transporters have several conformations are lacking. Experimental evidence, however, suggests the existence of more than one isozyme of sulfate transporter (e.g. Smith et al. 1995; Hawkesford and Prosser 2000; Takahashi et al. 2000).
Relative xylem loading of sulfate in poplar trees (Fig. 4) is comparable to beech (Kreuzwieser et al. 1996) and oak (Seegmüller et al. 1996) trees, but clearly lower than that observed in tobacco (Herschbach and Rennenberg 1991). This may be due to the fact that trees are perennials which predominantly store sulfur in the roots, and tobacco is an annual which invests sulfur more directly in growth and development. Part of the sulfate taken up is reduced in poplar roots, but sulfate reduction is clearly low compared to the shoot (Hartmann et al. 2000; Herschbach, personal communication). Also the concentration of sulfate in the xylem is much higher than that of reduced sulfur compounds (Herschbach et al. 2000). High sulfate concentrations in the roots (40–60 μmol g−1 FW, Herschbach et al. 2000) as a consequence of low relative xylem loading of sulfate indicate storage of sulfate in the roots. This storage of sulfate, its liberation from decaying roots, and its uptake by newly developed roots are considered a set of processes that keep sulfate in the rhizosphere. This may be required, because sulfate can be rapidly washed out from the soil by precipitation.
Regulation of sulfate uptake and xylem loading
Incubation of poplar roots in media containing regulatory substances and perfusion of the root xylem with a solution of the same regulator have in common that both treatments act via the apoplast. Therefore, it was expected that the regulatory effects are the same. The data presented in Figs. 5 and 7, however, reveal that this is not the case. Cys and GSH added to the incubation medium repressed sulfate uptake; Met repressed, SMM and OAS stimulated xylem loading of sulfate in perfusion experiments. Apparently, not only the regulatory compound itself, but also the site of application is of importance for the regulatory effect on sulfate uptake and xylem loading.
Together with Met, glutathione is the most abundant reduced sulfur compound in poplar trees (Herschbach et al. 2000). From previous studies glutathione is known to repress sulfate uptake and xylem loading (Fig. 7, see also Herschbach and Rennenberg 1991; Lappartient and Touraine 1996; Leustek et al. 2000; Vauclare et al. 2002). In poplar trees, however, not glutathione itself, but the SO42−/glutathione ratio in the phloem seems to be responsible for regulation of sulfate uptake and xylem loading (Herschbach et al. 2000). In these previous studies GSH was applied to the symplast (phloem), whereas both techniques used in this study provided GSH to the apoplastic space (medium, xylem). This difference in application could explain the differences in regulation of sulfate uptake and xylem loading observed between this and previous experiments.
The slight stimulatory effect of OAS on sulfate uptake and absolute xylem loading (Figs. 5, 7) confirms earlier findings on the regulatory properties of this compound (e.g. Kreuzwieser 1997; Clarkson et al. 1999; Hatzfeld and Saito 2000; Hirai et al. 2003). The absence of a stimulatory effect of OAS fed to the xylem on sulfate uptake might be due to the lability of OAS (Kredich 1993) that probably was metabolized before it could reach the root cortex.
Abbreviations
- CHES::
-
2-(Cyclohexylamino)-ethanesulfonic acid
- Ea::
-
Activation energy
- mBBr::
-
Monobromobimane
- MS::
-
Murashige and Skoog
- OAS::
-
O-Acetyl-l-serine
- OAS-TL::
-
O-Acetyl-l-serine thiol lyase
- SAT::
-
Serine acetyl transferase
- SMM::
-
S-Methyl-l-methionine
- SMMI::
-
Iodide salt of SMM
References
Bassirirad H (2000) Kinetics of nutrient uptake by roots: responses to global change. New Phytol 147:155–169
Benning C (1998) Biosynthesis and function of the sulfolipid sulfoquinovosyl diacylglycerol. Annu Rev Plant Physiol Plant Mol Biol 49:53–75
Biedlingmaier S, Schmidt A (1989) Sulfate uptake in normal and S-deprived Chlorella fusca. Z Naturforsch 44C:495–503
Bisswanger H (ed) (1979) Theorie und Methoden der Enzymkinetik. Eine Einführung für Biochemiker, Biologen und Mediziner. Chemie, Weinheim
Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S (1999) Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by l-cysteine. Plant Mol Biol 39:527–537
Borstlap AC (1983) The use of model-fitting in the interpretation of “dual” uptake isotherms. Plant Cell Environ 6:407–416
Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C, Herschbach C, Rennenberg H, Pimenta MJ, Shen T-L, Gage DA, Hanson AD (1999) S-methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase. Plant Cell 11:1485–1497
Cherest H, Davidian J-C, Thomas D, Benes V, Ansorge W, Surdin-Kerjan Y (1997) Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics 145:627–635
Clarkson DT, Smith FW, Vandenberg PJ (1983) Regulation of sulfate transport in a tropical legume, Macroptilium atropurpureum, cv. Sirato. J Exp Bot 34:1463–1483
Clarkson DT, Diogo E, Amânchio S (1999) Uptake and assimilation of sulfate by sulfur deficient Zea mays cells: the role of O-acetyl-l-serine in the interaction between nitrogen and sulfur assimilatory pathways. Plant Physiol Biochem 37:283–290
Coughlan S (1977) Sulfate uptake in Fucus serratus. J Exp Bot 28:1207–1215
Cuppoletti J, Segel IH (1974) Transinhibition kinetics of the sulfate transport system of Penicillium notatum. Analysis based on an iso uni uni velocity equation. J Membr Biol 17:239–252
Cuppoletti J, Segel IH (1975) Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons, and calcium. Biochemistry 14:4712–4718
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51
Hartmann T, Mult S, Suter M, Rennenberg H, Herschbach C (2000) Leaf age-dependent differences in sulfur assimilation and allocation in poplar (Populus tremula x P. alba) leaves. J Exp Bot 51:1077–1088
Hatzfeld Y, Saito K (2000) Regulation of sulfate transporter genes in Arabidopsis thaliana. In: Brunold C, Rennenberg H, Kok LJ, Stulen I, Davidian J-C (eds) Sulfur nutrition and sulfur assimilation in higher plants. Molecular, biochemical and physiological aspects. Paul Haupt, Berne, Switzerland, pp 269–271
Hawkesford MJ, Prosser IM (2000) The plant sulfate transporter family. In: Brunold C, Rennenberg H, Kok LJ, Stulen I, Davidian J-C (eds) Sulfur nutrition and sulfur assimilation in higher plants. Molecular, biochemical and physiological aspects. Paul Haupt, Berne, Switzerland, pp 263–264
Hawkesford MJ, Davidian J-C, Grignon C (1993) Sulfate/proton cotransport in plasma membrane vesicles isolated from roots of Brassica napus L.: increased transport in membranes isolated from sulfur-starved plants. Planta 190:297–304
Hell R (2003) Metabolic regulation of cysteine synthesis and sulfur assimilation. In: Davidian J-C, Grill D, Kok LJ, Stulen I, Hawkesford MJ, Schnug E, Rennenberg H (eds) Sulfur nutrition and sulfur assimilation in higher plants: regulation, interaction and signaling. Backhuys, Leiden, The Netherlands, pp 21–32
Herschbach C (2003) Whole plant regulation of sulfur nutrition of deciduous trees—influence of the environment. Plant Biol 5:233–244
Herschbach C, Rennenberg H (1991) Influence of glutathione (GSH) on sulfate influx, xylem loading and exudation in excised tobacco roots. J Exp Bot 42:1021–1029
Herschbach C, Rennenberg H (1995) Long-distance transport of 35S-sulfur in 3-year-old beech trees (Fagus sylvatica). Physiol Plant 95:379–386
Herschbach C, Rennenberg H (1996) Storage and remobilization of sulfur in beech trees (Fagus sylvatica). Physiol Plant 98:125–132
Herschbach C, Rennenberg H (1997) Sulfur nutrition of conifers and deciduous trees. In: Rennenberg H, Eschrich W, Ziegler H (eds) Trees—contributions to modern tree physiology. Backhuys, Leiden, The Netherlands, pp 293–311
Herschbach C, Rennenberg H (2001) Significance of phloem-translocated organic sulfur compounds for the regulation of sulfur nutrition. Prog Bot 62:177–193
Herschbach C, van der Zalm E, Schneider A, Jouanin L, De Kok L, Rennenberg H (2000) Regulation of sulfur nutrition in wildtype poplar trees overexpressing γ-glutamylcysteine synthetase as affected by atmospheric H2S. Plant Physiol 124:461–473
Herschbach C, Pilch B, Tausz M, Rennenberg H, Grill D (2002) Sulphate uptake and xylem loading of young pea (Pisum sativum L.) seedlings. Plant Soil 242:227–233
Hirai MY, Fujiwara T, Awazuhara M, Kimura T, Noji M, Saito K (2003) Global expression profiling of sulfur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-l-serine as a general regulator of gene expression in response to sulfur nutrition. Plant J 33:651–663
Holmern K, Vange MS, Nissen P (1974) Multiphasic uptake of sulfate by barley roots. II. Effects of washing, divalent cations, inhibitors, and temperature. Physiol Plant 31:302–310
Jones SL, Smith IK (1981) Sulfate transport in cultured tobacco cells. Plant Physiol 67:445–448
Kocsis MG, Nolte KD, Rhodes D, Shen T-L, Gage DA, Hanson AD (1998) Dimethylsulfoniopropionate biosynthesis in Spartina alterniflora. Evidence that S-methylmethionine and dimethylsulfoniopropylamine are intermediates. Plant Physiol 117:273–281
Kredich NM (1993) Gene regulation of sulfur assimilation. In: Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE (eds) Sulfur nutrition and assimilation in higher plants. SPB Academic, The Hague, The Netherlands, pp 37–47
Kreuzwieser J (1997) Sulfat- und Nitrattransport bei mykorrhizierten und nicht-mykorrhizierten Buchen (Fagus sylvatica L). Ph.D. Thesis, University Freiburg, Germany
Kreuzwieser J, Rennenberg H (1998) Sulfate uptake and xylem loading of mycorrhizal beech roots. New Phytol 140:319–329
Kreuzwieser J, Herschbach C, Rennenberg H (1996) Sulfate uptake and xylem loading of non-mycorrhizal excised roots of young Fagus sylvatica trees. Plant Physiol Biochem 34:409–416
Lappartient AG, Touraine B (1996) Demand-driven control of root ATP sulfurylase activity and SO42− uptake in intact canola. The role of phloem-translocated glutathione. Plant Physiol 111:147–157
Leustek T, Martin MN, Bick J-A, Davies JP (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol 51:141–165
Manabe T, Hasumi A, Sugiyama M, Yamazaki M, Saito K (2000) Alliinase (S-alk(en)yl-l-cysteine sulfoxide lyase) from Allium tuberosum (Chinese chive). In: Brunold C, Rennenberg H, Kok LJ, Stulen I, Davidian J-C (eds) Sulfur nutrition and sulfur assimilation in higher plants. Molecular, biochemical and physiological aspects. Paul Haupt, Berne, Switzerland, pp 419–420
Matsuda Y, Colman B (1995) Characterization of sulfate transport in the green alga Chlorella ellipsoidea. Plant Cell Physiol 36:1291–1296
Mead R, Curnow RN, Hasted AM (1993) Statistical methods in agriculture and experimental biology. Chapman and Hall, London
Mendel RR, Schwarz G (1999) Molybdoenzymes and molybdenum cofactor in plants. Crit Rev Plant Sci 18:33–69
Menon VKN, Varma AK (1982) Sulfate uptake in the cyanobacterium Spirulina platensis. FEMS Microbiol Lett 13:141–146
Mimura T, Reid RJ, Smith FA (1998) Control of phosphate transport across the plasma membrane of Chara corollina. J Exp Bot 49:13–19
Nissen P (1971) Uptake of sulfate by roots and leaf slices of barley: mediated by single, multiphasic mechanisms. Physiol Plant 24:315–324
Nissen P (1991) Multiphasic uptake mechanisms in plants. Int Rev Cytol 120:89–134
Rennenberg H, Polle A, Martini N, Thoene B (1988) Interaction of sulfate and glutathione transport in cultured tobacco cells. Planta 176:68–74
Ritchie RJ (1996) Sulfate transport in the cyanobacterium Synechococcus R-2 (Anacystis nidulans, Sleopoliensis) PCC 7942. Plant Cell Environ 19:1307–1316
Schneider A, Kreuzwieser J, Schupp R, Sauter JJ, Rennenberg H (1994) Thiol and amino acid composition of xylem sap of poplar trees (Populus × canadensis “robusta”). Can J Bot 72:347– 351
Schupp H, Rennenberg H (1988) Diurnal changes in the glutathione concentration of spruce needles (Picea abies L.). Plant Sci 57:113–117
Seegmüller S, Rennenberg H (2002) Transport of organic sulfur and nitrogen in the roots of young mycorrhizal pedunculate oak trees (Quercus robur L.). Plant Soil 242:291–297
Seegmüller S, Schulte M, Herschbach C, Rennenberg H (1996) Interactive effects of mycorrhization and elevated CO2 on sulfur nutrition of young pedunculate oak (Quercus robur L.) trees. Plant Cell Environ 19:418–426
Smith FW, Ealing PM, Hawkesford MJ, Clarkson DT (1995) Plant members of a family of sulfate transporters reveal functional subtypes. Proc Natl Acad Sci USA 92:9373–9377
Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vandenberg PJ, Belcher AR, Warrilow AGS (1997) Regulation of expression of a cDNA from barley roots encoding a high affinity sulfate transporter. Plant J 12:875–884
Steinkamp R (1984) Der Abbau von Glutathion in Suspensionskulturen von Nicotiana tabacum var. “Samsun.”. Ph.D. Thesis, University Köln, Germany
Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In: Smith JAC, Griffiths H (eds) Water deficits: plant responses from cell to community. Bios Scientific, Oxford, UK, pp 5–36
Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H (1995) Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula x P. alba) overexpressing glutathione synthetase. Plant J 7:141–145
Sunarpi, Anderson JW (1996) Effect of sulfur nutrition on the redistribution of sulfur in vegetative soybean plants. Plant Physiol 112:623–631
Tabe L, Droux M (2000) Sulfur metabolism in developing seeds of transgenic narrow leaf lupin expressing a sulfur-rich protein. In: Brunold C, Rennenberg H, Kok LJ, Stulen I, Davidian J-C (eds) Sulfur nutrition and sulfur assimilation in higher plants. Molecular, biochemical and physiological aspects. Paul Haupt, Berne, Switzerland, pp 317–318
Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2000) The roles of three functional sulfate transporters involved in uptake and translocation of sulfate in Arabidopsis thaliana. Plant J 23:171–182
Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos P, Krähenbühl U, Op den Camp R, Brunold C (2002) Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5’-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. Plant J 31:729–740
Yildiz FH, Davies JP, Grossmann AR (1994) Characterization of sulfur transport in Chlamydomonas reinhardtii during sulfur-limited and sulfur-sufficient growth. Plant Physiol 104:981–987
Acknowledgements
The authors thank Prof. E. Steudle for introducing us to the pressure probe technique. R. Nitschke and M. Eiblmeier are gratefully acknowledged for expert technical assistance. Dr. J. Kreuzwieser is gratefully acknowledged for helpful discussions. We thank Dr. G. Leubner and Dr. S. Kopriva for critically reading the manuscript. This research was financially supported by the German National Science Foundation (DFG) under project number Re-515/6-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Zalm, E., Schneider, A. & Rennenberg, H. Regulation of sulfate uptake and xylem loading of poplar roots (Populus tremula x P. alba). Trees 19, 204–212 (2005). https://doi.org/10.1007/s00468-004-0383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-004-0383-2