Abstract
Pathogens can cause chronic premature needle abscission in coniferous species. To assess the potential impacts on tree productivity, stomatal regulation was investigated in Douglas fir with chronic stomatal occlusion and defoliation from varying levels of the Swiss needle cast (SNC) fungus, Phaeocryptopus gaeumannii. Levels of SNC disease and subsequent defoliation were manipulated by choosing six sites with varying levels of disease and by foliar applications of fungicides on six trees per site. Diurnal measurements of leaf water potential (Ψleaf), stomatal conductance (g s) and vapor pressure deficit (D) were made on six fungicide treated and six control trees per site. In addition, leaf specific hydraulic conductance was calculated on a single branch (K L_B) from three trees per treatment per site. Stomatal conductance at D=1 kPa (g sref) was negatively correlated with fungal colonization (number of fruiting bodies present in needle stomata) and positively correlated with K L_B. Despite reduced needle retention in diseased trees, K L declined due to a reduction in sapwood area and permeability (i.e., increasing presence of latewood in functional sapwood). In general, stomatal sensitivity to D for all foliage was consistent with stomatal regulation based on a simple hydraulic model [g s=K L(Ψsoil−Ψleaf)/ D], which assumes strict stomatal regulation of Ψleaf. However, when fungal presence reduced maximum g s below the potential maximum supported by hydraulic architecture, stomatal sensitivity was lower than expected based on the theoretical relationship: dg s/dlnD=0.6·g sref. The results indicate that losses in productivity associated with physical blockage of stomata and defoliation are compounded by additional losses in K L and a reduction in g s in remaining functional stomata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In healthy plants, regulation of leaf water loss is achieved by control of stomatal conductance (g s). Typically, when light and soil moisture are not limiting, g s declines exponentially with increasing vapor pressure deficit (D) (Monteith 1995; Bond and Kavanagh 1999; Oren et al. 1999). Although stomatal closure may be related to D (Monteith 1995; Oren et al. 1999), increasing evidence suggests that the regulation of stomata occurs by a feedback response linked to leaf specific hydraulic conductance (K L) (Sperry and Pockman 1993; Bond and Kavanagh 1999; Hubbard et al., 2001) and maintenance of leaf water potential (Ψleaf) at or above a threshold (Jones 1990; Saliendra et al. 1995; Hubbard et al. 2001). Thus, avoiding a Ψleaf capable of causing physiological stress or injury (e.g., Kavanagh et al. 1999; Tezara et al. 1999).
Assuming plants are an inelastic system, Ψleaf can be determined from the following relationship between transpirational water loss and supply (Jones and Sutherland 1991; Dewar 1995; Whitehead 1998; Bond and Kavanagh 1999):
where g s is stomatal conductance, D is the leaf-to-air vapor pressure gradient, K L is whole plant leaf-specific conductance, Ψsoil and Ψleaf are water potential of the soil and leaf, respectively.
Based on the relationship described in Eq. 1, plants with a higher g s at a low D are hypothesized to close stomata more rapidly as D increases (McNaughton and Jarvis 1991). A number of recent articles have explored this relationship either directly (Oren et al. 1999, 2001; Schäfer et al. 2000; Hubbard et al. 2001) or through modeling studies (Bond and Kavanagh 1999; Oren et al. 1999). In particular, Oren et al. (1999) showed that for a number of tree species a single consistent relationship exists between stomatal sensitivity to increasing D (dg s/dlnD) and g s at D=1 kPa (g sref) such that dg s/dlnD=0.6·g sref. The balance between water supply and water loss illustrated in Eq. 1 and the relationship explored by Oren et al. (1999) should be affected by pathogen-induced defoliation and changes in g s and K L. For example, a number of studies have shown that increasing g s is a short-term response to defoliation (Meinzer and Grantz 1990, Reich et al. 1993; Kramer and Boyer 1995; Pataki et al. 1998; Tausend et al. 2000) associated with declining leaf area to sapwood area ratios (LA:SA) and higher K L values (Meinzer and Grantz 1990; Pataki et al. 1998; Tausend et al. 2000). However, long-term chronic losses in leaf area and the potential feedback on the ability of the stem to maintain high K L and g s have not been well studied.
In this study, we investigated stomatal regulation in Douglas fir (Pseudotsuga menziesii var. menziesii) with varying levels of chronic defoliation from an intensifying foliar disease, Swiss needle cast (SNC). In 1996, aerial surveys in coastal Oregon detected 196,000 ha with moderate to severe disease levels (Hansen et al. 2000). The fungal pathogen of SNC, Phaeocryptopus gaeumannii (Rhode) Petrak, occludes stomata causing a reduction in maximum stomatal conductance (g smax) (Manter et al. 2000; Manter et al. 2003) and chronic premature needle abscission (Hansen et al. 2000). The disease cycle begins in the spring when newly emerging needles are infected by airborne spores. Following deposition, spores germinate and enter needles through stomata where they grow intercellularly, eventually producing fruiting bodies (pseudothecia) that emerge from needle stomata (Hansen and Lewis 1997; Capitano 1999). Reductions in g smax are positively correlated with occluded stomata (i.e., visual estimates of stomata harboring fungal structures), and no impact of P. gaeumannii on gas exchange is detected prior to their emergence (Manter et. al. 2000).
Our objective was to determine the effects of a chronic physical occlusion of stomata and premature needle abscission of older needle age classes on stomatal regulation in the remaining foliage. We hypothesize (1) that the declining g s associated with fungal presence, will decrease g sref and concurrent changes in dg s/dlnD will be consistent with dg s/dlnD=0.6·g sref and (2) this decline in g sref and premature needle abscission will result in a higher K L and g sref on remaining non-occluded foliage. Such a pattern of stomatal regulation will result in a reduced impact of P. gaeumannii infection on g s over the course of a typical day as D increases. Our future efforts to understand the full impact of SNC on needle CO2 assimilation will be strongly influenced by diel patterns of g s (Harley et al. 1992; Williams et al. 1996) and the ability of the stem to maintain K L. For example, the potential impact of P. gaeumannii on total daily CO2 assimilation rates (assuming a constant c i) will be comparatively lower than determined from maximum rates alone (i.e., Manter et al. 2000), if dg s/dlnD is reduced in P. gaeumannii-infected foliage.
Materials and methods
Study sites
Paired permanent plots were created on north- and south-aspect slopes (10–30%) at each of three 12- to 15-year-old Douglas-fir plantations with varying levels of SNC (ca. 2, 2.5, and 3.5 years of foliage retention). The high disease site is located on the Siuslaw National Forest, near Beaver, Ore. [Beaver north (BN) plot—N45°17.55′ W123°46.46′; Beaver south (BS) plot N45°17.43′ W123°46.35′], the medium disease site is located on the Siuslaw National Forest near Hebo, Ore. [Hebo north (HN) plot – N45°13.87′ W123°48.51′; Hebo south (HS) N45°13.85′ W123°48.19′], and the low disease site is located on the MacDonald-Dunn State Forest near Corvallis, Ore. [Mac north (MN) plot – N44°37.68′ W123°19.45′; Mac south (MS) plot N44°37.48′ W123°19.57′]. Both the Beaver and Hebo sites are located in an area where symptoms of SNC disease have been present since at least 1990 (Hansen et al. 2000). Each permanent plot consisted of a group of six partially defoliated infected trees and six trees with a significant reduction in infection and defoliation. Sample trees with minimal infection and therefore defoliation were created by spraying them with a fungicide to prevent new P. gaeumannii infections (Chlorothalonil, rate =66 ml / 3.78 l, applied until run-off). Fungicide applications were conducted in 1998, 1999, and 2000 at bud break (90% trees had broken bud) and 1 month following bud break, successfully prevented fungal infections in all new needle cohorts beginning in 1998.
Disease assessment
Prior to the start of the experiment (May 1999), a survey for the presence of P. gaeumannii and the amount of defoliation at each site was conducted. Two sun-exposed, south-facing branches from each study tree were collected, and each needle cohort were visually analyzed for pseudothecia density (PD, percent of stomata with visible fungal structures, predominately fruiting bodies) and needle retention (0, 25, 50 or 100%) as outlined in Manter et al. (2000).
Gas exchange measurements
On 4 sunny days in June and July 1999, diurnal measurements of g s and Ψleaf were measured on all trees at each site. Concurrent measurements at both plots within a site (i.e., north- and south-facing slopes) were conducted using two sets of equipment. Diurnal measurements on one sun-exposed branch per tree included pre-dawn Ψleaf, bi-hourly g s on all needle cohorts present (Li-1600, LiCor, Lincoln, Neb.), and Ψleaf (Scholander-Hammel pressure chamber, PMS Instruments, Corvallis, Ore.) measurements commencing at dawn or immediately after foliage dried off. At the end of the day, all measured foliage was removed and measured for one-sided projected leaf area (Agimage, Decagon Devices, Pullman, Wash.). In order to avoid any equipment biases, each set of equipment (Li-1600 and pressure chamber) was crosschecked each morning and randomly assigned to a site at each time point. Photosynthetically active radiation (PAR), relative humidity and air temperature measurements were collected from radiation-shielded sensors (Spectrum Technologies, Plainfield, Ill.) located in an opening within each stand (radius >5 m) at a height of 1.5 m.
Analysis of the relationship between g s and D; g s = b−m·lnD where b is g sref and m is dg s/dlnD, was determined from the porometry data for plot-treatment-needle age combination by regression analysis (Systat 8.0, Evanston, Ill., USA) of the upper boundary line when 1< D <3 kPa (e.g., Schäfer et al. 2000). Similar to Oren et al. (1999), we set the reference g s (g sref) at D=1 kPa which was common to all sample sites.
Hydraulic conductance
In September 2000, one 4-year-old branch from each of the three non-infected and three infected trees at each plot was harvested, sealed in a plastic bag, and stored at 0°C. Within 48 h stem water flux was measured on a ca. 4 cm segment from the base of the main stem on each of the current-, 1-, and 2-year-old nodes as outlined in Kavanagh et al. (1999). Four consecutive 60 s flux measurements were made on each stem segment by connecting one end of the stem segment to tubing containing a solution of degassed dH2O pressured to 12.5 kPa. Total leaf area (LA) from each age class on a sample branch was determined by multiplying the leaf area to dry weight ratio determined from a sub-sample of ca. 50 needles by the total needle dry weight present on the branch. From this data a branch-leaf specific conductance (K L_B, mmol m−2 s−1 MPA−1) was calculated as K L_B=(F / p) / LAs, where F is the average flux (mmol s−1), p is the pressure gradient (0.0125 MPa), LAs is the total "supplied" leaf area downstream of the stem segment, and SA is the segment cross-sectional area (calculated from the average diameter at both ends). For all branches, the percent of latewood present in each annual increment was also visually estimated (40× magnification) for each study branch. All reported values of percent latewood are the average of all annual increments present in each sample branch.
Results and discussion
Disease and defoliation level
The first needle cohort treated with fungicides had a significant reduction in fungal infection as indicated by the sharp reduction in pseudothecia emerging from needles compared to non-sprayed needles (data not shown). For example, the site with the highest level of disease had a 100% reduction in pseudothecia emerging from fungicide-treated 1-year-old needles. Since needle retention decreased linearly as P. gaeumannii colonization increased (Fig. 1), subsequent applications of fungicides result in increasing levels of foliar retention (Fig. 2) especially on sites with high to moderate levels of disease. These results indicate that the fungicide is effective in controlling infection and the elimination of the fungal infection results in foliar retention of all treated cohorts.
Supplied leaf area on a 4-year-old branch at three Douglas-fir plantations (September 2000). The first letter of the site code is the site (B Beaver, H Hebo, M Mac), the second letter is the aspect (N north, S south) and the last two are the treatment (I infected, NI non-infected). Bars are the mean (n=3) and error bars are one standard error. For each site, means with different letters are significantly different (P<0.05) using a t-test
Stomatal regulation
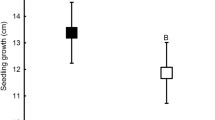
The hydraulic model of stomatal regulation (Bond and Kavanagh 1999; Oren et al. 1999) depends upon the underlying assumption that plants are isohydric, i.e., stomatal control of g s maintains Ψleaf at or above a threshold. Previous studies have shown that healthy Douglas-fir trees maintain Ψleaf ≥−2.1 MPa (Bond and Kavanagh 1999), above the Ψleaf required for the induction of xylem cavitation (Kavanagh et al. 1999). Over the course of our study, the regulated (i.e., mid-day) Ψleaf, for all site-slope-treatment combinations, also typically hovered around −2.1 MPa (Fig. 3), and never fell below −2.3 MPa. Threshold Ψleaf was not influenced by changes in Ψsoil (assumed to be pre-dawn Ψleaf).
Emergence of P. gaeumannii fruiting bodies caused a reduction in g s. Data from all six plots shows a significant negative linear relationship (P<0.01) between g sref (g s at D=1 kPa) and pseudothecia presence (Fig. 4). This weaker relationship between g sref and pseudothecia presence (r 2=0.354) as compared to the relationship between g smax and pseudothecia presence described in Manter et al. (2000) (r 2=0.927) may be due to the control of between tree and climatic variation in the latter greenhouse study and the influence of K L on observed g sref values as discussed below. In addition, the greenhouse study was conducted on newly inoculated potted seedlings that had only 1 year of infected foliage. Whereas, our field sites had variable levels of defoliation and had several needle age classes with stomatal occlusion.
The response of g sref to increasing presence of Phaeocryptopus gaeumannii in needle stomata with high soil moisture (pre-dawn Ψleaf =−0.3 MPa) and fully illuminated foliage. Each observation is the mean (n=6) and error bars are one standard error. Current-, one- and two-year refer to the needle age
Based on results from short-term defoliation studies (e.g., Pataki et al. 1998) the loss of LA in diseased trees should increase K L and g sref in residual foliage. In order to avoid the confounding effects of pseudothecia presence on g sref, we tested for an effect of P. gaeumannii-associated defoliation on g sref by comparing g sref values from current-year foliage in trees with high and low needle retention at the Beaver and Hebo sites only (see Fig. 2). Interestingly, g sref values from trees with high needle retention were greater than the low needle retention trees (paired t-test, mean difference =19.3, t=5.36, P=0.013, n=4) suggesting that K L was actually lower in trees that experienced chronic defoliation and fungal-mediated reductions in g s.
Leaf specific hydraulic conductance
To determine one possible source of the loss in K L we measured the branch component (K L_B) of K L and two of the underlying factors influencing K L_B: LA:SA and sapwood specific conductance (K s) as represented by % latewood. As suggested from the observed patterns of g sref, K L_B was significantly lower in the low needle retention trees compared to uninfected or high needle retention trees at the Beaver and Hebo sites (see Fig. 5). The observed reductions in K L_B could not be attributed to changes in LA, since LA:SA ratios were conserved for all sample branches (Fig. 6) by a simultaneous decline in SA (data not shown). However, the percent of latewood present was negatively correlated with declining values of K L_B (Fig. 7) indicating that reductions in K L are a function of decreasing Ks as latewood percentage increases. A decline in K s with increasing latewood presence occurs due to the comparatively smaller diameter of latewood cells relative to earlywood cells (Zimmerman 1983; Megraw 1986).
Branch leaf specific conductance (K L_B) on 4-year-old branches at three Douglas-fir plantations (September 2000). The first letter of the site code is the site (B Beaver, H Hebo, M Mac), the second letter is the slope-aspect (N, north; S, south) and the last two are the treatment (I infected, NI non-infected). Bars are the mean (n=3) and error bars are one standard error. For each site, means with different letters are significantly different (P<0.05) using a t-test
Branch leaf specific conductance (K L_B) versus the percent of latewood present in sapwood cores taken at breast height from field trees with varying levels of Phaeocryptopus gaeumannii infection and defoliation. Each observation is based on one tree, conductance values for a given branch node (e.g., 1998) are plotted against the percent latewood for the corresponding annual increment (e.g., 1998)
The findings from other studies on the hydraulic impacts of defoliation differ from our results. For example, when K L was measured following a single defoliation episode K L increased due to a decline in LA:SA ratios, thereby allowing gs to increase on the remaining foliage (Meinzer and Grantz 1990; Reich et al. 1993; Kramer and Boyer 1995; Pataki et al. 1998; Tausend et al. 2000). However, our results indicate that the maintenance of a constant LA:SA ratio and reduced K s, in chronically defoliated trees, not only prevented the expected increase in g sref, but actually caused a decrease in g sref relative to refoliated treatments. We propose that the increase in K L is a short-term response to defoliation, and that following repeated and/or severe defoliation any increases in K L will fade as the quantity and quality (i.e., lower sapwood permeability) of new functional sapwood declines. This decline is not unexpected. Since a non-linear relationship between g s and CO2 assimilation rate (e.g., Jones 1992) exists; increases in CO2 assimilation rates will be less than increases in g s. Therefore, the loss of photosynthetically active leaf area is not fully compensated, reducing the total amount of photosynthate available for the production of new functional sapwood. We suggest that a more detailed time-series experiment of hydraulic conductance responses to defoliation are needed in order to determine if increases in K L following defoliation are transient as suggested from our current study of P. gaeumannii-infected Douglas fir.
Patterns and processes influencing stomatal sensitivity
Based on the empirical and theoretical analysis presented by Oren et al. (1999) dg s/dlnD is typically equal to 0.6·g sref. As shown in Fig. 8, this relationship was not necessarily observed for our field porometry data and differed depending upon the presence of P. gaeumannii. For example, when P. gaeumannii was absent: dg s/dlnD=−20.8+0.798·g sref (r 2=0.884); and when P. gaeumannii was present: dg s/dlnD=−41.62+0.877·g sref (r 2=0.797).
Relationship between g sref (stomatal conductance at D=1 kPa) and stomatal sensitivity to increasing vapor pressure deficit (dg s/dlnD) from field trees with varying levels of P. gaeumannii infection. Each point was determined from a regression analysis of the upper boundary line determined from the 1999 porometery data. Pseudo is the percent of stomata with visible fungal structures
One of the underlying assumptions of the theoretical analysis presented by Oren et al. (1999) is that maximum stomatal conductance (g smax)>g sref. One consequence of this assumption is that g smax (i.e., due to leaf anatomical features or stomatal density and maximum aperture width) must be greater than or equal to g smax_hyd [i.e., due to hydraulic features or K L·(Ψsoil–Ψleaf)/D]. However, it is plausible that the presence of P. gaeumannii in needle stomata imparts an additional constraint on g smax such that g smax <g smax_hyd. To explore the impact of this assumption, we conducted a theoretical analysis of Eq. 1, where estimates of g s were calculated over a range of D (1, 1.5, 2, 2.5 and 3 kPa), K L values (0.07, 0.09, 0.11, and 0.13 mmol m−2 s−1 MPa−1), and g smax values [g smax=100, 75 and 50% of g smax_hyd, where g smax_hyd=K L·(Ψsoil−Ψleaf)/D and D=1 kPa]. As in Oren et al. (1999) we assumed a strict stomatal regulation of Ψleaf, except that Ψsoil–Ψleaf was set to 1.9 MPa (i.e., Ψsoil=−0.3 and Ψleaf=−2.1 MPa, based on Fig. 3).
Figure 9 shows the theoretical g s versus lnD curves when g smax is 100, 75 and 50% of g smax_hyd and K L=0.13 mmol m–2 s–1 MPa−1. Notice that the fit of the negative linear relationship decreases as g smax deviates from g smax_hyd [r 2 (P-value)=0.977 (0.002), 0.969 (0.002), and 0.668 (0.091), respectively]. Plotting g sref versus dg s/dlnD for the simulated data (Fig. 10) shows that stomatal sensitivity (dg s/dlnD) decreases as g smax declines below g smax_hyd. Based on this theoretical data: (1) if g smax <g smax_hyd, then dg s/dlnD=−847.4·K L+1.095·g sref (r 2=1.0), and (2) if g smax≥g smax_hyd, then dg s/dlnD=0.633·g sref (r 2=1.0), similar to the result's of Oren et al. (1999). Thus, dg s/dlnD may be influenced by both g sref and reductions in K L, and when g smax<g smax_hyd then dg s/dlnD may be less than 0.633·g sref.
Theoretical response of g s to increasing D based on Eq.1 with an increasing limitation imposed on g smax. Estimates of g s were calculated using D =1, 1.5, 2, 2.5 and 3 kPa, K L=0.13 mmol m–2 s–1 MPa−1, and g smax =100, 75 and 50% of g smax_hyd. Hidden points are identical for all three levels of g smax
Relationship between g sref (stomatal conductance at D =1 kPa) and stomatal sensitivity to increasing vapor pressure deficit (dg s/dlnD) based on Eq. 1. Each point was determined from a regression analysis of the response of gs to increasing D. Within each level of leaf-specific conductance (K L), estimates were determined when g smax is 100, 75 and 50% of g smax_hyd
In the discussion above, it was observed that the presence of P. gaeumannii is associated with a reduction in g sref; therefore it is plausible that their relationship between dg s/dlnD and g sref, which fell below the theoretical 0.6 line (see Fig. 8), could have arisen from g smax being less than g smax_hyd. We tested the fit of the above theoretical regression analysis to our field data, and found a significant regression: dg s/dlnD=−847.4·(0.001·K L_B)+1.095·g sref (r 2=0.683), where 0.001 is an estimated scaling factor relating K L_B and K L. As was the case for g sref, stomatal sensitivity to D is dependent upon K L and can be modeled according to Eq. 1. However, the relationship between g sref and dg s/dlnD departs from the theoretical 0.6 line (Oren et al. 1999) when P. gaeumannii is present in needle stomata. This observation is consistent with our theoretical analysis of Eq. 1, which assumes that some unknown constraint limits g s such that g smax is less than g smax_hyd.
Conclusions
Stomatal response to chronic blockage of carbon uptake and defoliation did not result in a reduced impact of P. gaeumannii infection on g s (i.e., g s infected / g s uninfected) over the course of a typical day as D increases. In this paper we demonstrate that stomatal regulation in Douglas fir can be explained by a simple hydraulic model, which assumes strict regulation of Ψleaf. In healthy needles, g s is directly related to K L, and stomatal regulation conforms to the theoretical relationship outlined by Oren et al. (1999). However, when P. gaeumannii is present in needle stomata, the direct relationship between g sref and K L is altered such that g smax (i.e., leaf limited) may become less than g smax_hyd (i.e., hydraulic limited). Indicating that shifts in hydraulic architecture following chronic blockage in carbon uptake and defoliation eliminates any short-term compensation that may have resulted in increasing g s in remaining foliage.
A similar approach, investigating the response of g s to D, may be useful for determining the underlying factors influencing g s in other systems. For example, the causal mechanisms responsible for a decline in g s as trees age, and grow in size, have been variously attributed to maturational changes (e.g., Day et al. 2001) and/or hydraulic limitations (e.g., Ryan and Yoder 1997), among other factors. Based on the theoretical and empirical observations presented here, if g smax is limited by leaf processes then dg s/dlnD may be <0.6·g sref; whereas, if g smax is limited by hydraulic architecture then dg s/dlnD should equal 0.6·g sref.
References
Bond BJ, Kavanagh KL (1999) Stomatal behavior of four woody species in relation to leaf-specific hydraulic conductance and threshold water potential. Tree Physiol 19:503–510
Capitano B (1999) The infection and colonization of Douglas-fir by P. gaeumannii. MS thesis, Oregon State University, Corvallis
Day ME, Greenwood MS, White AS (2001) Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiol 21:1195–1204
Dewar R.C (1995) Interpretation of an empirical model for stomatal conductance in terms of guard cell function. Plant Cell Environ 18:365–372
Hansen EM, Lewis KJ (1997) Compendium of conifer diseases. APS, St. Paul, Minn.
Hansen EM, Stone JK, Capitano BR, Rosso P, Sutton W, Winton L, Kanaskie A, McWilliams MG (2000) Incidence and impacts of Swiss needle cast in forest plantations of Douglas-fir in coastal Oregon. Plant Dis 84:773–778
Harley PC, Thomas RB, Reynolds JF Strain BR (1992) Modeling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282
Hubbard RM, Ryan MG, Stiller V, Sperry JS (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24:113–121
Jones HG (1990) Physiological aspects of the control of water status in horticultural crops. Hortic Sci 25:19–26
Jones HG (1992) Plants and microclimate, 2nd edn. Cambridge University Press, Cambridge
Jones HG, Sutherland RA (1991) Stomatal control of xylem embolism. Plant Cell Environ 14:607–612
Kavanagh KL, Bond BJ, Aitken SN, Gartner BL, Knowe S (1999) Shoot and root vulnerability to xylem cavitation in four populations of Douglas-fir seedlings. Tree Physiol 19:31–37
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Manter DK, Bond BJ, Kavanagh KL, Rosso PH, Filip GM (2000) Timing and mechanism of impact of the Swiss needle cast fungus, Phaeocryptopus gaeumannii, on Douglas-fir needle gas exchange and rubisco activation. New Phytol 148:481–491
Manter DK, Bond BJ, Kavanagh KL, Stone JK, Filip GM (2003) Modelling the impacts of the foliar pathogen, Phaeocryptopus gaeumannii, on Douglas fir physiology: net canopy carbon assimilation, needle abscission and growth. Ecol Model (in press)
McNaughton KG, Jarvis PG (1991) Effects of spatial scale on stomatal control of transpiration. Agric For Meteorol 54:279–301
Megraw RA (1986) Douglas-fir wood properties. In: Oliver CD, Hanley DP, Johnson JA (eds) Douglas-fir: stand management for the future. University of Washington, Institute of Forest Resources, Contribution No. 55, Seattle, pp 81–96
Meinzer FC, Grantz DA (1990) Stomatal and hydraulic conductance in growing sugarcane: stomatal adjustment to water transport capacity. Plant Cell Environ 13:383–388
Monteith JL (1995) A reinterpretation of stomatal response to humidity. Plant Cell Environ 18:357–364
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N (1999) Intra- and interspecific responses of canopy stomatal conductance to vapour pressure deficit. Plant Cell Environ 22:1515–1526
Oren R, Sperry J, Ewers B, Pataki D, Phillips N, Megonigal J (2001) Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in a flooded Taxodium distichum L. forest: hydraulic and non-hydraulic effects. Oecologia 126:21–29
Pataki DE, Oren R, Phillips N (1998) Responses of sap flux and stomatal conductance of Pinus taeda L. trees to stepwise reductions in leaf area. J Exp Bot 49:871–878
Reich PB, Walters MB, Krause SC, Vanderlien DW, Raffa KF Tabone T (1993) Influence of pre-dawn water potential and soil-to-leaf hydraulic conductance on maximum daily leaf diffusive conductance in two oak species. Funct Ecol 3:719–726
Ryan M, Yoder B (1997) Hydraulic limits to tree height and tree growth. BioScience 47:235–242
Saliendra, NZ, Sperry JS, Comstock MP (1995) Influences of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196:356–366
Schäfer KVR, Oren R, Tenhunen JD (2000) The effect of tree height on crown level stomatal conductance. Plant Cell Environ 23:365–375
Sperry JS, Pockman WT (1993) Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant Cell Environ 16:279–287
Tausend PC, Meinzer FC, Goldstein G (2000) Control of transpiration in three coffee cultivars: the role of hydraulic and crown architecture. Trees 14:181–190
Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Whitehead D (1998) Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiol 18:633–644
Williams M, Rasletter EB, Fernandes DN, Goulden ML, Wofsy SC, Shaver GR, Melillo JM, Munger JW, Fan S-M, Nadelhoffer KJ (1996) Modeling the soil-plant-atmosphere continuum in a Quercus–Acer stand at Harvard Forest: the regulation of stomatal conductance by light, nitrogen and soil/plant hydraulic properties. Plant Cell Environ 19:911–927
Zimmerman MH (1983) Xylem structure and the ascent of sap. Springer, Berlin Heidelberg New York
Acknowledgements
We are grateful to Dr. Barbara Bond for the loan of several LiCor gas exchange analyzers. This research was funded through the Swiss Needle Cast Cooperative at Oregon State University—a consortium of industrial, tribal, federal, and state landowners in Oregon and Washington.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manter, D.K., Kavanagh, K.L. Stomatal regulation in Douglas fir following a fungal-mediated chronic reduction in leaf area. Trees 17, 485–491 (2003). https://doi.org/10.1007/s00468-003-0262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-003-0262-2