Abstract
To assess the effects of stand density and canopy environment on tree physiology, we measured gas exchange responses of the same needle age class of 16-year-old loblolly pines (Pinus taeda L.) in thinned (512 trees ha−1) and non-thinned treatment plots (2,863 trees ha−1) in central Louisiana. Physiological data were collected in the upper and lower canopy positions on 26 sunny days between July 1996 and June 1997 (one-half of the leaf life span). Mean net photosynthesis was highest (4.3 µmol m−2 s−1) in the spring and closely corresponded with light intensity in the canopy. Photosynthesis in the winter was nearly 3.0 µmol m−2 s−1, indicating that loblolly pine enables substantial carbon fixation all year around in the Gulf Coastal Plain region. Mean transpiration and stomatal conductance were highest in the summer and lowest in the winter. With increased light availability after thinning, needle photosynthesis, transpiration and stomatal conductance rose 84, 40 and 23%, respectively, in the lower canopy of the thinned-treatment trees. Light-saturated photosynthetic capacity of the lower canopy needles was 5.2 µmol m−2 s−1 for the thinned treatment and 4.2 µmol m−2 s−1 for the non-thinned treatment. It is concluded that thinning-induced light penetration through the canopy enhances physiological activities in the lower canopy foliage of residual trees, and that light availability is the only significant variable for predicting needle-level photosynthesis rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Loblolly pine (Pinus taeda L.) is extensively planted for timber and fiber production in the southern United States due to its rapid growth and responsiveness to forest management practices (Schultz 1997). Forest researchers have revealed that tree growth (stem diameter and volume and fine roots) of the species responds substantially to thinning manipulations (Dean and Baldwin 1996; Sword et al. 1998; Yu et al. 1999). Positive growth responses are chiefly attributed to increasing live crown ratio, foliage growth and site resource availability (Brix 1983; Snowdon and Waring 1995; Peterson et al. 1997). However, the physiological mechanisms that govern post-thinning growth responses are poorly understood.
Leaf gas exchange sensitivity to climate change has been investigated for commercially important conifers such as radiata pine (P. radiata D.) (Whitehead et al. 1996), Scots pine (P. sylvestris L.) (Irvine et al. 1998) and slash pine (P. elliottii Engelm.) (Teskey et al. 1994). Several studies have also reported that loblolly pine responded physiologically to elevated CO2 and air temperature (Teskey 1997) and components of environmental change (Ellsworth 2000). These studies have been conducted mainly on Carolina piedmont and eastern Gulf Coastal Plain sites. However, seasonal physiological responses of loblolly pine in relation to environmental variation and stand density are not fully investigated in the western Gulf Coastal Region where the climate is characterized by abundant rainfall, hot summers and mild winters. Experiments are needed to better understand how forest management practices affect the physiological processes of loblolly pine trees.
Recently, research efforts have been made to model stand growth relationships with carbon dynamics and resource availability (Wang and Jarvis 1990; Baldwin et al. 2000). Application of forest models suggests that the accurate prediction of carbon gain and stand productivity requires an understanding of variability in canopy physiology in response to natural environment and forest management practices (Johnsen et al. 2000). The purpose of this study was (1) to determine seasonal physiological responses of the same needle age class of 16-year-old loblolly pine trees after repeated thinning treatments, and (2) to evaluate relationships between net CO2 exchange and canopy environment at two stand densities. Our hypothesis was that canopy position and thinning manipulation could affect seasonal photosynthetic responses and water relations of loblolly pine trees. Response equations were developed to estimate net photosynthesis rates of loblolly pine needles.
Materials and methods
This experiment was carried out in a loblolly pine plantation (31°11′N, 92°41′W) located about 20 km southwest of Alexandria, Louisiana. The soil was a Beauregard silt loam (fine-silty, siliceous, thermic, Plinthaquic Paleudults), moderately drained and poor in available nutrients (Shoulders and Tiarks 1983). Mean annual temperature was 19°C and annual precipitation was approximately 1,500 mm. In May 1981, the plantation was established at a spacing of 1.8×1.8 m with 14-week-old containerized seedlings. In November 1988, eight research plots (13 rows x13 trees each) were installed for this experiment. Two levels of thinning were randomly applied to those plots in a completely random design with four replications. In the thinned treatments, 75% of trees were removed by mechanically cutting every other row of trees and every other tree in remaining rows, leaving 721 trees ha−1. The non-thinned treatments remained at the original density of 2,990 trees ha−1. In early 1995, the initially thinned treatments were rethinned to 512 trees ha−1. The non-thinned treatments remained unthinned and had 2,860 trees ha−1. Herbicides were periodically used to control understorey vegetation. Forty-eight access towers were permanently erected to facilitate ecophysiological data collection in the canopy. Wooden walkways were constructed in the upper canopy (approximately 13 and 14 m high from the ground in the thinned and non-thinned treatments, respectively) and the lower canopy (nearly 9 and 12 m high in the thinned and non-thinned treatments).
During July 1996, needle gas exchange in the upper and lower canopy positions was measured in the expanding fascicles of first flush shoots that were initiated in April 1996. Thereafter, the physiological measurements continued approximately every 2 weeks through December 1996. Similar measurements were made in the same needle age class in February 1997 and continued in April through June 1997. A Li-6200 photosynthesis system (Li-Cor, Lincoln, Neb., USA) associated with a 250-ml leaf chamber (pine needle type) was used for data collection. In order to examine diurnal variability of gas exchange, we conducted the physiological measurements between 0930 and 1130 hours and between 1300 and 1500 hours. The measured variables were net photosynthesis (P n), transpiration (T r) and stomatal conductance to water vapor (g s). Meanwhile, photosynthetic photon flux density (PPFD), air temperature (T a) and vapor pressure deficit from leaf to air (VPD) in the canopy were also monitored. An instrument service in January and overcast weather conditions in March prevented sampling during these months.
Two of the four treatment-plot replications (two thinned and two non-thinned plots) were sampled daily. The sampling scheme was that on the first day of each period, upper canopy needles were measured followed by lower canopy needles. On the second day, the sampling order was reversed for the two canopy positions. At the beginning of each sampling day, three south-side branches per canopy position were randomly selected from three interior trees in each treatment. On each branch, two needle fascicles in the middle section of the terminal first-flush shoot were enclosed in the leaf chamber and the physiological variables of the sample were measured in their natural orientation in the canopy. Forty-eight measurements per day were made. The physiological variables were expressed on a needle surface area basis.
Needle water status was determined with a pressure chamber (PMS Instruments, Corvallis, Ore., USA). Predawn xylem pressure potential (Ψpd) was measured in conjunction with daytime physiological measurements. Three fascicles per canopy position per plot, each from three branches of the interior trees, were detached before dawn (0430 and 0530 hours) and placed in a plastic bag for Ψpd determinations. Daytime xylem pressure potentials (Ψd) of the excised fascicles were recorded 5 min following each gas exchange measurement. Three time domain reflectometer sensors (Soil Moisture Equipment, Santa Barbara, Calif., USA) were installed horizontally at the 15-cm soil depth in each plot and soil volumetric moisture content (SMC) was measured twice each month.
The data set consisted of 1,248 observations and grouped into four seasons: summer (July–August 1996 and June 1997), autumn (September–November 1996), winter (December 1996–February 1997) and spring (April–May 1997). The effects of thinning (thinned and non-thinned), season (summer, autumn, winter and spring), canopy position (upper and lower) and diurnal period (morning and afternoon) on gas exchange physiology were determined statistically at P≤0.05, using an analysis of variance (SAS 2000). General additive models were constructed to evaluate interrelationships between P n and environmental variables. The regression models were tested for statistical differences between the thinning treatments and canopy positions at P≤0.05.
Results
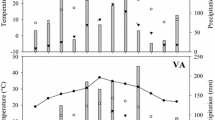
P n (per unit needle surface area) averaged 3.5 µmol m−2 s−1 over the study period. Needle physiology significantly varied with season (Table 1). Mean P n was lowest in the winter and highest in the spring (Fig. 1A). Winter P n rates were 70% of the annual peak during the spring. Differences in T r and g s were significant among the seasons, with the highest rates in the summer and the lowest rates in the winter (Fig. 1B, C). Winter PPFD in the canopy was lowest of the four seasons and represented 60% of the spring value (Fig. 1D). Mean T a and VPD in the canopy were highest during the summer and lowest during the winter (Fig. 1E, F). Mean SMC and needle Ψpd and Ψd varied seasonally (Fig. 2A–C), but no significant correlation was found among SMC, Ψpd, Ψd, P n and T r.
Physiological attributes were statistically different vertically in the canopy (Table 1). The upper canopy needles had higher mean P n, T r, and g s rates and more negative Ψd than the lower canopy needles. Diurnal measurements showed that the trees maintained significantly higher needle T r and lower Ψd in the afternoon compared to the values in the morning (Table 2). With a significant increase in PPFD, afternoon T a in the canopy was 5°C higher and VPD doubled over the morning values. However, there was no significant difference in P n and g s rates between the morning and afternoon measurements.
Thinning produced a significant effect on needle physiology (Table 1). Overall, mean P n was 3.9 µmol m−2 s−1 for the trees in the thinned treatment, a 26% increase over the P n rate (3.1 µmol m−2 s−1) for the trees in the non-thinned treatment. Mean T r was significantly higher in the thinned treatment than in the non-thinned treatment, but needle Ψpd and Ψd did not differ statistically between the two treatments. A significant thinning-by-canopy position interaction was found for the physiological variables. Needle P n, T r, g s and Ψpd in the upper canopy position was the same for the thinned-treatment and non-thinned-treatment trees (Table 2). However, lower canopy needle P n, T r, and g s in the thinned treatment were 85, 50 and 23%, respectively, greater than the rates in the same canopy position of the non-thinned treatment. Needle Ψd of the lower canopy foliage was more negative in the thinned-treatment trees relative to the non-thinned-treatment trees. The observed physiological responses coincided with a significantly higher PPFD in the lower canopy of the thinned treatment.
The P n response pattern was similar for all thinning-by-canopy position combinations (thinned-upper, non-thinned-upper, thinned-lower and non-thinned-lower canopy). Non-thinned-lower canopy needles had little P n increase above PPFDs of 600 µmol m−2 s−1. For other combinations, no additional P n increase was observed as PPFD exceeded 1,000 µmol m−2 s−1. In order to predict P n responses, we chose the uppermost 5% of the PPFD-P n data points for multivariate regression analyses. The results indicated that the regressions differed significantly for the upper and lower canopy positions (P=0.006). PPFD at both upper and lower canopy positions was positively correlated with P n rates and explained 71–79% of the total variation in P n for the thinning-by-canopy position combinations (Table 3). The light-saturated P n was significantly higher for the upper canopy position than for the lower canopy. Needles of the thinned-lower canopy had a significantly higher light-saturated P n compared to the needles in the non-thinned-lower canopy. However, apparent quantum efficiency of photosynthesis (Φ, initial slope of P n response curve) was not statistically different among the thinning-by-canopy position combinations. T a and VPD in the canopy were not significant covariates for P n.
Discussion
Data from this study indicate that in July, the P n rate (3.9 µmol m−2 s−1) of young needles (3 months old) in the upper canopy had already attained 65% of light-saturated P n (6.0 µmol m−2 s−1). Sword et al. (1996) found that on Gulf Coastal Plain sites, new fascicle needles of loblolly pine begin elongating in early April and achieve 75% of annual maximum needle elongation by the end of June. The photosynthetic capacity of developing foliage and rapid leaf area expansion early in the growing season may allow loblolly pine to take advantage of spring and early-summer field conditions that appear to be optimal for carbon uptake and tree growth.
Hot, humid summers and mild winters are common in the Gulf Coastal Plain region. When light is not limiting, water stress associated with periodic droughts can reduce carbon gain substantially by limiting foliar growth and photosynthesis rates (Teskey et al. 1987). Ellsworth (2000), examining the crown physiology of 12-year-old loblolly pine trees for three years, reported that summer drought caused the largest year-to-year variation in gas exchange rates during the growing season and that drought-induced water deficit decreased daily carbon assimilation by 45% on sunny days. In our study, Ψpd and SMC data suggest that no significant drought stress occurred over the study period. Thus, as young needles continued expanding in summer and became mature in autumn, their P n rates kept increasing gradually (Fig. 1A). After maturity, the same needle age class had the lowest P n in winter. Teskey et al. (1986) observed high P n rates in loblolly pine needles at 20°C. It is unlikely that winter T a (19.3±1.5°C) in our study area caused the 15% decline in P n between autumn and winter. However, winter PPFD (690 µmol m−2 s−1) was 24% below the autumn level and 40% below the optimal level (1,000 µmol m−2 s−1), leading to the significant decrease in winter P n. The highest P n was observed in the spring, when ambient conditions were most favorable. The photosynthate exported from 1-year-old foliage during that period may have been one of the major carbon sources for flush shoot elongation and needle growth (Dickson 1989; Sword et al. 1996). During the early growing season, therefore, tree carbon balance could be significantly affected by non-optimal environmental conditions.
This experiment presents strong evidence that loblolly pine growing on western Gulf Coastal Plain sites can sustain considerable carbon fixation throughout mild winters. The winter P n from this study is comparable with the rates of the same needle age class reported by Murthy et al. (1996) and Ellsworth (2000) for trees grown on Carolina piedmont sites. However, our winter P n rate is almost twice as high as the winter P n found by Teskey et al. (1994) for 23-year-old slash pine in Florida and much higher than the rates documented by Schaberg et al. (1995) for red spruce in Vermont. In a related study at our site, Kuehler et al. (1999) reported that the root starch concentration of trees increased from November through March. High carbon fixation of foliage in late autumn through winter may be responsible for the continued winter accumulation in root starch (Ford and Deans 1977; Kuhns and Gjerstad 1991). Additionally, Maier et al. (1998) found that branch and stem maintenance respiration of 9-year-old loblolly pine ranged from 0.4 to 1.0 µmol m−2 s−1 in winter (November-February). Compared to their rates, our winter P n should be sufficient to meet these respiration costs and maintain carbon gain for starch accumulation during mild winters.
Our study provides insight to understanding spatial variability in canopy environment and gas exchange physiology of southern pines in response to silvicultural management practices. The photosynthetic response models illustrate that PPFD below the light saturation is the most significant factor that controls carbon uptake. The light saturation level and maximum photosynthetic capacity were substantially low in the lower-canopy needles of non-thinned-treatment trees, a characteristic of shade foliage adapted to a light-limiting environment (Cregg et al. 1993; Zhang et al. 1997). In contrast, PPFD in the lower canopy of the thinned treatment increased drastically after thinning. As a result, the P n rate of the lower canopy needles increased 85% in the thinned treatment (Table 2). The light-saturated P n of the lower canopy foliage also increased significantly in the thinned-treatment trees, which could make a substantial contribution to whole-tree carbon fixation. Thus, we conclude that as light availability in the canopy of thinned treatments rises, the photosynthetic activity of lower canopy foliage is greatly enhanced.
Needle T r and Ψd were significantly different with season, chiefly due to incident PPFD in the canopy. The increase in solar radiation during the spring and summer led to higher T a, VPD and T r, and more negative Ψd relative to other seasons (Figs. 1, 2). We also observed that the upper and lower canopy foliage significantly increased needle T r and decreased Ψd in the afternoon in response to increased solar radiation and T a and reduced relative humidity. However, g s and P n were not different between the morning and afternoon measurements, suggesting that stomatal closure of the upper or lower canopy needles did not occur during the measurement period. A significant interaction of thinning by canopy position on needle physiology demonstrates that non-thinned-treatment trees had greater T r and g s in the upper canopy needles than in the lower canopy needles, whereas, in the thinned treatments, T r and g s were similar for needles of both canopy positions (Table 2). After thinning, more solar radiation was intercepted by the lower canopy foliage in the thinned treatments and resulted in a significant increase in T r and g s rates of the lower canopy needles. Our results of needle T r and g s are consistent with the findings reported by Cregg et al. (1990) and Gravatt et al. (1997), who found a positive relationship between light penetration, crown exposure and tree water relations in response to thinning practice.
We used a generalized additive model to predict P n responses to stand density and canopy environmental variability. Within-canopy PPFD was the only significant variable for the P n rate of 1-year-old needles (Table 3). Our response equations strongly suggest that process models must be developed separately for managed and non-managed pine stands. However, we found no significant correlation between net photosynthesis and air temperature, vapor pressure deficit, and soil water content. This result, however, should be further verified including environmental extremes over a more extensive geographic range of loblolly pine.
References
Baldwin VC, Burkhart HE, Westfall JA, Peterson KD (2000) Linking growth and yield and process models to estimate impact of environmental changes on growth of loblolly pine. For Sci 47:77–82
Brix H (1983) Effects of thinning and nitrogen fertilization on growth of Douglas-fir: relative contribution of foliage quantity and efficiency. Can J For Res 13:167–175
Cregg BM, Hennessey TC, Dougherty PM (1990) Water relations of loblolly pine trees in southeastern Oklahoma following precommercial thinning. Can J For Res 20:1508–1513
Cregg BM, Teskey RO, Dougherty PM (1993) Effect of shade stress on growth, morphology, and carbon dynamics of loblolly pine branches. Trees 7:208–213
Dean TJ, Baldwin VC (1996) Growth in loblolly pine plantations as a function of stand density and canopy properties. For Ecol Manage 82:49–58
Dickson RE (1989) Carbon and nitrogen allocation in trees. Ann Sci For 46 [Ssuppl]:631–647
Ellsworth DS (2000) Seasonal CO2 assimilation and stomatal limitations in a Pinus taeda canopy. Tree Physiol 20:435–445
Ford ED, Deans JD (1977) Growth of a Sitka spruce plantation: spatial distribution and seasonal fluctuations of lengths, weights and carbohydrate concentrations of fine roots. Plant Soil 47:463–485
Gravatt DA, Chambers JL, Barnett JP (1997) Temporal and spatial patterns of net photosynthesis in 12-year-old loblolly pine five growing seasons after thinning. For Ecol Manage 97:73–83
Irvine J, Perks MP, Magnani F, Grace J (1998) The response of Pinus sylvestris to drought: stomatal control of transpiration and hydraulic conductance. Tree Physiol 18:393–402
Johnsen K, Samuelson L, Teskey R, McNulty S, Fox T (2000) Process models as tools in forestry research and management. For Sci 47:2-8
Kuehler EA, Sword MA, Andries CD (1999) Seasonal fine-root carbohydrate and growth relations of plantation loblolly pine after thinning and fertilization. In: Haywood JD (ed) Proceedings of the Tenth Biennial Southern Silvicultural Research Conference, USDA Forest Service Southern Research Station, Ashville, North Carolina, pp 420–425
Kuhns MR, Gjerstad DH (1991) Distribution of 14C-labeled photosynthate in loblolly pine (Pinus taeda) seedlings as affected by season and time after exposure. Tree Physiol 8:259–271
Maier CA, Zarnoch SJ, Dougherty PM (1998) Effects of temperature and tissue nitrogen on dormant season stem and branch maintenance respiration in a young loblolly pine (Pinus taeda) plantation. Tree Physiol 18:11–20
Murthy R, Dougherty PM, Zarnoch SJ, Allen HL (1996) Effects of carbon dioxide, fertilization, and irrigation on photosynthetic capacity of loblolly pine trees. Tree Physiol 16:537–546
Peterson JA, Seiler JR, Nowak J, Ginn SE, Kreh RE (1997) Growth and physiological responses of young loblolly pine stands to thinning. For Sci 43:529–534
SAS (2000) The SAS System. Version 8. SAS Institute Inc., SAS Campus Drive, Cary, NC, USA
Schaberg PG, Wilkinson RC, Shane JB, Donnelly JR, Cali PF (1995) Winter photosynthesis of red spruce from three Vermont seed sources. Tree Physiol 15:345–350
Schultz RP (1997) Loblolly pine—the ecology and culture of loblolly pine (Pinus taeda L.). Agricultural Handbook 713. USDA Forest Service, Washington, D.C.
Shoulders E, Tiarks AE (1983) A continuous function design for fertilizer rate trials in young pine plantations. In: Jones EP (ed) Proceedings of the Second Biennial Southern Silvicultural Research Conference. General Technical Report SE-24. USDA Forest Service Southeastern Forest Experiment Station, Ashville, NC, pp 352–356
Snowdon P, Waring HD (1995) Response of young densely stocked stands of Pinus radiata to thinning and refertilization. New For 10:207–223
Sword MA, Gravatt DA, Faulkner PL, Chambers JL (1996) Seasonal branch and fine root growth of juvenile loblolly pine five growing seasons after fertilization. Tree Physiol 16:899–904
Sword MA, Haywood JD, Andries CD (1998) Seasonal lateral root growth of juvenile loblolly pine after thinning and fertilization on a Gulf Coastal Plain site. In: Waldrop TA (ed) Proceedings of the Ninth Biennial Southern Silvicultural Research Conference, USDA Forest Service Southern Research Station, Ashville, NC, pp 194–201
Teskey RO (1997) Combined effects of elevated CO2 and air temperature on carbon assimilation of Pinus taeda trees. Plant Cell Environ 20:373–380
Teskey RO, Fites JA, Samuelson LJ, Bongarten BC (1986) Stomatal and non-stomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiol 2:131–142
Teskey RO, Bongarten BC, Cregg BM, Dougherty PM, Hennessey TC (1987) Physiological and genetics of tree growth response to moisture and temperature stress: an examination of the characteristics of loblolly pine (Pinus taeda L.). Tree Physiol 3:41–61
Teskey RO, Gholz HL, Cropper WP (1994) Influence of climate and fertilization on net photosynthesis of mature slash pine. Tree Physiol 14:1215–1227
Wang YP, Jarvis PG (1990) Influence of crown structural properties on PAR absorption, photosynthesis, and transpiration in Sitka spruce: application of model (MAESTRO). Tree Physiol 7:297–316
Whitehead D, Livingston NJ, Kelliher FM, Hogan KP, Pepin S, McSevery TM, Byers JN (1996) Response of transpiration and photosynthesis to a transient change in illuminated foliage area for a Pinus radiata D. Don tree. Plant Cell Environ 19:949–957
Yu S, Cao QV, Chambers JL, Tang Z, Haywood JD (1999) Managing leaf area for maximum volume production in a loblolly pine plantation. In: Haywood JD (ed) Proceedings of the Tenth Biennial Southern Silvicultural Research Conference, USDA Forest Service Southern Research Station, Ashville, NC, pp 455–460
Zhang S, Hennessey TC, Heinemann RA (1997) Acclimation of loblolly pine (Pinus taeda) foliage to light intensity as related to leaf nitrogen availability. Can J For Res 27:1032–1040
Acknowledgements
We gratefully appreciate Jim Scarborough for his dedication and effort in building towers and recording plant water potential and Dan Andries for collecting soil moisture data. We also wish to thank anonymous reviewers for their critical reviews of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Z., Chambers, J.L., Sword, M.A. et al. Seasonal photosynthesis and water relations of juvenile loblolly pine relative to stand density and canopy position. Trees 17, 424–430 (2003). https://doi.org/10.1007/s00468-003-0256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-003-0256-0