Abstract
Background
Dyslipidemia is a potentially modifiable risk factor in patients with chronic kidney disease (CKD). Information on the safety and efficacy of statins in pediatric CKD is limited.
Methods
Patients with CKD stage 2–5 and aged 5–18 years with low-density lipoprotein cholesterol (LDL-C) > 130 mg/dL and/or non-high-density lipoprotein cholesterol (non-HDL-C) > 145 mg/dL were enrolled from September 2019 to February 2021. All patients were administered atorvastatin 10 mg/day, which was escalated to 20 mg/day if LDL-C remained > 100 mg/dL and/or non-HDL-C > 120 mg/dL at 12 weeks. Proportion of patients achieving target lipid levels (LDL-C ≤ 100 mg/dL and non-HDL-C ≤ 120 mg/dL) and adverse events were assessed at 24 weeks.
Results
Of 31 patients enrolled, target lipid levels were achieved in 45.2% (95% CI 27.8–63.7%) at 24 weeks; 22 patients required dose escalation to 20 mg at 12 weeks. There was no difference in median lipid level reduction with 10 (n = 9) versus 20 mg/day (n = 22, P = 0.3). Higher baseline LDL-C (OR 1.06, 95% CI 1.00–1.11) and older age (OR 36.5, 95% CI 2.57–519.14) were independent predictors of failure to achieve target lipid levels with 10 mg/day atorvastatin. None had persistent rise in AST/ALT > 3 times upper normal limit (UNL) or CPK > 10 times UNL. No differences were noted in adverse events due to atorvastatin 10 or 20 mg/day.
Conclusion
Atorvastatin (10–20 mg/day) administered for 24 weeks was safe and effectively reduced LDL-C and non-HDL-C in children with CKD stages 2–5. Patients with higher baseline LDL-C required higher doses to achieve the target.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease accounts for almost one-third of mortality in children and adolescents with chronic kidney disease (CKD) requiring dialysis [1]. Multiple cardiovascular risk factors in pediatric CKD predispose to accelerated atherosclerosis due to vascular endothelial dysfunction and coronary artery calcification [1]. The American Heart Association (AHA) classifies children with kidney failure in the highest risk group and those with pre-dialysis CKD at moderate risk for development of cardiovascular disease and its sequelae [2].
Dyslipidemia is an important modifiable cardiovascular risk factor in children with CKD, with a prevalence of 45–75% in cohorts from North America, Europe, and Korea [3,4,5,6]. Studies have shown an association of dyslipidemia in children with CKD with increased carotid intimal media thickness, a marker of subclinical atherosclerosis [7]. While statins are routinely used in adults, it has not been proven that they improve the hard endpoints such as mortality or progression to kidney failure. Almost three-quarters of dyslipidemia in pediatric kidney diseases remains untreated [8]. The AHA recommends initiation of statins along with lifestyle measures simultaneously if LDL-C is > 130 mg/dL in children > 10 years of age with pre-dialysis CKD and two additional cardiovascular risk factors [9]. The National Heart, Lung, Blood Institute (NHLBI) also recommends statins for children > 10 years of age with LDL-C > 130 mg/dL with two high-risk conditions despite lifestyle modifications; high-risk conditions include CKD, hypertension, body mass index > 97th centile, and smoking [10]. However, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, in the absence of high-quality evidence for lipid-lowering therapies in pediatric CKD, recommends chiefly lifestyle changes for management, particularly for children less than 10 years of age [11].
There are limited studies on safety and efficacy of statin use for pediatric CKD. We designed this prospective interventional pilot study to assess the safety and efficacy of escalating doses of atorvastatin for control of dyslipidemia in children with pre-dialysis CKD.
Methods
We prospectively screened patients aged 5–18 years and diagnosed with CKD with estimated glomerular filtration rate (eGFR; modified Schwartz equation [12]) 15–89 mL/min/1.73 m2, not on kidney replacement therapy (KRT) from the outpatient pediatric nephrology clinic in this hospital. Patients were screened in two phases from September 2019 to March 2020 and August 2020 to August 2021 due to lockdowns during the pandemic. Patients with low-density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL and/or non-high-density lipoprotein cholesterol (HDL-C) ≥ 145 mg/dL, detected on two occasions 1 week apart, were enrolled following institutional ethics committee approval and informed written consent. Patients with hepatic dysfunction (aspartate or alanine aminotransferase (AST/ALT) ≥ 3 times upper limit of normal), creatine phosphokinase (CPK) ≥ 3 times upper limit of normal, history of hypersensitivity to lipid-lowering drugs, or therapy with beta-blockers, isotretinoin, antiretrovirals, or oral prednisolone at a dose > 2 mg/kg/day or variable doses of immunosuppressive medication in the past 3 months, were excluded.
Clinical details and anthropometry were recorded; weight-for-age, height-for-age, and body mass index-for-age standard deviation scores (SDS) were derived using WHO MGRS growth charts [13]. Obesity was defined as body mass index more than the equivalent of 27 kg/m2 in Indian adults using growth charts of Indian children [14]. Blood pressure was recorded twice at enrolment and every subsequent visit, and mean systolic and diastolic pressures were used to derive age-sex-height specific SDS [15]. Hypertension was defined as blood pressure ≥ 95th centile for age, height, and sex [16], or receiving any antihypertensive medications. Investigations, performed at baseline and follow-up, included blood levels of urea, creatinine, electrolytes, calcium, phosphate, alkaline phosphatase, hemoglobin, albumin, alanine and aspartate aminotransferases (AST and ALT), fasting blood sugar, and CPK. Patients were classified as CKD stage G2, G3a, G3b, or G4 with eGFR of 60–89, 45–59, 30–44, and 15–29 mL/min/1.73 m2, respectively [17]. Twenty-four-hour urine protein excretion was measured. Serum total cholesterol, low-density lipoprotein (LDL-C), very low-density lipoprotein (VLDL-C), high-density lipoprotein (HDL-C), and triglycerides were estimated following a 12-h overnight fasting period. Total cholesterol and triglyceride levels were measured using enzymatic endpoint method [18]. HDL-C was estimated after precipitation of LDL-C and VLDL-C using phosphotungstic acid and magnesium [19]. Non-HDL-C was the difference between total cholesterol and HDL-C [20]. Hypertriglyceridemia was defined as triglycerides > 100 mg/dL in children below 10 years and ≥ 130 mg/dL in children 10–19 years; HDL-C < 40 mg/dL was considered low. LDL-C was calculated by the Friedewald equation [21]:
Patients were initiated on atorvastatin at a dose of 10 mg once daily in the evening irrespective of last meal and followed up at 12 and 24 weeks (Fig. 1). Target lipid level was defined as reduction of LDL-C ≤ 100 mg/dL and non-HDL-C ≤ 120 mg/dL at 12 and 24 weeks based on the NHLBI guidelines [10]. The daily dose of atorvastatin was escalated to 20 mg at 12 weeks if target lipid levels were not attained. The initial dietary assessment showed total fat intake of 15–30% in the present cohort. Throughout the study period, patients were instructed to follow the CHILD-2 diet ([10] (total, saturated, and mono-polyunsaturated fat intake of 25–30, 7, and 10% of the daily total caloric intake respectively). Patients were encouraged to have high dietary fiber intake and at least 1 h of moderate to vigorous physical activity daily, and to limit daily screen time to < 2 h. Compliance to medications was ensured by pill count at 12 and 24 weeks. All patients received standard management of CKD, including supplements of iron, multivitamins, calcium carbonate (250 to 500 mg), and vitamin D if required. Patients with eGFR > 30 mL/min/1.73 m2 received an angiotensin-converting enzyme inhibitor for control of hypertension or proteinuria.
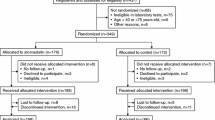
Flow of patients screened, enrolled, and followed up during study period. Eligible patients were administered atorvastatin 10 mg/day that was escalated to 20 mg/day if LDL-C > 100 mg/dL and/or non-HDL-C > 120 mg/dL at 12 weeks. Proportion of patients achieving target lipid levels (LDL-C ≤ 100 mg/dL and non HDL-C ≤ 120 mg/dL) at 12 and 24 weeks is depicted. CKD, chronic kidney disease; HDL, high density lipoprotein; LDL, low density lipoprotein
Adverse events were assessed using Common Terminology Criteria for Adverse Events (CTCAE) guidelines [22], and causality assessment was conducted when a severe adverse event was suspected using WHO Uppsala Monitoring Centre (UMC) system [23]. Criteria for withdrawal from study were two consecutive measurements of CPK > 10 times upper limit of normal or AST/ALT > 3 times upper limit of normal, at least 2 weeks apart [24].
Statistical analysis
Data are summarized as median (interquartile, IQR) or as proportions and analyzed using Stata version 14.0 (Stata Corp, College Station, TX). Tests for significance within or between groups included Wilcoxon signed rank and rank sum tests. Strength of association between variables was assessed using Spearman correlation. Determinants of failure to achieve target lipid levels following atorvastatin dose of 10 mg/day for 12 weeks were estimated as odds ratios (OR) by univariate and multivariable analyses using logistic regression; variables with P < 0.1 on univariate analysis were included in the multivariable models. Receiver operator characteristic (ROC) curves were used for estimating the threshold of LDL-C and non-HDL-C at onset that determined failure to achieve target lipid levels at 12 weeks. Two-tailed P < 0.05 was considered significant.
Results
Of 97 consecutive patients screened, 31 patients aged 15 (12, 17) years, 22 (71.0%) boys were included (Fig. 1). The baseline characteristics of the patients are summarized in Table 1. Patients were stunted (21, 70.9%) and undernourished (4, 12.9%). Estimated GFR was > 60 mL/min/1.73 m2 at enrolment in 20 (64.5%) patients. The etiology of CKD was chiefly glomerular, including membranoproliferative glomerulonephritis (21) and steroid-resistant nephrotic syndrome (3); other causes were reflux nephropathy (2), posterior urethral valve (3), cystinosis, and multicystic dysplastic kidney (1 each). Nephrotic range proteinuria (> 1 g/m2/day) was present in 22 (71.0%). All patients were receiving therapy with enalapril for proteinuria or hypertension (blood pressure controlled in 61.3%). Baseline lipid profile values are shown in Table 1. Hypertriglyceridemia and low HDL-C were additionally present in 25 (80.6%) and 3 (9.7%) patients, respectively. Higher baseline LDL-C and non-HDL-C were correlated with higher 24-h urine protein (rs = 0.53, P = 0.002 and rs = 0.52, P = 0.003, respectively) and lower blood albumin levels (rs = – 0.33, P = 0.072 and rs = – 0.36, P = 0.049, respectively). These lipid levels were not significantly correlated with age, BMI SDS, eGFR, systolic SDS, and diastolic SDS (P > 0.5, data not shown).
Upon initiation of atorvastatin at a dose of 10 mg/day, at 12-week follow-up, 9 (29.0%, 95%CI 15.2–48.2) achieved the target lipid levels; the other 22 patients required escalation of atorvastatin dose to 20 mg/day. At 24 weeks, 7 additional patients (31.8%, 95% CI 14.7–54.9) achieved the target lipid levels on 20 mg/day atorvastatin. Overall, in the entire cohort, the target lipid levels were achieved in 45.2% (95% CI 27.8–63.7) patients at 24 weeks with atorvastatin of 10–20 mg/day.
The median reduction in LDL-C, non-HDL-C, and TG at 24 weeks was 61, 72, and 27 mg/dL, corresponding to median 40.7, 39.4, and 19.0% relative reductions from baseline, respectively (P < 0.0001; Table 2). There was no significant difference in percentage change in lipid levels from baseline to 24 weeks between patients receiving atorvastatin doses of 10 and 20 mg (Table 2).
Over 24 weeks, median change in eGFR and 24-h urine protein was – 2.7 (– 11.6, 2.1) mL/min/1.73 m2 and – 0.2 g/m2/day (– 2.9, 0.9). Three patients required initiation of KRT due to disease progression at 6–7 months from enrolment. Median changes in LDL-C and non-HDL-C from baseline to 24 weeks were not significantly correlated with change in BMI SDS, blood albumin levels, 24-h urine protein, and eGFR (P > 0.5, data not shown).
Predictors of non-response to atorvastatin
Factors associated with failure to achieve target lipid levels on 10 mg/day atorvastatin on multivariable analysis were higher baseline LDL-C levels (OR1.06, 95% CI 1–1.11), higher baseline non-HDL-C (OR 1.03, 95% CI 1–1.07), age > 12 years (OR 36.5, 95% CI 2.57–519.14), higher doses of atorvastatin, both dosed as mg per kg and mg per body surface area, predicted response (OR 0.0001, 95% CI < 0.001–0.40, and OR 0.63, 95% CI 0.40–1.00) (Table 3). Twenty-four-hour urine protein was not a significant predictor of response on univariate analysis (OR 1.00, 95% CI 0.99–1.00, P = 0.336). The percentage of patients who had nephrotic range proteinuria and failed to achieve target LDL-C levels after atorvastatin therapy was 13 of 22 (59.1%) compared to 4 of 9 patients (44.4%) without significant proteinuria with failure to achieve target lipid levels (P = 0.7). ROC analysis showed that baseline LDL-C > 161.7 mg/dL or non-HDL-C > 211.5 mg/dL predicted non-response to 10 mg/day atorvastatin at 12 weeks with sensitivity of 82.0 and 73% and specificity of 56.0 and 89.0%, with area under the curve (AUC) of 0.69 and 0.81.
Adverse events
A total of 40 adverse events were observed (Table 4). Elevated AST/ALT > 3 upper normal limit (UNL) and CPK > 10 UNL meriting withdrawal from study did not occur in any patient. Asymptomatic elevation of CPK to four times upper limit of normal (CTCAE grade 1) was noted in a patient who also had deranged thyroid function test; hence, this was not attributed to atorvastatin therapy. Transient and mildly elevated AST (41–58 U/L, n = 4), ALT (46–86 U/L, n = 2), and CPK (227–304 U/L, n = 4) were present in few patients, which were perhaps likely associated with statin treatment. Three patients had one episode of leg cramps each, which resolved spontaneously, with no associated rise in CPK. Muscle cramps, previously reported more frequently with atorvastatin [25], was also likely due to statins. Adverse events not related to atorvastatin were dyspnea (CTCAE grade 2) in a patient with dilated cardiomyopathy, upper respiratory tract infection (13 episodes), and fever without any focus (7 episodes); these adverse events have not been reported with statins.
Discussion
The present study showed that in patients 5–18 years old with predialysis CKD and dyslipidemia, target levels of LDL-C ≤ 100 mg/dL and non-HDL-C ≤ 120 mg/dL were achieved in 45.2% at 24 weeks with escalating doses of atorvastatin. With this dosing strategy, the relative reduction in LDL-C and non-HDL-C from baseline were 40.7 and 39.4%, respectively. Higher LDL-C (> 162 mg/dL) and non-HDL-C levels (> 212 mg/dL), older age, and lower weight- and body surface area-based dose of atorvastatin were associated with requirement of dose escalation due to failure to achieve target lipid levels. Atorvastatin at a dose of 10–20 mg/day was safe and well tolerated.
The AHA guidelines consider predialysis CKD a moderate cardiovascular risk factor; two or more additional comorbidities (including dyslipidemia, hypertension, family history of premature cardiovascular disease, smoking, and obesity) classify such patients as high risk with the need to initiate statins along with lifestyle measures simultaneously if LDL-C > 130 mg/dL [9]. In the current study, all patients had elevated lipid levels and were hypertensive at baseline (receiving one anti-hypertensive agent), therefore having two additional risk factors to qualify in the high-risk category. An LDL-C target < 100 mg/dL is appropriate as per NHLBI and AHA guidelines while on statin therapy for high-risk patients [9, 10]. In the present study, 71% of patients failed to achieve target lipid levels at 12 weeks and required dose escalation of atorvastatin from 10 to 20 mg/day; one-third of these patients subsequently achieved target levels after 3 months, emphasizing a dose-dependent efficacy of atorvastatin to lower LDL-C. These findings are comparable to previous reports in pediatric hypercholesterolemia showing dose escalation from 10 to 20 mg was required in 54.3–62.5% of patients to achieve LDL-C < 130 mg/dL in 50–56% patients over 6–7 months [26, 27].
Daily atorvastatin at 10–20 mg for 24 weeks enabled reduction in LDL-C by median 61 mg/dL in the present study. This is similar to individual patient data meta-analysis of statin therapy in adults with predialysis CKD (n = 176,366; 28 trials) that showed a decline in LDL-C of 42.15 mg/dL over several years [28]. The change in LDL-C in the present study was slightly more than the reduction of 46.9 mg/dL and 44.9 mg/dL in randomized double-blind placebo controlled, crossover trials of atorvastatin (10 mg) administered for 8 weeks and simvastatin (weight-based, escalating doses) for 3 months, respectively, in children with CKD [28, 29]. While target LDL-C levels were achieved in a majority of patients in the simvastatin trial [29], the baseline LDL-C levels were lower than in the present study (133 mg/dL vs. 174 mg/dL respectively). The relative reduction in LDL-C following simvastatin was 33.7% [29] compared to the present study showing 40.7% decline. The change in LDL-C was higher than shown in a randomized, placebo-controlled trial in 30 children with unremitting nephrotic syndrome from this center (relative reduction of 15.8% with 10 mg/day atorvastatin), possibly due to presence of severe hypoalbuminemia in the latter [30]. Overall, relative decline in LDL-C in the present study was consistent with a meta-analysis in familial hypercholesterolemia showing reduction of 39% in children (atorvastatin 10–20 mg, n = 140) and 43% in adults (atorvastatin 20 mg, n = 7506) [31].
We demonstrated that higher baseline level of LDL-C was associated with non-response to a lower dose of atorvastatin in the present study, similar to a study on pediatric familial hypercholesterolemia [26]. Additionally, lower weight- and body surface area-based doses and older age were independent predictors for non-response to atorvastatin. The mean dose of atorvastatin used in the current study was 0.29 ± 0.13 mg/kg or 8.63 ± 2.53 mg/m2 until 3 months and 0.47 ± 0.18 mg/kg or 17.27 ± 5.06 mg/m2 thereafter. Contrary to our findings, a study on simvastatin in pediatric CKD did not show a correlation between change in lipid levels and dose in milligrams per kilogram or BMI [29]. Atorvastatin at a dose of 10–20 mg/day was safe and well tolerated in the present study without significant muscle or hepatic toxicity, similar to prior studies that have demonstrated long-term tolerability of doses of atorvastatin up to 20 mg/day in children older than 8 years with familial hypercholesterolemia [26, 27]. However, it is possible that mild elevation in AST/ALT or CPK and muscle cramps in a few patients were related to statin therapy. While none of the patients < 10 years of age had mild elevation of AST/ALT or CPK, more studies are required to understand the degree of elevation in CPK or AST/ALT that should be considered as a significant adverse effect of statin therapy on children of this age. While the recommended atorvastatin dose for adults ranges from 10 to 80 mg/day, based on the desired intensity of LDL-C lowering [9], administration of statins in early childhood is fraught with concerns of adverse effects on neurological and pubertal development [27, 31]. Therefore, further research is required on whether a dose of atorvastatin above 20 mg/day could be adapted for children on the basis of dosing titrated to weight or body surface area.
While it seems logical to treat hyperlipidemia for prevention of accelerated atherosclerosis, the role of LDL-cholesterol in the arteriopathy and calcification seen in children with CKD is perhaps less important than that in classical atherosclerosis where it is a major player. There is evidence of higher levels of small dense LDL and oxidized LDL in CKD, which are highly atherogenic and have higher affinity for uptake by macrophage receptors to increase fatty streak formation [32,33,34]. However, current evidence from prospective cohort studies and arterial biopsy studies shows little, if any, signs of LDL cholesterol as a major risk factor for the prevailing early arterial changes, which are driven mainly by hypertension, inflammation, and disturbances in mineral metabolism [1]. Therefore, while there is no proven benefit of statins on overall cardiovascular morbidity and mortality in children with CKD, information on efficacy, tolerability, and safety of statins in CKD patients is required. This is the first interventional pilot study assessing safety and efficacy of dose escalation of atorvastatin to decrease LDL-C levels below currently recommended cut-offs. The study is, however, limited by a small number of participants enrolled, uncontrolled design, and short-term duration. Additionally, a majority of patients in the present study were at significantly higher risk of hyperlipidemia due to the presence of nephrotic-range proteinuria; this might be a confounding factor when comparing dose escalation treatments, especially since we did not find 24-h urine protein to predict response to atorvastatin. Based on this study, we suggest atorvastatin 20 mg/day (or equivalent) be initiated in patients older than 12 years with pre-dialysis CKD stages 2–5 and with LDL-C levels > 160 mg/dL despite therapeutic lifestyle changes. A dose of 10 mg/day may suffice in others. Robust safety and efficacy data is required to further explore doses based on body weight or surface area.
References
Weaver DJ, Mitsnefes M (2018) Cardiovascular disease in children and adolescents with chronic kidney disease. Semin Nephrol 38:559–569
de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS et al (2019) Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 139:e603–e634
Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL (2012) CKiD (CKD in Children) Prospective cohort study: a review of current findings. Am J Kidney Dis 60:1002–1011
Saland JM, Kupferman JC, Pierce CB, Flynn JT, Mitsnefes MM, Warady BA et al (2019) Change in dyslipidemia with declining glomerular filtration rate and increasing proteinuria in children with CKD. Clin J Am Soc Nephrol 14:1711–1718
Baek HS, Kim SH, Kang HG, Choi HJ, Cheong HI, Ha IS et al (2020) Dyslipidemia in pediatric CKD patients: results from KNOW-pedCKD (Korean cohort study for Outcomes in patients With Pediatric CKD). Pediatr Nephrol 35:1455–1461
Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A et al (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28
Khandelwal P, Murugan V, Hari S, Lakshmy R, Sinha A, Hari P et al (2016) Dyslipidemia, carotid intima-media thickness and endothelial dysfunction in children with chronic kidney disease. Pediatr Nephrol 31:1313–1320
Ashoor IF, Mansfield SA, O’Shaughnessy MM, Parekh RS, Zee J, Vasylyeva TL et al (2019) Prevalence of cardiovascular disease risk factors in childhood glomerular diseases. J Am Heart Assoc 8:e012143
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS et al (2018) AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/apha/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139:e1187
Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents (2011) National Heart, Lung, and Blood Institute summary report. Pediatrics 128:13–56
Sarnak MJ, Bloom R, Muntner P, Rahman M, Saland JM, Wilson PWF et al (2015) KDOQI US commentary on the 2013 KDIGO clinical practice guideline for lipid management in CKD. Am J Kidney Dis 65:354–366
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667
Khadilkar VV, Khadilkar AV (2015) Revised Indian Academy of Pediatrics 2015 growth charts for height, weight and body mass index for 5–18-year-old Indian children. Indian J Endocrinol Metab 19:470–476
Flack JM, Adekola B (2020) Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med 30:160–164
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR et al (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904
Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J et al (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease Improving Global Outcomes (KDIGO). Kidney Int 67:2089–2100
Wentz PW, Cross RE, Savory J (1976) An integrated approach to lipid profiling: enzymatic determination of cholesterol and triglycerides with a centrifugal analyzer. Clin Chem 22:188–192
Steele BW, Koehler DF, Azar MM, Blaszkowski TP, Kuba K, Dempsey ME (1976) Enzymatic determinations of cholesterol in high-density-lipoprotein fractions prepared by a precipitation technique. Clin Chem 22:98–101
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P et al (1987) Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317:1237–1245
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the Common Terminology Criteria for Adverse Events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 112:90–92
Behera SK, Das S, Xavier AS, Velupula S, Sandhiya S (2018) Comparison of different methods for causality assessment of adverse drug reactions. Int J Clin Pharm 40:903–910
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ et al (2016) ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 37:2999–3058
Thompson PD (2003) Statin-associated myopathy. JAMA 289:1681
Gandelman K, Glue P, Laskey R, Jones J, Labadie R, Ose L (2011) An eight-week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia. Pediatr Cardiol 32:433–441
McCrindle BW, Ose L, Marais AD (2003) Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr 143:74–80
Cholesterol Treatment Trialists’ (CTT) Collaboration, Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD et al (2016) Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 4:829–839
García-de-la-Puente S, Luis Arredondo-García J, Gutiérrez-Castrellón P, Bojorquez-Ochoa A, Reyna Maya E, Del Pilar P-M (2009) Efficacy of simvastatin in children with hyperlipidemia secondary to kidney disorders. Pediatr Nephrol 24:1205–1210
Hari P, Khandelwal P, Satpathy A, Hari S, Thergaonkar R, Lakshmy R et al (2018) Effect of atorvastatin on dyslipidemia and carotid intima-media thickness in children with refractory nephrotic syndrome: a randomized controlled trial. Pediatr Nephrol 33:2299–2309
Avis HJ, Vissers MN, Stein EA, Wijburg FA, Trip MD, Kastelein JJP et al (2007) A systematic review and meta-analysis of statin therapy in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 27:1803–1810
Anderson JLC, Gautier T, Nijstad N, Tölle M, Schuchardt M et al (2017) High density lipoprotein (HDL) particles from end-stage renal disease patients are defective in promoting reverse cholesterol transport. Sci Rep 7:41481
Binder V, Ljubojevic S, Haybaeck J, Holzer M, El-Gamal D et al (2013) The myeloperoxidase product hypochlorous acid generates irreversible high-density lipoprotein receptor inhibitors. Arterioscler Thromb Vasc Biol 33:1020–1027
Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S et al (2012) Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: novel pathways generating dysfunctional high-density lipoprotein. Antioxid Redox Signal 17:1043–1052
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramesh, P.L., Khandelwal, P., Lakshmy, R. et al. Short-term safety and efficacy of escalating doses of atorvastatin for dyslipidemia in children with predialysis chronic kidney disease stage 2–5. Pediatr Nephrol 38, 2763–2770 (2023). https://doi.org/10.1007/s00467-023-05887-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-05887-0