Abstract
Background
Regional citrate anticoagulation (RCA) is the preferred continuous kidney replacement therapy (CKRT) anticoagulation strategy for children in the USA. Nafamostat mesilate (NM), a synthetic serine protease, is used widely for CKRT anticoagulation in Japan and Korea. We compared the safety and efficacy of NM to RCA for pediatric CKRT.
Methods
Starting June 2019, the most recent 100 medical records of children receiving CKRT with either RCA or NM were reviewed retrospectively, at one children’s hospital in Japan (NM) and one in the USA (RCA). The number of hours a single CKRT filter was in use, was the primary outcome. Safety was assessed by bleeding complications for the NM group and citrate toxicity leading to RCA discontinuation or electrolyte imbalance in the RCA group.
Results
Eighty patients received NM and 78 patients received RCA. Median filter life was longer for the NM group (NM: 38 [22, 74] vs. RCA: 36 [17, 66] h, p = 0.02). When filter life was censored for discontinuation other than clotting, the 60-h survival rate was higher for RCA (71% vs. 54%). The hazard ratio comparing NM over RCA varied over time (HR 0.7; 0.2–1.5, p = 0.33 at 0 h to HR 5.5; 1.3–23.7, p = 0.334 at 72 h). The lack of difference in filter survival persisted controlling for filter surface area, catheter diameter, and pre-CKRT platelet count. Major bleeding rates did not differ between groups (NM: 5% vs. RCA: 9%).
Conclusions
RCA and NM provide satisfactory anticoagulation for CKRT in children with no difference in major bleeding rates.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for AKI recommend regional citrate anticoagulation (RCA) as the preferred anticoagulation method for continuous kidney replacement therapy (CKRT) in patients without contraindications [1]. However, RCA has potential adverse effects which include alkalosis, lactic acidosis, hypernatremia, and hyperglycemia. Although recent literature [2,3,4,5] shows the safety of RCA in patients with liver failure, some centers avoid RCA due to risk of citrate accumulation. A recent study revealed an association between citrate intolerance and lactic acidosis as the citric acid cycle is oxygen dependent [6]. Liver failure and inadequate oxygen delivery are not uncommon in the critically ill population requiring CKRT. RCA requires frequent monitoring of circuit and patient ionized calcium levels, which adds to the clinical team workload [7]. Finally, recent IV calcium shortages highlight the need for other safe and effective CKRT anticoagulation strategies.
Nafamostat mesilate (NM), a synthetic serine protease inhibitor placed on the market by Japan Tobacco in 1986, is approved to treat pancreatitis, DIC and for CKRT anticoagulation in patients with bleeding risk in Japan [8]. NM has been used for CKRT anticoagulation in Japan and Korea. NM conjugates with thrombin and blocks its coagulative activity, and suppresses the activated coagulative factors including factor XIIa, Xa, plasmin, kallikrein and complement [9]. NM also inhibits platelet aggregation by suppressing secretion of arachidonic acids including phospholipase A2 [10]. The biological half-life of NM is approximately eight minutes [11]. Thus, while adequate circuit anticoagulation is maintained when NM is infused into the access side of the CKRT circuit, its systemic concentration decreases to a level which does not cause anticoagulation in the patient [12]. Therefore, NM does not need a reversal agent like RCA and does not cause systemic anticoagulation like heparin. NM is not contraindicated in liver failure, shock status or for patients at risk of bleeding. While NM has longer filter life compared to no anticoagulation usage [13,14,15], its efficacy compared to heparin remains controversial [13, 16, 17].
There is paucity of data for CKRT NM anticoagulation in children. The only previously published study comparing NM to either unfractionated heparin (UFH) or no anticoagulation showed no major bleeding events in the NM group. However, the NM cohort had only 25 patients and while filter life of NM was superior to UFH, NM was only used for patients with high risk of bleeding [18]. Therefore, this indication bias renders conclusions regarding efficacy speculative.
In the current study, we compared the safety and efficacy of NM anticoagulation to RCA for CKRT in children.
Materials and methods
We conducted a two-center retrospective observational cohort study comparing NM and RCA in children receiving CKRT. Data for the NM group were collected from the pediatric ICU (mixed unit with cardiac population) at National Center for Child Health and Development (NCCHD), Tokyo, Japan. Data for the RCA group were collected from the pediatric ICU and cardiac ICU at Cincinnati Children’s Hospital Medical Center (CCHMC), Cincinnati, OH. CCHMC served as the data coordinating site for this study.
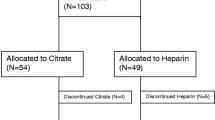
Records from patients at NCCHD were reviewed by study investigators. Data were included from patients (i) aged 0 to 21 years, (ii) receiving CKRT, and (iii) CKRT was not accompanied by ECMO. Records were excluded if (i) filters received no anticoagulation or methods other than RCA or NM or (ii) concomitant use of multiple anticoagulation methods (e.g., the CKRT filter was anticoagulated with RCA while patient was receiving UFH for another device or a hypercoagulable state). Data from filters with an anticoagulation method switch in the middle of the same filter were also excluded. Data from patients who received different anticoagulation methods, but the switch was made between filters, were included under a different study identification number. When the CKRT treatment course had an interval > 48 h between CKRT procedures, a new study ID was created (Fig. 1).
Study flow diagram. Abbreviations: CKRT, continuous kidney replacement therapy; CCHMC, Cincinnati Children’s Hospital Medical Center; NCCHD, National Center for Child Health and Disease; DVT, deep vein thrombosis; VAD, ventricular assist device; UFH, unfractionated heparin; ACG, anticoagulation; RCA, reginal citrate anticoagulation; NM, nafamostat mesilate
Data from all patients who received CKRT at CCHMC and were included in the ongoing quality improvement project, under IRB 2019–0466, were enrolled if they met all the inclusion criteria and none of the exclusion criteria. Patients were identified through IRB study 2019–0466, but additional data were collected for the purposes of this study. This study was approved by CCHMC IRB 2019–1252 and by NCCHD IRB 2019–089. CKRT courses were screened from most recent backwards starting June 2019 until 100 CKRT courses were reached for each anticoagulation method group. The requirement for subject/caregiver informed consent was waived given the retrospective design of the study.
Exposure and outcome measures
We collected demographic information including age, sex, weight, Pediatric Index of Mortality 2 (PIM2) score [19], baseline and admission diagnosis, and CKRT indication. Baseline laboratory measurements included blood pH, lactate, potassium, hemoglobin, platelet count, prothrombin time-international normalized ratio (PT-INR), active partial thromboplastin time (aPTT), kidney function, and liver function before CKRT initiation.
The anticoagulation method was the primary exposure. Filter characteristics included the following: anticoagulation dosage range, anatomic location and size of hemodialysis catheter, size and type of filter. CKRT settings included modality, blood pump flow rate, total effluent rate, post or pre filter replacement fluid rate, dialysis fluid rate, ultrafiltration rate, highest hematocrit for each filter, and filtration fraction (%). Concomitant use of other extracorporeal therapies including therapeutic plasma exchange (TPE), selective cytopheretic device (SCD; SeaStar Medical, Inc., Denver, CO) filter [20], and/or a polymyxin B-immobilized polystyrene derivative fibers (PMX; Toraymyxin 20-R; Toray Industries, Inc, Tokyo, Japan) were noted.
The primary outcome was filter life in hours defined from the time of CKRT circuit initiation to when the filter was changed or the CKRT treatment course was completed. Elective cessation of the CKRT circuit (i.e., other than filter clotting) was treated as censored data. The reason for the filter change and transmembrane pressure (TMP) before filter discontinuation were obtained. The secondary outcome was incidence of a major bleeding episode during CKRT, defined as new or worsened bleeding on CKRT which required transfusion of 15 mL/kg or more of packed red blood cells (PRBCs) or accompanied by a decrease in hemoglobin level of ≥ 2 g/dL. The definition was modified from an adult study [17]; we used 15 mL/kg, a common volume for pediatric PRBC transfusion, to recapitulate the adult protocol, considering the smaller and diverse size of the patient population. Minor bleeding, ICU mortality, incidence of citrate toxicity or other electrolyte imbalance in RCA, anaphylaxis in NM were described as well. Anaphylaxis in this study is defined in the Supplementary reference [21].
Anticoagulation method
Each center followed local practice with respect to the anticoagulation (ACG) method. The protocols are listed in Table S1. RCA was accomplished using an ACD-A™ (Anticoagulant Dextrose-A, Baxter Healthcare, Deerfield, IL) infusion via arterial limb of the CKRT circuit to maintain a circuit ionized calcium level between 0.2 and 0.4 mmol/L. The patient systemic ionized calcium is maintained between 1.1 and 1.3 mmol/L by administration of calcium infusion (either calcium chloride or calcium gluconate, based on pharmacy availability) into the venous limb of CKRT or a separate central line. Paired circuit and systemic ionized calcium levels were assessed with following intervals; 5 min and 2 h post-initiation and every 8 h thereafter, or 30–60 min after a change in rate. The 5-min paired assessment is the CCHMC standard as a safety measure, to ensure that the ACD-A™ is administered to the circuit and the calcium to the patient. If these are reversed, the circuit and patient serum ionized calcium concentrations would be similar and would lower the patient ionized calcium to potentially dangerous low concentrations.
NM was accomplished by using Fusan™ (Nichi-Iko, Toyama, Japan) given pre-filter into the CKRT circuit with starting doses of 1 mg/kg/h followed by 1 mg/kg bolus with a post filter activated clotting time (ACT) target of 280–360 s using a Glass Activated (P214) ACT tube (International Technidyne Corporation, Edison, NJ, USA) or 200–250 s using a Celite (FTCA510) ACT tube (International Technidyne Corporation, Edison, NJ, USA). The ACT was measured every 3 h when in the target range and 1 to 2 h after a dosage change.
For patients who received TPE simultaneously with CKRT, the TPE circuit was integrated in parallel to the CKRT circuit with RCA at a fixed ratio to the blood flow rate (CCHMC) or serial/parallel to CKRT with a fixed NM rate of 0.5 mg/kg/h (NCCHD).
CKRT machine and filters
The Prismaflex™ (Baxter Healthcare, USA) was used at CCHMC. Two different filter types; HF series (polyarylethersulfone; PAES) with surface area of 0.2–1.1 m2 and M-series (AN69) with 0.6–0.9 m2 were utilized in this study population. The TR55X™ machine (Toray, Inc, Japan) was used at NCCHD. The UT series (cellulose triacetate, CTA) filters with a surface are of 0.3–2.1 m2 were used. Patients with sepsis were often supported with an AN69 or SCD filter (in line with CKRT) at CCHMC and a PMX filter at NCCHD. Filter choice was based on patient size or pathophysiology. Both centers employ extracorporeal circuit blood priming when the extracorporeal volume exceeds 10% of the patient’s blood volume.
CCHMC employs a routine circuit change at or near 72 h of filter life per manufacturer recommendations. No manufacturer recommendations for routine circuit change exist for the TR-55X™; therefore, no routine circuit change was employed for the NM group.
Statistical analysis
Patient demographics, filter characteristics, and outcome were shown by anticoagulation method and compared by Wilcoxon rank-sum test for continuous measures and chi-square test for counts. Distribution normality was assessed by Shapiro–Wilk test. Filter life was censored for survival analysis if electively discontinued or when discontinuation was unrelated to the filter clot (e.g., mandatory discontinuation due to gain/loss limit) and compared using Kaplan–Meier analysis with the Cox proportional hazards model. Although this initial analysis did not adjust for confounders, Cox regression was needed to adjust for correlation among filters within the same patients using a shared frailty approach. We assessed unadjusted hazard ratios at 60 h and 72 h for robustness as previous studies mainly reported 60 h survival rate [22]. Further univariable analyses were performed to assess potential factors associated with filter survival other than anticoagulation method. Variables that showed significance in univariable analysis (cutoff p < 0.05) were used for multivariable analysis as confounders. The multivariable, shared frailty Cox model that included anticoagulation method and confounders was examined for non-proportional hazards and we found that the effect of NM changed significantly over time (i.e., demonstrated non-proportional hazards). Therefore, our final model includes the main effect of NM versus RCA, as well as a linear time term for NM to capture changes in the hazard ratio over time. Two-tailed p values were reported for all analyses, and α < 0.05 was considered statistically significant. Point estimates are reported with 95% confidence intervals (95% CIs). We undertook a sub-group analysis among patients with liver dysfunction. Liver dysfunction was defined if the patient had one of the conditions below. As this is post hoc analysis, our data are limited as we do not have information regarding mental status information or bilirubin level: (i) hyperammonemia OR, (ii) INR > 1.5 with > 3 × upper normal ALT OR, (iii) ALT > 5 × upper limit of normal. Using these criteria, 16 patients in RCA group and 58 patients in NM group were identified with liver dysfunction.
We conducted daily uninterrupted CKRT cost comparison between RCA and NM anticoagulation based on a 30 kg patient receiving CKRT with a blood pump flow rate of 150 mL/min (Supplementary file). All statistical analyses were conducted using STATA/IC (version 16.1; StataCorp LLC, College Station, TX, USA).
Results
Patient demographics
Two hundred fifty-three circuits receiving RCA (78 patients) and 202 circuits receiving NM (80 patients) were analyzed (Fig. 1). Baseline characteristics are shown in Table 1. CCHMC and therefore RCA patients were older. The most common primary diagnosis in the CCHMC group was primary kidney disease (23%) with the most common CKRT indication being AKI (55%), while the most common diagnosis in the NM group was liver disease (28%) with metabolic derangement (63%, mostly hyperammonemia) as an indication.
Anticoagulation method, circuit survival, and clotting rates
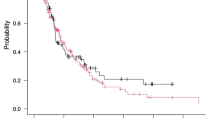
Filter characteristics are shown in Table 2. Median filter life was longer in the NM group (NM: 38 [22, 74] vs. RCA: 36 [17, 66] h, p = 0.02). The elective filter discontinuation rate was 74% for the RCA group vs. 54% in the NM group, and those were censored for Kaplan Meier analysis (Fig. 2). Filter clotting was observed in 45/253 (18%) and 83/202 (41%) for RCA and NM groups, respectively (p < 0.001). Median time to filter clotting was 22 [8, 40] h and 34 [21, 68] h for the RCA and NM groups, respectively. Seventy-one percent of RCA and 54% of NM circuits were functional at 60 h (HR 1.38; 0.84–2.28, p = 0.21), and 69% of RCA and 47% of NM circuits were functional at 72 h (HR 1.51; 0.92–2.47, p = 0.10).
Kaplan–Meier curves of filter survival rate comparing anticoagulation type, RCA vs. NM for the first 72 h. Filters were censored for elective discontinuation or discontinuation unrelated to the filter clot (e.g., due to gain/loss limit or machine failure). Average HR 1.51; 0.92–2.47, p = 0.09 over the 72 h (adjusted for correlation among filters within the same patients). Sixty-hour filter survival; 71% for RCA vs. 54% for NM (HR 1.38; 0.84–2.28, p = 0.21)
Larger filter surface area (m2), catheter diameter (Fr), and lower pre-CKRT platelet count were associated with longer filter survival in univariable analysis (Table S2). We observed no difference in filter survival between the ACG groups after adjusting for filter surface area, catheter diameter, and pre-CKRT platelet count (Table 3).
Complications
Seven RCA patients had evidence of major bleeding including pulmonary, gastrointestinal, cerebral, cerebellar, biopsy site, or catheter site hemorrhage. Four NM patients developed major bleeding including pulmonary and gastrointestinal hemorrhage. Neither major nor minor bleeding rates differed between groups (Table 4). Nineteen filters were removed prior to analysis for anticoagulation switch during the same filter. The reason for the switch from RCA or NM to the others were not due to bleeding.
Eleven RCA patients demonstrated citrate toxicity (serum total calcium to ionized calcium ratio of greater than 2.5), and three patients developed metabolic alkalosis. Eleven patients receiving RCA developed citrate toxicity; all but one of these patients had evidence of liver failure (elevated LFT with an INR > 1.5) and/or was less than 2 years of age. Citrate toxicity was managed by decreasing the citrate infusion rate or increasing the CKRT clearance rate. Five of these 11 cases resulted in RCA discontinuation. Three cases were discontinued due to high total calcium to ionized calcium ratio associated with patient ionized calcium of < 1.0 mmol/L refractory to decreasing citrate infusion rate or increasing CKRT clearance. The other three cases were discontinued due to refractory metabolic alkalosis. Five cases resulted in RCA discontinuation and one case resulted in ACG switch from RCA to UFH which resulted in minor bleeding. No episodes of anaphylaxis were observed in the NM group.
We conducted a subgroup analysis of patients with liver dysfunction: 16 patients (comprising 44 CKRT circuits) receiving RCA and 58 patients (comprising 171 CKRT circuits) receiving NM, were compared (Tables S3 and S4). Patient characteristics in the liver dysfunction sub-group mirrored the comparisons for the overall patient cohorts: patients in the RCA group were older, larger, and started CKRT later in their ICU course. The RCA group had more secondary liver dysfunction and the NM cohort had more primary liver dysfunction. Both groups had only mechanically ventilated patients. The pre-CKRT serum creatinine concentrations were higher in the RCA group while the serum ammonia levels were higher in the NM group (p = 0.0001 for each). The median circuit survival was longer in the NM cohort (38.4 [21.7–76] vs. 22.3 [15.8–55.9] h, p = 0.02).
At the present time, NM anticoagulation is approximately 1/3 the cost of RCA (daily cost $154 vs. $527), although NM is available as a generic form in Japan, whereas there is no generic form of ACD-A™ available in the USA (Supplementary file).
Discussion
We compared the safety and efficacy of NM to RCA for pediatric CKRT using retrospective data from two quaternary pediatric centers. Our data suggest RCA and NM both provide satisfactory anticoagulation for CKRT in children with no significant differences in bleeding.
The difference in unadjusted filter life seems to be largely driven by differences in practice and patient demographics. The RCA group was subject to recommended routine circuit change at 72 h, whereas the NM group was not. The higher circuit clotting rate, yet longer median time to clot for NM likely results from this practice difference. Filter survival analysis showed tendency of longer filter survival with RCA at 60 h and at 72 h, but no difference when adjusted for correlation among filters within the same patients or confounders. The NM group was younger and smaller in size, and thus was prescribed lower blood pump flow rates. Filter survival did not differ between groups after controlling for filter surface area, catheter diameter and pre-CKRT platelet count.
While both a meta-analysis (11 RCT with 992 patients) [23] and single-center prospective study in children (20 patients with 226 circuits) [24] showed longer filter life in RCA over UFH, there is mixed evidence regarding the efficacy in NM comparing UFH in retrospective studies [16, 17]. There are two single-center RCTs of NM vs. no ACG in AKI in a high bleeding risk population and both showed improved filter life in NM over no ACG, without increasing bleeding events [13, 14].
Brain and colleagues reviewed non-anticoagulant factors associated with filter life (n = 7502) in CKRT and showed absence of robust evidence outside of anticoagulation strategies [25]. However, trends favor CVVHDF (or CVVHD) over CVVH. Among CVVH, pre-dilution vs. post-dilution strategies have been compared, and concluded that a pre-dilution strategy is favorable [26, 27]. In our study, distribution of CKRT modalities (more CVVHDF in RCA, more CVVHD in NM, CVVH only seen in NM) and dilution method (pre-dilution in RCA, post-dilution in NM) were different between groups. The mean filtration fraction was higher in the RCA group, which results from less CVVHD use and the higher effluent rate needed to account for volumes of citrate and calcium infusions that are part of RCA. A report from the ppCRRT registry showed an association of vascular access location and size on circuit survival in children [28], and an adult study showed an association between platelet count and circuit survival [29]. Miklaszewska and colleagues reported a surface area association with filter life in a pediatric population [30]. In our study, larger filter surface area (m2), catheter diameter (Fr), and lower pre-CKRT platelet count were associated with increased filter life.
No differences in major bleeding rates were observed between the groups. Both groups had more than 20% of patients with high bleeding risk (platelet counts < 50,000: 27% in RCA, 23% in NM, INR ≥ 2: 27% in RCA, 36% in NM). Bleeding complication rates are reported to be lower for both NM and RCA compared to UFH in multiple studies [16,17,18, 22,23,24]. A single-center retrospective study reported that none of 38 patients in the study who received NM for CKRT experienced major bleeding [11]. The safety of NM in high risk for bleeding patients is reported in two RCTs [13, 14]. Yang and colleagues studied the evolution of intracerebral hemorrhage on intermittent hemodialysis comparing NM to UFH and showed an advantage of NM in this population regarding the size of hemorrhage [9]. NM anticoagulation for extracorporeal membrane oxygenation (ECMO) [31,32,33], cardiac bypass [34], and leukopheresis [35] are also reported comparing UFH. For safety monitoring of NM, a single-center retrospective adult study (n = 76) showed correlation between high post-filter time-weighted ACT and bleeding complications [36]. Post-filter ACT has been a common monitoring method for NM and was used in our study population.
ACD-A™ has a glucose concentration of 225 mg/dL and sodium concentration of 224.4 mEq/L, which can cause hyperglycemia or hypernatremia. RCA requires citrate and calcium infusions that result in a high filtration fraction to remove the associated volume, while the volume for NM anticoagulation is negligible (7.4 ml/kg/h vs. 0.1 ml/kg/h). Reported adverse effects of NM include hyperkalemia, anaphylaxis and agranulocytosis when systemically used for pancreatitis and DIC.
Our study has several strengths. First, it comprises a comparison of 80 and 78 CKRT courses using NM or RCA in children, respectively, which allowed us to assess multiple potential confounders associated with filter survival in pediatric CKRT. Second, we chose to assess the 100 most recent CKRT courses in both institutions starting in June 2019, thereby mitigating bias with respect to ICU and CKRT ACG practice changes at each of the sites. Finally, we excluded circuits that had a change in ACG strategy or were connected to other extracorporeal circuits using UFH for ACG, so we could assess RCA or NM ACG outcomes directly.
Our study has limitations which require us to view our data with caution. First, standards for circuit discontinuation differed between the centers, with a 72-h mandate for the RCA group. Second, the CKRT modalities differed between the groups. Third, the NM group was younger and smaller, and had resultant lower CKRT circuit blood pump flow rates. Fourth, the NM group had almost half of the filters with concomitant use of TPE.
We suggest both NM and RCA can serve well as CKRT anticoagulation strategies with comparable safety. NM can be a viable alternative for patients not eligible for UFH for bleeding risk/developing heparin-induced thrombocytopenia and when RCA is complicated due to the risk of developing citrate toxicity in younger patients, or those with liver dysfunction. In addition, RCA has not been possible during times of national intravenous calcium shortage. Given the heterogeneity between the study groups, a prospective trial of RCA vs. NM anticoagulation in the same patient centers would be optimal to confirm our findings and support our conclusions.
References
(2012) Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group - KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int suppl 2:1–138
Rodriguez K, Srivaths PR, Tal L, Watson MN, Riley AA, Himes RW, Desai MS, Braun MC, Akcan Arikan A (2017) Regional citrate anticoagulation for continuous renal replacement therapy in pediatric patients with liver failure. PLoS One 12:e0182134
Schultheiss C, Saugel B, Phillip V, Thies P, Noe S, Mayr U, Haller B, Einwachter H, Schmid RM, Huber W (2012) Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: a prospective observational study. Crit Care 16:R162
Zhang W, Bai M, Yu Y, Li L, Zhao L, Sun S, Chen X (2019) Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care 23:22
Klingele M, Stadler T, Fliser D, Speer T, Groesdonk HV, Raddatz A (2017) Long-term continuous renal replacement therapy and anticoagulation with citrate in critically ill patients with severe liver dysfunction. Crit Care 21:294
Tan JN, Haroon SWP, Mukhopadhyay A, Lau T, Murali TM, Phua J, Tan ZY, Lee N, Chua HR (2019) Hyperlactatemia predicts citrate intolerance with regional citrate anticoagulation during continuous renal replacement therapy. J Intensive Care Med 34:418–425
Oudemans-van Straaten HM, Ostermann M (2012) Bench-to-bedside review: Citrate for continuous renal replacement therapy, from science to practice. Crit Care 16:249
Chen X, Xu Z, Zeng S, Wang X, Liu W, Qian L, Wei J, Yang X, Shen Q, Gong Z, Yan Y (2019) The molecular aspect of antitumor effects of protease inhibitor nafamostat mesylate and its role in potential clinical applications. Front Oncol 9:852
Yang JW, Han BG, Kim BR, Lee YH, Kim YS, Yu JM, Choi SO (2009) Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage. Ren Fail 31:668–675
Fuse I, Higuchi W, Toba K, Aizawa Y (1999) Inhibitory mechanism of human platelet aggregation by nafamostat mesilate. Platelets 10:212–218
Maruyama Y, Yoshida H, Uchino S, Yokoyama K, Yamamoto H, Takinami M, Hosoya T (2011) Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: a three-year retrospective cohort study. Int J Artif Organs 34:571–576
Hu ZJ, Iwama H, Suzuki R, Kobayashi S, Akutsu I (1999) Time course of activated coagulation time at various sites during continuous haemodiafiltration using nafamostat mesilate. Intensive Care Med 25:524–527
Lee YK, Lee HW, Choi KH, Kim BS (2014) Ability of nafamostat mesilate to prolong filter patency during continuous renal replacement therapy in patients at high risk of bleeding: a randomized controlled study. PLoS One 9:e108737
Choi JY, Kang YJ, Jang HM, Jung HY, Cho JH, Park SH, Kim YL, Kim CD (2015) Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial. Medicine 94:e2392
Baek NN, Jang HR, Huh W, Kim YG, Kim DJ, Oh HY, Lee JE (2012) The role of nafamostat mesylate in continuous renal replacement therapy among patients at high risk of bleeding. Ren Fail 34:279–285
Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY (2013) Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs 36:208–216
Makino S, Egi M, Kita H, Miyatake Y, Kubota K, Mizobuchi S (2016) Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy. Int J Artif Organs 39:16–21
Lee SC, Cho H (2014) The use of nafamostat mesilate as an anticoagulant during continuous renal replacement therapy for children with a high risk of bleeding. J Korean Soc Pediatr Nephrol 18:98–105
Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study Group (2003) PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285
Goldstein SL, Askenazi DJ, Basu RK, Selewski DT, Paden ML, Krallman KA, Kirby CL, Mottes TA, Terrell T, Humes HD (2021) Use of the selective cytopheretic device in critically ill children. Kidney Int Rep 6:775–784
Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O’Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW (2006) Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 117:391–397
Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, Rogers K, Barnett J, Blowey D, Baker C, Bunchman TE, Goldstein SL (2005) Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant 20:1416–1421
Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, Wang P, Li L, Jing R, Zhao L, Sun S (2015) Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med 41:2098–2110
Raymakers-Janssen P, Lilien M, van Kessel IA, Veldhoen ES, Wosten-van Asperen RM, van Gestel JPJ (2017) Citrate versus heparin anticoagulation in continuous renal replacement therapy in small children. Pediatr Nephrol 32:1971–1978
Brain M, Winson E, Roodenburg O, McNeil J (2017) Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrol 18:69
Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R (2003) Pre-dilution vs. post-dilution during continuous veno-venous hemofiltration: impact on filter life and azotemic control. Nephron Clin Pract 94:c94-98
van der Voort PH, Gerritsen RT, Kuiper MA, Egbers PH, Kingma WP, Boerma EC (2005) Filter run time in CVVH: pre- versus post-dilution and nadroparin versus regional heparin-protamine anticoagulation. Blood Purif 23:175–180
Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Alexander SR, Mahan JD, McBryde KD, Benfield MR, Goldstein SL (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121
Dunn WJ, Sriram S (2014) Filter lifespan in critically ill adults receiving continuous renal replacement therapy: the effect of patient and treatment-related variables. Crit Care Resusc 16:225–231
Miklaszewska M, Korohoda P, Zachwieja K, Kobylarz K, Stefanidis CJ, Sobczak A, Drozdz D (2017) Filter size not the anticoagulation method is the decisive factor in continuous renal replacement therapy circuit survival. Kidney Blood Press Res 42:327–337
Han SJ, Han W, Song HJ, Kim CS, Jeong SM, Kang MW (2018) Validation of nafamostat mesilate as an anticoagulant in extracorporeal membrane oxygenation: a large-animal experiment. Korean J Thorac Cardiovasc Surg 51:114–121
Park JH, Her C, Min HK, Kim DK, Park SH, Jang HJ (2015) Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation. Int J Artif Organs 38:595–599
Lim JY, Kim JB, Choo SJ, Chung CH, Lee JW, Jung SH (2016) Anticoagulation during extracorporeal membrane oxygenation; nafamostat mesilate versus heparin. Ann Thorac Surg 102:534–539
Murase M, Usui A, Tomita Y, Maeda M, Koyama T, Abe T (1993) Nafamostat mesilate reduces blood loss during open heart surgery. Circulation 88:II432–436
Sawada K, Ohdo M, Ino T, Nakamura T, Numata T, Shibata H, Sakou J, Kusada M, Hibi T (2016) Safety and tolerability of nafamostat mesilate and heparin as anticoagulants in leukocytapheresis for ulcerative colitis: post hoc analysis of a large-scale, prospective, observational study. Ther Apher Dial 20:197–204
Miyatake Y, Makino S, Kubota K, Egi M, Mizobuchi S (2017) Association between intra-circuit activated clotting time and incidence of bleeding complications during continuous renal replacement therapy using nafamostat mesilate: a retrospective pilot observational study. Kobe J Med Sci 63:E30–E36
Acknowledgements
We thank Dr. Heather Baer (Department of Epidemiology, Harvard T.H. Chan School of Public Health), Dr. John Orav (Department of Biostatistics, Harvard T.H. Chan School of Public Health) and Dr. Takahiro Kinoshita (Philips Research North America) for methodological advice.
Author information
Authors and Affiliations
Contributions
MJM designed the study, analyzed the data, and drafted the paper; KI and KAK contributed to IRB preparation and subject screening; MM and KT contributed data collection; SLG guided the study and critically revised the paper; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MJM serves as a consultant to Lowell Therapeutics, Inc, which has licensed NM in the US. At the current time, NM is not approved for marketing in the US. Lowell did not have input into the design of this study, access to the raw data or development of any aspect of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyaji, M.J., Ide, K., Takashima, K. et al. Comparison of nafamostat mesilate to citrate anticoagulation in pediatric continuous kidney replacement therapy. Pediatr Nephrol 37, 2733–2742 (2022). https://doi.org/10.1007/s00467-022-05502-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05502-8