Abstract
Background
Young children starting kidney replacement therapy (KRT) suffer high disease burden with unique impacts on growth and development, timing of transplantation and long-term survival. Contemporary long-term outcome data and how these relate to patient characteristics are necessary for shared decision-making with families, to identify modifiable risk factors and inform future research.
Methods
We examined outcomes of all children ≤ 5 years enrolled in the Australia and New Zealand Dialysis and Transplant Registry, commencing KRT 1980–2017. Primary outcomes were patient and graft survival. Final height attained was also examined. We used generalized additive modelling to investigate the relationship between age and graft loss over time post-transplant.
Results
In total, 388 children were included, of whom 322 (83%) received a kidney transplant. Cumulative 1-, 5- and 10-year patient survival probabilities were 93%, 86% and 83%, respectively. Death censored graft survival at 1, 5 and 10 years was 93%, 87% and 77%, respectively. Most children were at least 10 kg at transplantation (n = 302; 96%). A non-linear relationship between age at transplantation and graft loss was observed, dependent on time post-transplant, with increased risk of graft loss among youngest recipients both initially following transplantation and subsequently during adolescence. Graft and patient survival have improved in recent era.
Conclusions
Young children commencing KRT have good long-term survival and graft outcomes. Early graft loss is no reason to postpone transplantation beyond 10 kg, and among even the youngest recipients, late graft loss risk in adolescence remains one of the greatest barriers to improving long-term outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kidney failure in childhood is rare and distinct to that among adults [1]. Many children have congenital disorders of the kidney and urinary tract (CAKUT), and some also have congenital abnormalities in other organs [2,3,4]. The impact of kidney failure upon young children is also unique, with the normal patterns of rapid growth and development affected [5, 6].
The impact of kidney failure is likely to be even more profound in the very young. While transplantation is the preferred mode of kidney replacement therapy (KRT), the procedure is technically challenging in young children, who are at an increased risk of surgical complications, including graft thrombosis [7]. Although outcomes have improved more recently, possibly as a result of improved surgical techniques and peri-operative care, transplantation of children weighing less than 10 kg is considered high risk and usually avoided in the absence of strong clinical indications [4, 8, 9]. There are also age-related differences in the immune system, with rapid evolution and maturation during the first few years of life potentially resulting in different immunologic risks for this group [10]. These factors impact access to and outcomes of transplantation.
Both haemodialysis and peritoneal dialysis are potential options for all but the smallest infants. However, successful outcomes require a high level of medical, nursing and caregiver expertise, as well as substantial time and financial resource. The capacity for long-term peritoneal dialysis can be limited by higher rates of infection and chronic damage to the peritoneum. Due to the obligate fluid intake of infant formulas needed to attain adequate nutrition, fluid removal requirements can be high and challenging to achieve in the oligo-anuric child.
The aim of this study was to describe long-term outcomes, including patient and graft survival, among children 5 years and younger starting KRT in Australia and New Zealand since 1980. We also sought to investigate the relationship between age at transplant and graft survival, and whether this has changed across era.
Methods
Population
All children 5 years or younger commencing KRT in Australia and New Zealand were included, using data from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). All paediatric nephrology units in Australia and New Zealand participate in ANZDATA, which collects data from all patients receiving long-term KRT and operates on an ‘opt-out’ consent policy. The history and methods of the registry have previously been described in detail [11]. Key events are reported to the registry as they occur (start/change of dialysis modality, transplantation, graft failure, death) and other data are collected annually. These data are reliable when compared to other sources, such as the Australian National Death Index [12]. Given the relative paucity of data for this cohort and to allow comparison across eras, children commencing KRT between 1980 and 2017 were included. The study was approved by the University of Western Australia Human Research Ethics Committee. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’.
Outcomes
Mortality was reported in real time by participating centres and the most important factor nominated. Graft outcomes are reported yearly and, for the primary analyses in this paper, were considered after censoring for death and for the first transplant only. Total graft survival and the risk of graft loss, treating death as competing risk, were also reported.
Exposures of interest
The main exposure of interest was age, available from the registry. The initial dialysis modality was measured at 1 month, to allow for early changes in modality after patient stabilization. The dates of transplant and graft failure (initial and repeat transplants) were provided. When evaluating the association between graft failure and exposures of interest, only first transplants were included. Data on the following predictors of graft survival were available: recipient sex, recipient weight (to the nearest kilogram), aetiology of kidney failure, time on dialysis prior to transplantation (months), total ischemic time (hours), donor source, donor age, donor sex, donor weight (to the nearest kilogram), delayed graft function (dialysis within 72 h), number of human leukocyte antigen (HLA)-A/B/DR mismatches (one field) and baseline immunosuppression including induction therapy.
Statistical analysis
Normally distributed variables were expressed as mean and standard deviation (SD); otherwise, median and inter-quartile range (IQR) were used. Where groups were compared, an independent 2-samplet test or Wilcoxon rank sum test was used as appropriate. Life tables were used to determine survival and time-to-event data, stratified by clinically important characteristics, and presented with 95% confidence intervals (CI) around the Kaplan-Meier estimator. Graft survival was calculated using the same method. Associations between graft survival and exposures of interest were quantified using generalized additive modelling. Generalized additive models are an extension of generalized linear models, whereby the linear predictor is the sum of several smooth functions [13]. This method produces unbiased estimates of effect with stable and accurate coverage properties using Bayesian confidence intervals [14], an approach that has been validated using data from randomized trials in oncology and nephrology [15]. A key advantage to generalized additive models is the ability to incorporate variables that vary in a non-linear fashion against the outcome and across time (smooth non-linear, smooth time-varying effects), using a penalized smoothing function to minimize overfitting [16]. This is important because, in addition to age being a time-varying covariate that has a non-linear association with graft loss [17], the risk of early graft loss is increased among very young recipients (time-varying coefficient, or effect size), such that a 2-year-old recipient, 1 month following transplantation, has a higher hazard for graft loss at that point in time compared to a patient of the same age but 1-year post-transplant [18]. The impact of era on the association between age at transplant and graft survival was of particular interest given trends observed in other data. Hence, we also constructed a model including year of transplant as an additional smooth non-linear, smooth time-varying effect. Multivariable models were then constructed: firstly adjusting a priori for age at transplant and era, and then adjusting simultaneously for age at transplant, aetiology of kidney failure, era, donor type (deceased or living), donor age and sex and HLA mismatches. Additional covariates were to be included where significant in the era- and age-adjusted model. Delayed graft function was not included in the main analysis because of its role as a mediator on the casual pathway for other covariates such as donor type and era.
Sensitivity analyses included comparing the results of the generalized additive model to those from a relative survival model (piecewise Cox model) that included age as a time-varying covariate with a spline (penalized spline with 4 degrees of freedom) for the relationship between age and risk, producing a non-linear coefficient varying with age as time-dependent covariate, but where the shape of this association was time invariate [19]. The impact of removing graft losses within 30 days, transplantation prior to 1990, and the impact of delayed graft function stratified by donor type were also examined. Further sensitivity analyses included accounting for missing data by multiple imputation prior to constructing a Cox model of graft survival, to examine the impact of any missingness [20]. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R 4.0 (R Core Team, Vienna, Austria), including the generalized additive model R package mcgv [21].
Results
Patient characteristics
In total, 388 children initiated KRT, of whom 322 subsequently received a kidney transplant. The median age at initiation of KRT was 2.1 years (IQR 0.8–3.9). To our knowledge, no potential participants opted out of the registry during the study period. There were more males (64.4%), consistent with CAKUT, including posterior urethral valves, being the most common cause of kidney failure (Table 1). After 12 months of treatment, among the 248 who had not received a transplant, 183 (74%) were receiving peritoneal dialysis. Six patients recovered native kidney function by 12 months (2%), of whom three later returned to KRT.
Survival
There were 4535 person-years of follow-up (median per child 9.8 years, IQR 3.3–18.6 years), during which 77 (20%) patients died at a median age of 3.4 years (IQR 1.7–10.3). Expressed as a rate, crude mortality among the cohort overall was 16.9 per 1000 children per year; this was 25.4 among children less than 2 years old at initiation of KRT, and 11.1 among children 2 years and older at initiation of KRT.
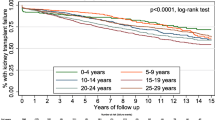
The cumulative 1-, 5- and 10-year survival probabilities were 93% (95% CI 91–96), 86% (95% CI 82–89) and 83% (95% CI 79–87) respectively. Mortality was greater among younger patients (Fig. 1a) and a strong era effect was observed, with improved survival in the more recent transplant eras (Fig. 1b). The 5- and 10-year survival for patients less than 6 months old at initiation of KRT was 65% (95% CI 51–78) and 61% (95% CI 46–76), respectively, improving to a 5-year survival of 72% (95% CI 56–89) in the most recent era (2008–2017). Infection was the most common cause of death overall (n = 27, 35%), followed by cardiovascular events (n = 20, 26%). Among the 26 children who died in the first year after starting KRT, infection was similarly common (n = 11, 42%), with cardiovascular events (n = 4, 15%) and withdrawal of care (n = 4, 15%) the next most frequent causes of death. There were 42 patients (11% of the total cohort) who died prior to receiving a transplant, at a median of 0.7 years after initiating dialysis (IQR 0.4–1.4).
Time to transplantation
The median age at transplantation was 3.6 years (IQR 2.5–5.1), with the youngest recipient aged 11 months (Table 2). Excluding pre-emptive recipients (n = 44; median age 3.7 years, IQR 2.3–4.5), the median time to transplantation was 1.5 years (95% CI 1.2–1.6). The small number of First Nations children in the cohort meant that it was difficult to formally assess any differences in access among these groups. The median time to transplantation was 1.1 years (95% CI 0.1–2.1) among Aboriginal Australian children, 1.8 years (95% CI 0.8–3.2) among Maori children and 2.0 years (95% CI 1.1–2.6) among Pacific children.
The graft was from a living donor in 210 (65%) transplants and from a deceased donor in 112 (35%), with a median donor age of 36 years (IQR 31–43) for living donors and 32 years (IQR 17–43) for deceased donors. About three-quarters of living donors were from a parent (n = 159, 76%). The median ischemic time for deceased donors was 13 h (IQR 9–18). At least one HLA-DR mismatch was present among 227 (76%) donor–recipient pairs.
Transplant outcomes
Death censored graft survival at 1, 5 and 10 years was 93% (95% CI 90–96), 87% (95% CI 83–91) and 77% (95% CI 72–83%) respectively. There was little difference in the 5-year death censored graft survival for children who received a transplant before 2 years of age and those aged 6 years or older, 81% (95% CI 69–93) compared to 83% (95% CI 72–93) (Fig. 1c). The 5-year death censored graft survival improved markedly across era, from 66% pre-1998 (95% CI 47–85) to 99% post-2008 (95% CI 96–100) (Fig. 1d). Few children died with a functioning graft (n = 12, 4% of transplant recipients). Total graft survival at 1, 5 and 10 years was 92% (95% CI 89–95), 84% (95% CI 80–89) and 74% (95% CI 68–79%) respectively. Treating death as a competing risk gave similar estimates (5-year graft survival 87%, 95% CI 83–90).
The proportional hazards assumption was not met for the main explanatory variable of interest, age at transplant, consistent with the repeatedly crossing survival curves when age was stratified (Fig. 1c). Generalized additive modelling confirmed the association between age and graft survival varied with time post-transplant and demonstrated this relationship to be non-linear(Fig. 2). The effect size was similar after adjustment for era and in the final multivariable model (Supplementary Material Figs. S2–3). Era was also a strong predictor of outcome in all models (Table 3). An increasing number of HLA mismatches were associated with poorer outcomes, although the effect size was attenuated after adjustment for age and era. Glomerulonephritis as a cause of kidney failure was associated with a greater hazard for graft loss. An increased risk of graft loss among recipients of deceased donor kidney transplants was seen in the first 10 years after transplantation. However, the survival curves converged after this point, leading to no difference in cumulative risk (Fig. S4).
Age at transplant and death censored graft survival. a The non-linear and time-varying effect of age at transplant as represented by period of follow-up on the x-axis, age at transplant on the z-axis and risk on the y-axis as measured by the Poisson generalized additive model coefficient. This figure shows that younger patients had the highest risk of early graft loss; the risk then fell among all patients before increasing again as children entered adolescence. b Cross-sectional representation of the hazard on the y-axis against time post-transplantation on the x-axis for a child aged 2, 4 or 6 years at transplantation
To further explore if the association between age at transplant and graft survival changed with era, we modelled both age and year of transplant as smooth non-linear, smooth time-varying effects (Supplementary Material, Fig. S5; p < 0.001 compared to era as a categorical variable, p < 0.01 compared to era as smooth non-linear but time invariant effect). The greatest contributor to improved outcomes among children transplanted in more recent era was a reduced risk of early allograft loss.
The final recorded height was higher among patients who received a transplant, and was also higher among patients starting KRT or transplanted in more recent era. The mean height z-scores for those who were not transplanted and who received a transplant (by era) were as follows: no transplant −2.1 (SD 1.8), before 1987 −2.4 (SD 2.1), 1988 to 1997 −2.0 (SD 1.7), 1998 to 2007 −1.7 (SD 1.6) and 2008 to 2017 −1.4 (SD 1.3) (p < 0.001; Fig. 3).
Last recorded height by transplant era. The grey lines are the 5th, 50th and 95th centiles respectively for height according to the Centre for Disease Control reference values [22]. The observations plotted are children’s last recorded height
Repeat transplantation
Of the 117 children with graft failure returning to dialysis, 76 (65%) received a second transplant. For 50 of these (66%) children, the repeat transplant was from a living donor. The median time to repeat transplantation was 2.4 years (95% CI 1.7–3.5); 28 patients received a repeat transplant within 1 year (26%, 95% CI 17–34%) and 44 within 2 years (42%, 95% CI 32–52). There were 14 children who went on to receive three transplants and one patient received four.
Sensitivity analysis
Results from the relative survival model were in close agreement to those obtained by the generalized additive models (Supplementary Material, Table S1, Figs. S6–7). Substituting total graft survival for death censored graft survival made little difference to the reported estimates (Table S2). Delayed graft function was a significant predictor of outcome; however, its inclusion in the multivariable model did not materially alter the other estimates presented (Table S3). Removing patients entering the registry prior to 1990 or with graft loss in the first 30 days following transplantation did not alter the results. The effect size for HLA mismatches did not differ by locus (HLA-A/B/DR).
The fraction of missing data was not more than 11% for any variable except donor weight (70% missing). As a sensitivity analysis, a Cox model was constructed after accounting for missing covariates by multiple imputation. This did not reveal any substantial inconsistencies from the results presented. Missing data fractions, and multiple imputation methods and results are presented in the Supplementary Material (Tables S4–7).
Discussion
These data are important in counselling families about expected outcomes for young children starting KRT. While there remains substantial mortality among very young patients, over 80% of patients went on to receive a kidney transplant, and substantial improvements were observed in both patient and graft survival over time.
The observed 10-year overall survival of 83% among our population is comparable to reports from other regions. The French Pediatric Nephrology Society detailed outcomes among 224 children, less than 2 years old, commencing KRT between 1992 and 2012, with a 10-year survival of 84% [4]. A large cohort including 1998 participants 5 years or younger from the European Society of Pediatric Nephrology, the European Renal Association, and European Dialysis and Transplant Association (ESPN/ERA-EDTA) Registry reported 82% and 88% 10-year survival among children aged 0–1 and 2–5, respectively, at KRT initiation [23]. In that cohort, cardiovascular disease was a slightly more common cause of death compared to infection among children overall, but this likely reflects differences in the mix of KRT, with infection being more common among those with a functioning transplant or on peritoneal dialysis. In our population, the subgroup of very young recipients, less than 6 months old at initiation of KRT, had a substantially lower survival of 65% at 5 years and 61% at 10 years. Survival improved in more recent eras, with a 72% 5-year survival for children less than 6 months initiating KRT between 2008 and 2017. Among infants commencing dialysis aged < 1 month old, participating in the North American Pediatric Renal Trials and Collaborative Studies, the 3-year survival improved from 69% for children starting dialysis between 1992 and 1999 to 79% among children starting between 2002 and 2012 [24]. Also for children < 1 month old, a collaborative study from the ESPN/ERA-EDTA, International Pediatric Peritoneal Dialysis Network, Japanese and ANZDATA registries reported a 76% 5-year survival [25]. These comparator data among young infants are better than reported among our cohort, but consistent with uncertainty about the estimate. As a cross-disease comparator, the 10-year survival for children with acute lymphoblastic leukaemia is now substantially better than that of young children with kidney failure, exceeding 90% [26].
The long-term graft survival rates for patients progressing to transplantation were good, with a 10-year death censored graft survival of 77% over the entire period, and 5-year death censored graft survival of 99% among children transplanted since 2008. The aforementioned French Pediatric Nephrology Society found a 10-year graft survival of 74% [4]. Among children aged less than 11 years in the USA (United Network for Organ Sharing and Scientific Registry of Transplant Recipients), who received a kidney transplant between 2007 and 2011, the 5-year graft survival for living donor kidney recipients was 91%, and between 80 and 85% for deceased donor recipients [27]. Among small recipients, single centres report 10-year graft survival of 80–84%, with more early graft losses among very small (less than 12 kg) recipients and fewer graft losses due to thromboses in more recent era, such analyses being limited by low event frequency [7, 28]. Using generalized additive modelling with a non-linear, time-varying smooth term to account for age at transplant, we were able to demonstrate variation in the effect of age at transplantation post-transplant with time post-transplant. Younger patients had an increased risk of graft loss in the early post-transplant period, which improved through mid-childhood, but then rebounded upon entering adolescence.
It would appear clinicians remain reluctant to transplant very young children, particularly those less than 10 kg. This is supported by our data demonstrating that very young recipients had an increased hazard in the early post-transplant period. However, the risk of early graft loss was less and long-term outcomes improved for those transplanted in more recent era. Furthermore, the risk of transplantation must be balanced against the physical and psychosocial impact of long-term dialysis. As previously described, the risk of graft loss increased markedly during adolescence, independent of other risk factors [17, 18]. Glomerulonephritis as a cause of kidney failure was also a strong predictor of outcome. Recurrent glomerulonephritis is recognized as an important contributor to graft loss among adults, and this finding translated to our population also [29]. Graft survival was better among recipients of living donor kidneys in the first 10 years after transplantation, but the survival curves subsequently converged, attenuating any initial benefit.
The ANZDATA registry includes only children initiating chronic KRT. Hence, data on the number of young children opting for conservative management (no dialysis treatment), or who died in the setting of acute dialysis prior to a decision about long-term treatment intention, were not available. As a result, the estimates of survival provided in this and other registries reported thus far are optimistic as compared to the expected outcome for all children with kidney failure. Inherent to intention-to-treat registries including ANZDATA is the inability at inclusion to define acute kidney injury and chronic disease with absolute certainty, highlighted by the recovery of function in a small number of children in this and other reports [25]. Other limitations included a lack of information on the presence of non-kidney congenital anomalies that may impact mortality and the likelihood of undergoing transplantation [4]. While age at transplant was the main explanatory variable of interest, age and weight are strongly correlated and we were unable to determine which might be the more important predictor of outcome. The usual policy among transplanting units in Australia and New Zealand is to aim for a recipient weight > 10 kg, limiting our ability to draw conclusions about the outcomes of smaller transplant recipients. The use of generalized additive models to investigate age at transplant allowed us to better model the complex relationship with graft loss over time and in relation to other covariates. Generalized additive models including the posterior simulation of confidence intervals surrounding smooth terms are well established [14]. However, hypothesis testing is an area of ongoing development [30]. This might be viewed as a limitation, but for our purposes was outweighed by the advantage of more accurately estimating the true relationship present, and is consistent with recommendations de-emphasizing the importance of hypothesis testing over accurate effect estimates and confidence intervals, which reflect both uncertainty and precision [31, 32]. Another advantage is that the use of time-varying effects does not require the proportional hazards assumption to be met, which is a common problem with the application of other methods to survival data.
This study has important implications for clinicians. We have confirmed that outcomes within Australia and New Zealand are comparable to those internationally, finding long-term patient survival among young children commencing KRT is good with most children receiving a kidney transplant. Survival was better among children in more recent era. The reasons for this are unclear, but in addition to gradually improving dialysis technology, the development and experience of multidisciplinary teams caring for children with kidney failure are likely to have played an important role [33]. Graft survival also improved substantially, mostly due to better early transplant outcomes. There are several potential explanations for this, including improved surgical techniques, peri-operative care and immunosuppression. Yet, the substantial, age-dependent hazard observed during adolescence has changed little. These data would support efforts to determine and establish effective evidence-based interventions for improving adolescent transplant outcomes, such as tailored young adult clinics, which provide peer-connection opportunities and promote positive psychosocial and behavioural traits, including engagement and resilience [34, 35].
Outcomes for small children commencing KRT have improved substantially, with excellent patient and graft outcomes. Yet, despite advances, high mortality rates for the youngest children remain, with only two-thirds of children starting KRT at less than 6 months of age surviving 10 years following initiation of KRT. Similarly, graft loss among young recipients subsequently entering adolescence remains high, with a time-specific risk exceeding that of any other period post-transplant.
References
McDonald SP, Craig JC (2004)Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662
Orr NIT, McDonald SP, McTaggart S, Henning P, Craig JC (2009) Frequency, etiology and treatment of childhood end-stage kidney disease in Australia and New Zealand. Pediatr Nephrol 24:1719–1726
Chesnaye NC, Schaefer F, Groothoff JW, Bonthuis M, Reusz G, Heaf JG, Lewis M, Maurer E, Paripović D, Zagozdzon I, van Stralen KJ, Jager KJ (2016) Mortality risk in European children with end-stage renal disease on dialysis. Kidney Int 89:1355–1362
Hogan J, Bacchetta J, Charbit M, Roussey G, Novo R, Tsimaratos M, Terzic J, Ulinski T, Garnier A, Merieau E, Harambat J, Vrillon I, Dunand O, Morin D, Berard E, Nobili F, Couchoud C, Macher MA, French Pediatric Nephrology Society (2018) Patient and transplant outcome in infants starting renal replacement therapy before 2 years of age. Nephrol Dial Transplant 33:1459–1465
Wightman AG, Freeman MA (2016) Ethics series update on ethical issues in pediatric dialysis: has pediatric dialysis become morally obligatory? Clin J Am Soc Nephrol 11:1456–1462
Rees L (2013) Paediatrics: infant dialysis--what makes it special? Nat Rev Nephrol 9:15–17
Chavers BM, Rheault MN, Matas AJ, Jackson SC, Cook ME, Nevins TE, Najarian JS, Chinnakotla S (2018) Improved outcomes of kidney transplantation in infants (age < 2 years): a single-center experience. Transplantation 102:284–290
Van Arendonk KJ, Boyarsky BJ, Orandi BJ, James NT, Smith JM, Colombani PM, Segev DL (2014) National trends over 25 years in pediatric kidney transplant outcomes. Pediatrics 133:594–601
Chiodini B, Herman J, Lolin K, Adams B, Hennaut E, Lingier P, Mikhalski D, Schurmans T, Knops N, Wissing KM, Abramowicz D, Ismaili K (2018) Outcomes of kidney transplantations in children weighing 15 kilograms or less: a retrospective cohort study. Transpl Int 31:720–728
Dharnidharka VR, Fiorina P, Harmon WE (2014) Kidney transplantation in children. N Engl J Med 371:549–558
McDonald SP (2015) Australia and New Zealand Dialysis and Transplant Registry. Kidney Int Suppl (2011) 5:39–44
Sypek MP, Dansie KB, Clayton P, Webster AC, McDonald S (2019) Comparison of cause of death between Australian and New Zealand Dialysis and Transplant Registry and the Australian National Death Index. Nephrology 24:322–329
Bender A, Groll A, Scheipl F (2018) A generalized additive model approach to time-to-event analysis. Stat Model 18:299–321
Marra G, Wood SN (2012) Coverage properties of confidence intervals for generalized additive model components. Scand J Stat 39:53–74
Argyropoulos C, Unruh ML (2015) Analysis of time to event outcomes in randomized controlled trials by generalized additive models. PLoS One 10:e0123784
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686
Ritchie AG, Clayton PA, McDonald SP, Kennedy SE (2018)Age-specific risk of renal graft loss from late acute rejection or non-compliance in the adolescent and young adult period. Nephrology 23:585–591
Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA (2011) Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92:1237–1243
Foster BJ, Mitsnefes MM, Dahhou M, Zhang X, Laskin BL (2018) Changes in excess mortality from end stage renal disease in the United States from 1995 to 2013. Clin J Am Soc Nephrol 13:91–99
Larkins NG, Craig JC, Teixeira-Pinto A (2018) A guide to missing data for the pediatric nephrologist. Pediatr Nephrol 34:223–231
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. CRC press, New York
Barlow SE, Expert Committee (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120(Suppl 4):S164–S192
Chesnaye NC, van Stralen KJ, Bonthuis M, Harambat J, Groothoff JW, Jager KJ (2018) Survival in children requiring chronic renal replacement therapy. Pediatr Nephrol 33:585–594
Carey WA, Martz KL, Warady BA (2015) Outcome of patients initiating chronic peritoneal dialysis during the first year of life. Pediatrics 136:e615–e622
van Stralen KJ, Borzych-Dużalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, Inward C, Rönnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P, ESPN/ERA-EDTA registry; IPPN registry; ANZDATA registry; Japanese RRT registry (2014) Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86:168–174
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373:1541–1552
Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, Wainright JL, Haynes CR, Snyder JJ, Kasiske BL, Israni AK (2018)OPTN/SRTR 2016 annual data report: kidney. Am J Transplant 18(Suppl 1):18–113
Lee E, Ramos-Gonzalez G, Staffa SJ, Rodig N, Vakili K, Kim HB (2019) Perioperative renal transplantation management in small children using adult-sized living or deceased donor kidneys: a single-center experience. Pediatr Transplant 23:e13553
Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, Teixeira-Pinto A, Wong G (2017) Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int 92:461–469
Wood SN (2012) On p-values for smooth components of an extended generalized additive model. Biometrika 100:221–228
Chavalarias D, Wallach JD, Li AH, Ioannidis JP (2016) Evolution of reporting p values in the biomedical literature, 1990-2015. JAMA 315:1141–1148
Wasserstein RL, Lazar NA (2016) The ASA statement on p-values: context, process, and purpose. Am Stat 70:129–133
Rees L, Schaefer F, Schmitt CP, Shroff R, Warady BA (2017) Chronic dialysis in children and adolescents: challenges and outcomes. Lancet Child Adolesc Health 1:68–77
Tong A, Gow K, Wong G, Henning P, Carroll R (2015) Patient perspectives of a young adult renal clinic: a mixed-methods evaluation. Nephrology 20:352–359
Quinn SM, Fernandez H, McCorkle T, Rogers R, Hussain S, Ford CA, Barg FK, Ginsburg KR, Amaral S (2019) The role of resilience in healthcare transitions among adolescent kidney transplant recipients. Pediatr Transplant 23:e13559
Acknowledgements
The data reported here have been supplied by the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA).
Funding
WHL and NGL are supported by a Clinical Research Fellowships from the Raine Foundation (University of Western Australia and Health Department of Western Australia), and (WHL) Jacquot Research Foundation (Royal Australasian College of Physicians). GW is supported by a National Health and Medical Research Council Career Development Fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, data interpretation and manuscript preparation. NGL performed the data analysis. All authors approved the final version for publication and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the Australia and New Zealand Dialysis and Transplant Registry.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 871 kb)
Rights and permissions
About this article
Cite this article
Larkins, N.G., Wong, G., Alexander, S.I. et al. Survival and transplant outcomes among young children requiring kidney replacement therapy. Pediatr Nephrol 36, 2443–2452 (2021). https://doi.org/10.1007/s00467-021-04945-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-04945-9