Abstract
Background
Although peritonitis causes significant morbidity and mortality in children receiving chronic peritoneal dialysis (CPD), little is known about costs associated with treatment.
Methods
We analyzed 246 peritonitis-related hospitalizations in the USA, linked by the Standardized Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) and Pediatric Health Information Systems (PHIS) databases. Multivariable logistic regression was used to assess the relationship between high-cost hospitalizations (at or above the 75th percentile) and patient characteristics. Multivariable modeling was used to assess differences in the service-line specific geometric mean between (1) high- and low-cost (below the 75th percentile) hospitalizations and (2) fungal versus other types of peritonitis. Wage-adjusted hospitalization charges were converted to estimated costs using reported cost-to-charge ratios to estimate the cost of hospitalization.
Results
High-cost hospitalizations were associated with the following: age 3–12 years, Hispanic ethnicity, intensive care unit (ICU) stay, length of stay (LOS), and fungal peritonitis. Whereas absolute standardized cost by service line was significantly different when comparing high- and low-cost hospitalizations, the percentage of total cost by service line was similar in the two groups. Cost per case for fungal peritonitis was higher (p < 0.001) in every service line except pharmacy when compared to other peritonitis cases. The median (IQR) cost of hospitalization for the treatment of peritonitis was $13,655 ($7871, $28434) USD.
Conclusions
Hospitalization-related costs for peritonitis treatment are substantial and arise from a variety of service lines. Fungal peritonitis is associated with high-cost hospitalization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common dialysis modality for children with end stage kidney disease (ESKD) throughout the world is chronic peritoneal dialysis (CPD), and it accounts for 37% of prevalent pediatric dialysis patients in the USA [1, 2]. Peritonitis and other CPD-related infections cause significant morbidity, as well as mortality in this population. Despite efforts to the contrary, between 2005–2009 and 2010–2014, there was a 13.1% increase in PD infection-related hospitalizations reported by the U.S. Renal Data System (USRDS). Additionally, during the same time, the hospitalization rate for infection in pediatric CPD patients was higher than the rates of hospitalization for infection in pediatric hemodialysis (HD) and transplant patients, respectively [2]. Although the infection-related mortality rate has declined in all pediatric ESKD patients, the 1-year adjusted mortality rate reported by the USRDS was still highest in pediatric PD patients when compared to HD and transplant patients [2]. Furthermore, reports from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) and the International Pediatric Peritonitis Network (IPPN) have both shown that peritonitis is a leading cause of dialysis modality change [3, 4].
The fiscal impact of ESKD management is also substantial, with the annual cost of ESKD care in the USA now exceeding 30 billion dollars [2]. In the case of ESKD patients on CPD, there are unique fiscal implications pertaining to the development and treatment of peritonitis. Clinical issues range from brief hospitalizations for initiation of antibiotic therapy to the need for PD catheter removal and transition to HD, dependent on the severity of the infection and the responsiveness of the patient to therapy. Although peritonitis rates in children are generally higher than those experienced in adults and are in turn, associated with a substantial cost for the pediatric CPD population, only a single study from Canada out of a regional tertiary care center and published more than two decades ago has addressed the issue of cost associated with peritonitis in pediatric patients [5]. While the authors of this study were able to examine costs associated with hospitalization for peritonitis, they only reported on what was attributed to direct costs and hospital-level overhead costs and were not able to report on what factors contributed to higher cost hospitalizations.
The Standardized Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative is a quality improvement initiative coordinated by the Children’s Hospital Association (CHA) and is comprised of a group of pediatric dialysis programs in the USA working together to reduce peritonitis rates in pediatric CPD patients by implementation of standardized PD catheter care bundles [6]. Through this effort, the collaborative has been able to demonstrate a statistically significant reduction in the rate of peritonitis [7]. At the same time, the collection of data pertaining to the treatment of peritonitis by the participating sites, coupled with information collected in the Pediatric Health Information Systems (PHIS) database, has made it possible to describe factors associated with high-cost hospitalizations and to estimate the per patient cost of the inpatient management of peritonitis in pediatric CPD patients in the USA.
Methods
SCOPE Collaborative
The SCOPE Collaborative is a partnership between the CHA and their quality improvement resources, a multi-disciplinary, multi-institutional core faculty, and the physicians, nurses, and staff from 45 participating pediatric dialysis programs throughout the USA. Data collected between October 1, 2011 and September 30, 2015 were included in this analysis. There were 30 hospitals participating in SCOPE during this time, listed in Table 3. Details of the structure of the collaborative and the quality improvement process used to assist teams in the implementation of standardized catheter care practices have been previously described [6]. The SCOPE Collaborative and its participating centers follow the Declaration of Helsinki. The SCOPE protocol was reviewed by an Institutional Review Board (IRB) at each center.
Pediatric Health Information System
PHIS is an administrative database maintained by the CHA (Lenexa, KS) that contains discharge data (demographics, diagnosis codes, procedure codes) and billing data from 49 tertiary pediatric hospitals in the USA. Professional and ambulatory charges are not captured in PHIS. Data warehousing for PHIS is managed by IBM Watson/Truven Health Analytics (Ann Arbor, MI), and data are subjected to validity and reliability checks prior to incorporation into the PHIS database. Whereas data are de-identified, all children in the database are assigned a unique, encrypted patient identifier so that they can be followed across multiple encounters.
Measures and data
Patients were entered into the SCOPE database following the insertion of a PD catheter for the performance of chronic dialysis. Demographic data collected from all patients entered into the SCOPE database included the following: age, race (White, Black, Hispanic, or other), sex, and cause of ESKD. Data on the number of catheters, number and microbiology of peritonitis episodes, outcome of peritonitis episodes, and compliance with SCOPE recommended catheter care bundle elements were collected at monthly intervals following the collaborative launch. Infection-related costs were obtained by linking the SCOPE data with the PHIS database.
Twenty-five of the 30 hospitals participating in SCOPE during the study period were also participating in PHIS and had information available for analysis. Peritonitis episodes from SCOPE were successfully linked to PHIS hospitalizations from 23 of these 25 centers (Fig. 1). A peritonitis episode was defined as an index peritonitis infection as well as any related relapses. The criteria used to diagnose peritonitis and a relapsing infection were as described in the 2012 update to the Consensus Guidelines for the Prevention and Treatment of Catheter-related Infections and Peritonitis in Pediatric Patients Receiving Peritoneal Dialysis, published by the International Society for Peritoneal Dialysis (ISPD) [8].
Peritonitis episodes from SCOPE-participating centers were linked indirectly with administrative and billing data from hospitalizations recorded in PHIS on the basis of the following: month of birth, year of birth, sex, date of peritonitis episode, and date of hospital admission. PHIS hospitalization records were required to have a diagnosis code indicating ESKD. The date of admission in PHIS was required to be within ± 30 days of the peritonitis infection date reported in SCOPE. Only SCOPE peritonitis episodes that could be uniquely linked to a PHIS hospitalizations based on the criteria described above were included in the analysis; peritonitis episodes that could potentially be linked to hospitalizations for more than one patient were excluded. Hospitalizations for both index peritonitis infections and relapses were included. Peritonitis episodes occurring during a PHIS hospitalization, but more than 48 h after admission were excluded from the analysis as peritonitis was not considered the cause of the hospitalization.
Statistical considerations
Frequencies and percentages were used to summarize categorical descriptive statistics; medians, first quartiles (Q1), and third quartiles (Q3) were used to summarize continuous descriptive statistics. Low-cost hospitalizations (below the 75th percentile) for treatment of peritonitis were compared with high-cost hospitalizations (at or above the 75th percentile) for treatment of peritonitis. The cost of hospitalization for treatment of fungal peritonitis was compared with the cost of hospitalization related to the treatment of all other (bacterial and culture negative) episodes of peritonitis. Costs by service line were examined in the following categories: pharmacy, lab, imaging, supply, clinical (includes dialysis-related costs), and other (room and board and intensive care unit). Categorical characteristics were compared using a chi-square test of association and continuous data were compared using a Wilcoxon rank-sum test. Multivariable logistic regression was used to assess the relationship between high-cost hospitalizations and patient characteristics. We also modeled the geometric mean of service-line costs using multivariable modeling techniques to compare differences in cost between (1) high-cost versus low-cost hospitalization and |(2) fungal versus other types of peritonitis. All multivariable models included a random hospital effect to account for clustering of peritonitis episodes within hospital. All analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, NC), and p values < 0.05 were considered statistically significant.
Outcome
The outcome measure for this analysis was cost of hospitalization for peritonitis in pediatric CPD patients participating in a multi-center quality improvement collaborative. Charges in PHIS are adjusted for the wage and price index (published annually in the Federal Register) to account for cost-of-living-differences; wage-adjusted hospitalization charges in PHIS were converted to estimated costs using reported cost-to-charge ratios either collected from a hospital’s Medicare cost report or reported directly by the hospital to CHA. This costing methodology has been described previously by Schwartz et al. [9]. Service-line level clinical charges were used to estimate hospital costs whenever possible. For hospitalizations in which clinical charges were missing (N = 32), aggregated charges were used to estimate costs.
Results
Overall, 553 peritonitis episodes were reported by SCOPE during the period of observation and 266 of those peritonitis episodes were linked with 278 PHIS hospitalizations. Of these 278 hospitalizations, specific service-line clinical charges were reported for 246 (N = 180 low-cost; N = 66 high-cost); 32 hospitalizations with missing clinical charges were omitted from the service-line analysis. See Fig. 2 for schematic explanation of hospitalizations included in cost analysis.
Cost of hospitalization for peritonitis and factors associated with high-cost hospitalizations
The median (IQR) cost of hospitalization for the treatment of peritonitis was $13,655 ($7871, $28434) USD. By univariate analysis, factors significantly associated with high-cost hospitalizations were as follows: patient age between 3 and 12 years, Hispanic race, intensive care unit (ICU) stay, and fungal peritonitis (Table 1). These factors remained significantly associated with high-cost hospitalizations even after adjustment in multivariable logistic regression modeling (p < 0.005 for all factors). Figure 3 shows a cost distribution of those factors. Additionally, length of stay (LOS) correlated with high-cost hospitalization. The median (Q1, Q3) LOS among high-cost hospitalizations was 13 (9, 25.5) days, whereas it was 4 (2, 5) days in low-cost hospitalizations (p < 0.001).
Services associated with high-cost hospitalization for peritonitis
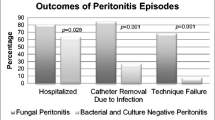
High-cost (n = 66) and low-cost (n = 180) hospitalizations for peritonitis were compared by service line, and standardized costs were found to be significantly different (p < 0.001) in every service line (Table 2). Despite these differences in absolute cost, the percentage of total costs by service line was similar for the high-cost and low-cost hospitalizations for the following: pharmacy (17 vs 15%, p = 0.63), lab (6 vs 7%, p = 0.30), imaging (3 vs. 3%, p = 0.85), supply (3 vs. 3%, p = 0.98), clinical (26 vs. 30%, p = 0.33), and other (44 vs. 47%, p = 0.18). Hospitalization cost per case of fungal peritonitis was significantly higher than for all other causes of peritonitis for every service line except pharmacy (Fig. 4).
Discussion
In this analysis we found the following factors to be associated with high-cost hospitalization: age between 3 and 12 years, Hispanic race, ICU stay, length of stay, and fungal peritonitis. When hospitalizations were broken down by service line, we found that although the absolute cost per service line was significantly different in all categories when low- and high-cost hospitalizations were compared, the percentage of costs by service line was the same between the two groups. Noteworthy is the finding that hospitalization cost per case of fungal peritonitis was significantly higher in every service line examined except pharmacy, likely due to a more prolonged hospitalization, a higher acuity necessitating an ICU stay, the need for removal of the PD catheter and placement of an HD catheter, and in-hospital hemodialysis costs that are typically associated with management of fungal peritonitis. The association of Hispanic ethnicity with high-cost hospitalization was also unique and further study is required to delineate factors that may contribute to this finding. We found the median cost of hospitalization for treatment of peritonitis in a pediatric PD patient in the USA to be $13,655 ($7871, $28434) USD.
Our findings represent one of only two studies to date that have analyzed the costs associated with peritonitis in pediatric CPD patients. Coyte et al. published an economic evaluation of the two types of ambulatory dialysis provided to pediatric patients in Canada (hospital-based ambulatory HD and home-based PD, including both CCPD and CAPD) in which they reported an annualized increase in the cost of care per patient due to peritonitis to be roughly ~ $2546 USD. This cost estimate differs from those presented in our analysis as their estimates represented the financial burden on their entire CPD population, in contrast to our figure which was determined by using costs pertaining only to those patients who were hospitalized for treatment of peritonitis. Whereas the analysis by Coyte et al. limited cost of care data to those patients over the age of 2 years and those weighing more than 20 kg, our analysis included patients of all ages and did not have a weight cutoff. The Canadian study included both direct and indirect costs and derived their estimate using data from a single tertiary care center collected over only 1 year, while our analysis was more representative of the spectrum of costs associated with the treatment of peritonitis as we included data from 23 pediatric dialysis programs over a period of 4 years and evaluated only direct costs. Most importantly, our estimate included costs of inpatient dialysis while theirs did not. Finally, their costs estimates are over 20 years old, were not based on patient-level utilization, and were not based on charges, while our cost estimates were based on the ratio of cost to charge (RCC) methodology and reflect patient-level utilization of resources across multiple service lines during a hospitalization [5, 9].
In addition to our analysis and the analysis by Coyte et al., two studies examining the economic impact of peritonitis in adults have been published. In 1990, Piraino et al. estimated the median total cost per hospitalization for peritonitis with associated exit site or tunnel infections at $5122 USD. This study looked at all of the patients in one US center’s dialysis program over a 6-month period; in this timeframe, 12 of their patients were diagnosed with peritoneal dialysis catheter-related infections, 4 of whom were hospitalized with peritonitis [10]. Costs were determined using charge data from billing records and patient charts. Only direct costs were included in this analysis and it is explicitly stated that dialysis-related costs (which made up a substantial proportion of the costs in our analysis, especially in the high-cost cases) were excluded. The largest expenses attributable to their peritonitis cases were those related to hospitalization and diagnostic testing, which is similar to our analysis.
In the most recent study of costs associated with peritonitis, Makhija et al. described fiscal data associated with their peritoneal dialysis continuous quality improvement program in Colombia and found that the cost per case of peritonitis was ~ $250 USD. This analysis included both hospitalized and ambulatory patients. To generate their cost estimate, the authors used patient-level data available for all patients seen at Renal Therapy Services (RTS) Clinics, comparing 200 cases with peritonitis and 200 controls without peritonitis, from 2006 to 2014; this analysis only included patients 18 years of age or older. As RTS records do not include hospital visits, a subset of patients from RTS that were also in the Coomeva EPS (public health insurance provider) were identified and linked to obtain data related to hospitalizations. Costs were determined using a case control (199 cases and 199 controls) analysis by subtracting the weekly cost of care for patients without peritonitis from the weekly cost of care for patients with peritonitis, converting to a per episode cost based on the average duration of peritonitis, which was based on antibiotic prescription information. Of the costs attributable to peritonitis, 68% were related to hospitalization. The largest components of hospitalization costs were pharmacy, supplies, and PD solutions. Data were not available regarding what proportion of patients were hospitalized [11].
As can be seen from the three previously published studies reported above, there is considerable variation in the reported economic impact of peritonitis depending on what service lines are included in the analysis, whether direct and/or indirect costs are included, and how costs are determined. Other factors that make cost comparisons from the published data difficult are the varying sizes of the patient numbers included in each analysis, as well as the recognition that some of the analyses were single-center over short timeframes and some were multi-center over longer time frames. Despite these differences, our analysis found hospitalization-related costs and pharmacy costs to be significant contributors to total cost, which is similar to the findings from other studies described above.
Our contribution to the literature is unique for several reasons. First, our data provide an estimate of the cost of hospitalization due to peritonitis derived from patient-level data from multiple pediatric centers, over multiple years. Additionally, since delivery of hemodialysis is generally more expensive than delivery of peritoneal dialysis, our data on costs related to dialysis of patients hospitalized with peritonitis helps to account for one of the larger expenditures related to peritonitis that was not necessarily accounted for in the other studies. Finally, but very importantly, our study is the first to describe factors associated with high-cost hospitalization for peritonitis. While these findings are likely applicable to pediatric dialysis care outside the USA as well, further study of the economics of peritonitis management in children in other geographic regions should be encouraged.
Our study has several limitations. Matching of SCOPE cases of peritonitis with PHIS hospitalizations was done on the basis of month of birth, year of birth, sex, and dates of peritonitis episode and hospital admission. There was also a requirement for the PHIS hospitalization to have the diagnosis code of ESKD in order to be included in the analysis, which might have led to missed cases, as the incidence of errors in clinical coding is well documented in the literature and is widespread [12, 13]. Indirect linkage between SCOPE peritonitis cases and PHIS hospitalizations could potentially result in missed matches (which would mean missed cases) or mismatches (cases of peritonitis matched with an incorrect hospital encounter). Data routinely captured by SCOPE suggests that 62% of all peritonitis cases require hospitalization. Therefore, for 553 episodes of peritonitis reported during the study period, approximately 342 hospitalizations would be anticipated. Given that this analysis includes a total of 278 hospitalizations, we were able to capture roughly 80% of expected peritonitis-related hospitalizations. Although the RCC cost methodology is well-described, does well with estimating average costs per diagnosis related group (DRG), and also has good reliability in comparing relative costs for patients in a DRG in one hospital to the average cost of patients in the same DRG in a group of hospitals, it can over- or under-estimate costs by up to 10% of the gold standard of RVU-calculated costs. Additionally, RCC cost methodology has found to be inaccurate for predicting costs of a single hospitalization, but has been shown to perform well when estimating average costs [9]. Finally, cost estimates included in this study are generalizable only within the USA. Differences in healthcare models, access to care, and services covered by insurance between the USA and other healthcare markets preclude any generalization of our cost estimates to other countries.
Despite the fact that the costs we reported are specific to the US health care system, we believe, as noted above, that our findings on factors associated with high-cost hospitalization are generalizable. In summary, the considerable additional cost of hospitalization due to the treatment of peritonitis highlights the importance of continued peritonitis prevention efforts in both the inpatient and outpatient setting, and provides justification for the cost of quality initiatives aimed at decreasing peritonitis like those conducted by SCOPE and the Colombian health care system. Fungal peritonitis in particular has substantial clinical consequences and is likely associated with a much higher cost than other types of peritonitis in countries besides the USA given the its association with hemodialysis and longer hospital stay. Additional efforts should thus be directed at reducing this specific type of peritonitis, which if successful would not only reduce the cost of care per patient in a PD population, but would also reduce overall ESKD management costs, given that patients with fungal peritonitis more commonly need to abandon peritoneal dialysis. Further investigation should be conducted to delineate factors that may contribute to the association of higher cost hospitalization with Hispanic race and in the non-infant age group.
References
Fadrowski JJ, Alexander SR, Warady BA (2012) The demographics of dialysis in children pediatric dialysis. In: Warady BA, Schaefer F, Alexander SR (eds) Pediatric Dialysis, 2nd edn. Springer, New York, pp 37–52
United States Renal Data System (2017) 2017 USRDS annual data report: volume 2: end-stage renal disease in the United. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
2011 NAPRTCS Annual Report. North American Pediatric Renal Trials and Collaborative Studies website https://web.emmes.com/study/ped/announce.htm. Accessed February 5, 2018
Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, Mir S, Ha IS, Fischbach M, Simkova E, Watson AR, Moller K, von Baum H, Warady BA (2007) Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 72(11):1374–1379
Coyte PC, Young LG, Tipper BL, Mitchell VM, Stoffman PR, Willumsen J, Geary DF (1996) An economic evaluation of hospital-based hemodialysis and home-based peritoneal dialysis for pediatric patients. Am J Kidney Dis 27(4):557–565
Neu AM, Miller MR, Stuart J, Lawlor J, Richardson T, Martz K, Rosenberg C, Newland J, McAfee N, Begin B, Warady BA, SCOPE Collaborative participants (2014) Design of the standardizing care to improve outcomes in pediatric end stage renal disease collaborative. Pediatr Nephrol 29(9):1477–1484
Neu AM, Richardson R, Lawlor J, Stuart J, Newland J, McAfee N, Warady BA, SCOPE Collaborative Participants (2016) Implementation of standardized follow-up care significantly reduces peritonitis in children on chronic peritoneal dialysis. Kidney 89(6):1346–1354
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yak HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32(Suppl 2):S32–S86
Shwartz M, Young DW, Siegrist R (2015–2016) The ratio of costs to charges: how good a basis for estimating costs? Inquiry 32(4):476–481
Piraino B, Bernardini J, Jonston JR (1990) Cost analysis of peritoneal catheter infections. Perit Dial Int 10(3):241–242
Makhija DU, Walton SM, Mora JP, Sanabria RM (2017) Economic impact of a peritoneal dialysis continuous quality improvement program in Colombia. Perit Dial Int 37(2):165–169
Hsia DC, Krushat WM, Fagan AB, Tebbutt JA, Kusserrow RP (1988) Accuracy of diagnostic coding for Medicare patients under the prospective payment system. N Engl J Med 318(6):352–355 Erratum in N Eng J Med (1990) 322(21)
Heywood NA, Gill MD, Charlwood N, Brindle R, Kirwan CC, Northwest Research Collaborative (2016) Improving accuracy of clinical coding in surgery: collaboration is key. J Surg Res 204(2):490–495
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The Institutional Review Board (IRB) at each participating center approved the collaborative protocol and informed consent was obtained where required by the institution’s IRB.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SCOPE Investigators: complete list of participating centers available in Table 3 in the Appendix
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Redpath Mahon, A.C., Richardson, T., Neu, A.M. et al. Factors associated with high-cost hospitalization for peritonitis in children receiving chronic peritoneal dialysis in the United States. Pediatr Nephrol 34, 1049–1055 (2019). https://doi.org/10.1007/s00467-018-4183-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4183-0