Abstract
Background
Childhood steroid-sensitive nephrotic syndrome (SSNS) has previously been assumed to be a disease of childhood. This has been challenged by few studies reporting that some patients with childhood SSNS may continue to relapse into adulthood. The aim of this study was to investigate the long-term outcome of childhood SSNS presenting data from an unselected well-defined cohort of Danish patients.
Methods
We conducted a retrospective study of the clinical outcome from a population of patients consecutively admitted to the pediatric departments in the central and northern region of Denmark from 1998 to 2015. Patients were followed until August 2017. Data were collected from the patient’s medical records.
Results
Long-term outcome was studied in 39 adult patients with childhood onset SSNS. A total of 31% (12/39) had active disease in adulthood. Univariate analysis showed that more severe forms of SSNS (e.g., steroid dependent/frequent relapsing (SD/FR) nephrotic syndrome) in childhood were associated with active disease in adulthood. Comparing adult patients with SD/FR showed a significantly higher number of relapses/patient/year from late childhood and adolescence in the group with active disease vs. non-active disease (1.06 (95%CI: 0.32–1.81) vs. 0.19 (95%CI: 0.06–0.31, p = 0.005).
Conclusion
In general, one third of all patients with SSNS during childhood continue to have active disease during early adulthood, in particular patients with SD/FR continue to suffer from active disease. The present data illustrates that SSNS is not just a disease of childhood but persists in adulthood in a significant number of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome (INS) is a common condition in pediatric nephrology. INS is, however, a rare condition in the society, with an annual incidence of 2–7 cases per 100,000 children below the age of 16 years [1,2,3]. Although childhood steroid-sensitive nephrotic syndrome (SSNS) is characterized by steroid responsiveness in > 80% of cases [4], approximately 80–90% of the patients relapse after their first episode of nephrotic syndrome (NS) [5], and 40–50% of these children develop a steroid-dependent (SD) or frequent relapsing (FR) nephrotic syndrome [4, 6, 7]. Only few studies regarding the long-term outcome of childhood SSNS have been published. Previously, the long-term outcome of childhood SSNS has been considered excellent. Studies from the early 1980s have reported that no more than 10% of patients with childhood onset minimal change primary NS relapses into adulthood, and the vast majority of patients come of age with regard to sustained remission [5, 8]. However, this has recently been challenged by studies reporting that 33–42% of children with SSNS relapse until adolescence and into adulthood despite treatment with immunosuppressive drugs [9, 10]. The present study was undertaken to investigate the clinical long-term prognosis of childhood SSNS from an unselected population of children residing in a well-defined geographic area of Denmark.
Methods
Study population
Clinical data were obtained from medical records of 39 patients with childhood onset SSNS admitted to one of the six pediatric departments in the central and northern region of Denmark. Standard computerized inpatient and outpatient statistics were used to identify patients coded with ICD-10 codes associated with NS (N04.0-N04.9) and/or proteinuria (R80). The search was performed in the Danish National Patient Registry [11] covering the period from January 1, 1998 to December 31, 2015. All patients listed from the search were evaluated in relation to the inclusion and exclusion criteria in order to detect any misclassification of the patients. Patients included in this study were followed until last visit regarding NS or until last day follow-up (August 1, 2017).
Due to national consensus regarding treatment of children with NS, none of these patients are treated by general practitioners but at the hospital by pediatricians. The pediatric departments cover all pediatric hospital service in this area of Denmark. They serve as secondary referral clinics. One of the centers additionally serves as a tertiary referral center in this geographical area.
Inclusion and exclusion criteria
The inclusion criteria comprised of (1) a fulfilled definition of SSNS, (2) age at onset < 15 years, (3) residency in the geographic area covered by the six pediatric departments, and (4) > 18.0 years of age at follow-up (August 2017). The patients were excluded from the study if (1) they were referred to the pediatric departments from other hospitals covering residency outside the two regions, and (2) if the patients were classified with steroid-resistant nephrotic syndrome (SRNS).
Definition
Nephrotic syndrome (NS) was defined by proteinuria (albumin/creatinine ratio > 2200 mg/g or proteinuria > 40 mg/m2/h or 1 g/day) and plasma albumin < 25 g/L. Remission of NS was defined by protein-free urine for more than 3 days. Relapse of NS was defined by 3 consecutive days with 2 + or more on urinary albumin dipstick. Steroid-sensitive NS (SSNS) diagnosis was made when remission of NS was obtained within the period of 4 weeks of adequate treatment with prednisolone 60 mg/m2/day, maximum 80 mg/day. Steroid-resistant NS (SRNS) if they had continuous proteinuria despite 4 weeks of adequate treatment with prednisolone 60 mg/m2/day. Steroid dependency (SD) was defined by recurrence of proteinuria despite ongoing therapy with steroid or within 2 weeks after cessation of treatment [12]. Frequent relapsing (FR) was defined as two or more relapses within 6 months following the initial treatment or four relapses within any 12-month period [13]. Infrequent Relapsing NS (IFR) was defined by less than two relapses within 6 months of the initial response or less than four relapses for any year thereafter. Non-relapsing NS (NRNS) was defined as no experience of relapse after the first attack of NS. Active disease was defined by relapse within the last 12 months at follow-up or ongoing immunosuppressive treatment.
Treatment protocols
The standard steroid regimen for the initial episode of NS changed during the period from 1998 to 2016. Before 2003, the standard steroid regimen for the initial episode of NS was prednisone (2 mg/kg/day or 60 mg/m2) for 4 weeks followed by 4 weeks of alternative day treatment (40 mg/m2) (pred-short) [4]. In 2003, the steroid regimen was changed to the prolonged treatment regimen (60 mg/m2/day) for 6 weeks followed by 6 weeks of alternate day prednisolone (40 mg/m2) (pred-long) [6]. Treatment of relapses consisted of daily high-dose prednisolone (60 mg/m2) until 3 consecutive days with no proteinuria on urinary albumin dipstick followed by 4 weeks of alternative day prednisolone (40 mg/m2).
Cytotoxic agents were administered to children with SD/FR NS for maintenance of SSNS remission [14,15,16]. Cyclophosphamide (2 mg/kg/day) were administrated for 8–12 weeks. Cyclosporine A (CyA), Tacrolimus (Tac), and mycophenolate mofetil (MMF) therapy were started at a daily dose of 6 mg/kg, 0.1–0.2 mg/kg, and 600 mg/m2, respectively. Rituximab was also used in the treatment for more severe forms of SSNS [17], in doses of 375 mg/m2 given twice or four times at start and 1–2 times at later stages, and were not administrated in patients below the age of 6 years.
Statistical analysis
Continuous variables are expressed as mean and 95% confidence interval or median and range. Categorical variables are presented as frequencies and percentage. Test of statistical differences was performed using chi-squared test, Fisher’s exact test, Student’s t test, and Mann-Whitney U test, where appropriate. The Kaplan-Meier method was used to illustrate the time to remission from debut; the time point for this event to occur was estimated based on 1 year without relapses and 1 year without active treatment with steroids or secondary immunosuppressive drugs. If a patient has had more than one of these periods of remission throughout the follow-up period, the last period of remission was used. All tests were two-tailed, and p values < 0.05 were considered as statistically significant. All statistical analysis was carried out using Stata software, release 13 (College Station, TX) for Mac.
Results
Study population
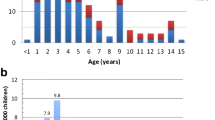
During the period from 1998 to 2016, 126 children with childhood INS were admitted to the six pediatric departments in the central and northern region of Denmark. Of these, 112 (89%) patients provided with a written informed consent to participate in the study. Two patients were excluded due to unavailability of medical records, which totaled 110 patients with INS (92 SSNS patients and 18 SRNS patients). In August 2017, 42% (39/92) of the patients with childhood SSNS had grown into adulthood (Fig. 1). The patients were followed for a mean time of 4.9 (range 0.1–13) years from the age of 18 years. Table 1 lists the characteristics of the clinical course in adulthood for patients with active disease vs. non-active disease. A total of 12 of the 39 patients (31% (95%CI: 0.19–0.46)) still had active disease during adulthood, of which all of them were classified with SD/FR NS in childhood. The two groups did not differ regarding gender distribution, age at onset, or ethnicity. The time from debut to the first relapse was significantly shorter for patients with active disease vs. non-active disease (201 (95%CI: 59.7–343.0) vs. 216 (244.8–575.2), p = 0.048).
Follow-up and time to remission
The patients were followed for at mean duration of 14.4 (range 7.8–19.3) years with a mean age of 22.8 (range 18.0–30.9) years at last day of follow-up. Figures 2 and 3 show the proportion of patients with active disease from debut and over a period of 10 years. As illustrated on both figures, all patients were in active treatment for 1.2 year after onset of NS. Patients treated for a longer period were all experiencing relapses, as they were classified with IFR or SD/FR NS (Fig. 2). After a period of 3.5 years from debut, patients with IFR NS all went into sustained remission. Patients with SD/FR NS had active disease for a significantly longer period compared to patients with IFR and NRNS (p < 0.001). After a period of 7 years from debut, 73% of SD/FR patients still had active disease.
Kaplan-Meier survival analysis of the 39 patients illustrating the number of patients with active disease in the period from debut until sustained remission. Sustained remission was defined as 1-year period without relapses and/or active treatment with steroids and/or secondary immunosuppressive treatment
Kaplan-Meier analysis of the patients divided in groups of infrequent relapsing nephrotic syndrome (IFR), non-relapsing nephrotic syndrome (NRNS), and steroid-dependent/frequent relapsing nephrotic syndrome (SD/FR). Sustained remission was defined as 1-year period without relapses and/or active treatment with steroids and/or secondary immunosuppressive treatment
Adulthood outcome data
Of the 12 patients (31%) who were classified with active disease, seven patients (58%) had experienced at least one relapse during adulthood (range 1–4 relapses) and did receive NS treatment in relation to this. The remaining five patients (42%) still received prophylactic treatment with steroids or secondary immunosuppressive drugs at the last date of follow-up but did not experience any relapse during adulthood. All relapses during adulthood were treated with high-dose steroids with responsiveness in all cases. The mean time to remission in adulthood was 16.5 (95%CI: 9.7–23.3) days and all were in remission within 5 weeks. At last follow-up, all 39 patients were reported with a normal systolic and diastolic blood pressure, and none of the patients were in treatment with antihypertensive drugs. Regarding the renal outcome, all 39 patients were reported with a normal plasma creatinine and estimated glomerular filtration rate (eGFR).
SD/FR nephrotic syndrome in adulthood
Table 2 compares of SD/FR patients in groups of active disease and sustained remission. A total of 19 patients were classified with SD/FR NS in childhood and of whom 12 (63%) patients still had active disease and seven (37%) patients were in sustained remission in adulthood (non-active disease). No significant difference regarding gender distribution, age at onset, duration of follow-up period, or age at last follow-up was found between the two groups. Also, no differences were found when comparing the two groups, neither regarding the initial treatment regimen nor regarding the use of second immunosuppressive drugs. Even though both groups were classified with SD/FR NS in childhood, the mean number of relapses during follow-up was significantly higher in patients with active disease vs. non-active disease (5.7 (95%CI: 5.6–17.1) vs. 4.4 (95%CI: 3.93–4.92), p = 0.030). From the age of 8–12 years, there was a significant difference in the number of relapses, as the mean number of relapses per patient per year was found to be significantly higher in the group of patients with active disease vs. non-active disease in the age interval 8–12, 12–15, 15–18, and 18–31 years (Fig. 4).
Discussion
Childhood SSNS has usually been considered self-limiting as relapses become less frequent towards puberty, where permanent remission is expected in many as the disease subsides with age. The present study reappraises this concept and illustrates that a significant number of patients with childhood SSNS continue suffering from active disease as adults suggesting that childhood SSNS is not entirely a disease of childhood. Particularly, patients with SD/FR NS are in higher risk of having continued and active disease as adults.
Long-term prognosis of childhood SSNS reviewed in 2016 by Hjorten et al. [18] in 2016 concluded that several studies regarding long-term follow-up reported persistent disease and a need of additional immunosuppressive treatment in adulthood. Our data presents an important contribution to the few available studies investigating the long-term outcome of SSNS because of the unselected cohort of patients. Data from previously published studies concerning adulthood relapsers have also indicated a risk of adulthood disease. An early study published by Trompeter et al. [8] reported that only 6% of patients still experiencing relapses during adulthood. There is a significant risk of recall bias to this study as data collection was based on a questionnaire followed by a clinical examination of patients with debut of disease in the 1960s. Moreover, the follow-up period did not extend beyond the age of 20 years. Contrary to the method used by Trompeter, all data in the present study were collected directly from the patient’s electronical medical records.
Later studies reported that 16% [19], 19% [20], and 33% [10] of SSNS patients relapsed into adulthood. As for this present study, the studies are limited by a relative small number of patients, for which reason, conclusions should be drawn carefully. Furthermore, these studies lack information on the length of the follow-up period in adulthood. Also, the studies by Ruth et al. [10] and Lewis et al. [20] report data from single medical center contributing to the risk of selection bias. Similarly, the risk of selection bias could be a factor influencing on the data presented by Fakhouri et al. [9] reporting as many as 42% of their patients experiencing at least one relapse during adulthood. This high proportion could be explained by the inclusion of patients from a second-referral center, and thereby inclusion of more severe cases of SSNS (e.g., SD/FR). However, the proportion of patients with more severe cases of NS in the study by Fakhouri et al. is unclear, as the group only reports the proportion of patients with FR NS [9]. In contrast to these studies, data in the present study is collected from a well-defined geographical area including a non-selected cohort of patients both presenting data from patients with a single episode of NS and from patients with multiple NS relapses during childhood, which is a major strength in our study.
Our data presents that the risk of having active disease during adulthood is particularly evident in the group of patients with SD/FR NS, as 63% of these children continue having from active disease during adulthood. Similar findings were found by Rüth et al. [10] as 60% (18/30) of the patients with SD/FR NS were reported with relapses during adulthood. According to the International Study of Kidney Disease in Children (ISKDC), the number of relapses during the first 6 months of disease was found to be a powerful predictor of disease severity in childhood [21]. The present study and both the studies by Fakhouri et al. [9] and Rüth et al. [10] did not confirm this observation. Young age at onset has also been found as a risk factor of relapsing in adulthood [8, 9]. This association was not supported by our study as well as studies by Rüth et al. [10] and Lewis et al. [20]. Interestingly, our group of patients seems to be older at first episode compared to children in previously published literature. This age difference could influence the risk of having active disease in adulthood. Furthermore, the present study did not find any association between gender, treatment with secondary immunosuppressive drugs, and the risk of having active disease in adulthood. However, we observed that patients having active disease in adulthood had a significant higher number of relapses per year during late childhood and adolescence compared to the group of patients with non-active disease in adulthood. This is supported by the findings of Rüth et al. [10] and Fakhouri et al. [9] suggesting that children with more aggressive disease during childhood are more prone to suffer from active disease in adulthood. One of the factors to predict the treatment response is genetic variations in NS. Genetics of SSNS is rather unknown compared to SRNS, probably due to the variability in clinical course of SSNS. HLA region on chromosome 6 has shown strong association with SSNS phenotypes. Gene defects in genes as PLCE1, EMP2, and limited evidence with APOL1 [22] have been observed in children with SSNS. Ethnic trends, where patients with Asian, African American, and Hispanic descent, are more likely to have SSNS with a clinical course as frequent relapses or steroid dependence has been reported [23]. With next generation sequencing (NGS) technologies, it is possible to screen genetic factors associated with NS, despite the ever-growing list of genes associated with NS. Gene panels as Bristol NGS panel (https://www.nbt.nhs.uk/severn-pathology/pathology-services/bristol-genetics-laboratory-bgl) provide a possibility of comprehensive and faster diagnosis, better prognosis, and patient management [24]. A patient database such as ours, together with the newly identified therapy options, CPF, genetic and laboratory biomarkers, enables the selection of right patients to accelerate clinical trial possibilities.
Despite the fact that childhood SSNS may continue into adulthood, the long-term renal prognosis is still good. In our cohort of patients with a mean follow-up of 5 years, all remained steroid-sensitive. Also, all 39 patients were reported with a normal renal function at follow-up despite the fact that one third of patients had received treatment with CyA or other calcineurin inhibitors with potential of nephrotoxicity. Similar results were reported by Rüth et al. and Fakhouri et al. [9, 10]. Furthermore, none of the patients in the present study were reported with hypertension or in treatment with antihypertensive drugs at the last date of follow-up.
Like studies performed previously regarding the long-term outcome, the retrospective design is a limitation in the present study. To reduce the risk of reporting more severe forms of SSNS, as it could seem like in the study of Fakhouri et al. [9], our study cohort was included from an unselected population of children with INS. Additionally, the unique possibility to follow the patients through the Danish health care system enabled us an almost complete follow-up on all patients, a fact that may compensate for the relatively small number of patients included.
Another limitation of the study was that the treatment protocols have changed during the period of the study, both with respect to steroid regimes but also with respect to secondary immunosuppression. However, no difference in the long-term outcome between the two steroid protocols was observed in this study. This observation is supported by recent studies whom all argue against prolonged steroid protocol during treatment of the initial episode of NS [25, 26]. Additionally, even with massive secondary immunosuppressive therapy including cyclophosphamide in the late 1990s, calcineurin inhibitors, or with the introduction of rituximab in the 2000s, the data presented here clearly illustrates that a curative therapy of SSNS is still not available as the most severe forms of childhood NS (e.g., SD/FR) are at significant risk of persistent disease into adulthood.
In conclusion, the present study illustrates that SSNS is not only a disease of childhood but may continue into adulthood in a significant proportion of patients in an unselected cohort of Danish children. Especially children with more severe course of SSNS in childhood (i.e., SD/FR) are prone to have active disease as adults, and the number of relapses during late childhood and adolescence is associated with active disease in adulthood. Prospective studies are needed to further illustrate that SSNS in childhood does not subside in a significant number of patients.
Abbreviations
- FR:
-
Frequent relapsing
- IFR:
-
Infrequent relapsing
- INS:
-
Idiopathic nephrotic syndrome
- NRNS:
-
Non-relapsing nephrotic syndrome
- NS:
-
Nephrotic syndrome
- SD:
-
Steroid dependency
- SSNS:
-
Steroid-sensitive nephrotic syndrome
References
Wong W (2007) Idiopathic nephrotic syndrome in New Zealand children, demographic, clinical features, initial management and outcome after twelve-month follow-up: results of a three-year national surveillance study. J Paediatr Child Health 43(5):337–341
Rothenberg MB, Heymann W (1957) The incidence of the nephrotic syndrome in children. Pediatrics 19(3):446–452
McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM (2001) Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 16(12):1040–1044
International Study of Kidney Disease in Children. Report (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr 98(4):561–564
Koskimies O, Vilska J, Rapola J, Hallman N (1982) Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57(7):544–548
Ehrich JH, Brodehl J (1993) Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Eur J Pediatr 152(4):357–361
Arbeitsgemeinschaft für Padiatrische Nephrologie (1988) Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1(8582):380–383
Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS (1985) Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1(8425):368–370
Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux MF, Landais P, Lesavre P, Chauveau D, Knebelmann B, Broyer M, Grunfeld JP, Niaudet P (2003) Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 41(3):550–557
Ruth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ (2005) Children with steroid-sensitive nephrotic syndrome come of age: long-term outcome. J Pediatr 147(2):202–207
Lynge E, Sandegaard JL, Rebolj M (2011) The Danish national patient register. Scand J Public Health 39(7 Suppl):30–33
Gordillo R, Spitzer A (2009) The nephrotic syndrome. Pediatr Rev 30(3):94–104 quiz 105
International Study of Kidney Disease in Children. Report (1982) Early identification of frequent relapsers among children with minimal change nephrotic syndrome. J Pediatr 101(4):514–518
International Study of Kidney Disease in Children. Report (1974) Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Lancet 2(7878):423–427
Niaudet P (1992) Comparison of cyclosporin and chlorambucil in the treatment of steroid-dependent idiopathic nephrotic syndrome: a multicentre randomized controlled trial. The French society of paediatric nephrology. Pediatr Nephrol 6(1):1–3
Pravitsitthikul N, Willis NS, Hodson EM, Craig JC (2013) Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 10:CD002290. https://doi.org/10.1002/14651858.CD002290.pub4
Hodson EM, Craig JC (2014) Rituximab for childhood-onset nephrotic syndrome. Lancet 384(9950):1242–1243
Hjorten R, Anwar Z, Reidy KJ (2016) Long-term outcomes of childhood onset nephrotic syndrome. Front Pediatr 4:53
Skrzypczyk P, Panczyk-Tomaszewska M, Roszkowska-Blaim M, Wawer Z, Bienias B, Zajgzkowska M, Kilis-Pstrusinska K, Jakubowska A, Szczepaniak M, Pawlak-Bratkowska M, Tkaczyk M (2014) Long-term outcomes in idiopathic nephrotic syndrome: from childhood to adulthood. Clin Nephrol 81(3):166–173
Lewis MA, Baildom EM, Davis N, Houston IB, Postlethwaite RJ (1989) Nephrotic syndrome: from toddlers to twenties. Lancet 1(8632):255–259
Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the international study of kidney disease in children. J Am Soc Nephrol 8(5):769–776
Adeyemo A, Esezobor C, Solarin A, Abeyagunawardena A, Kari JA, El Desoky S, Greenbaum LA, Kamel M, Kallash M, Silva C, Young A, Hunley TE, de Jesus-Gonzalez N, Srivastava T, Gbadegesin R (2018) HLA-DQA1 and APOL1 as risk loci for childhood-onset steroid-sensitive and steroid-resistant nephrotic syndrome. Am J Kidney Dis 71(3):399–406
Karp AM, Gbadegesin RA (2017) Genetics of childhood steroid-sensitive nephrotic syndrome. Pediatr Nephrol 32(9):1481–1488
Bierzynska A, Saleem M (2017) Recent advances in understanding and treating nephrotic syndrome. F1000Res 6:121. https://doi.org/10.12688/f1000research.10165.1
Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, Ishikura K, Hataya H, Honda M, Ito S, Shima Y, Kaito H, Nozu K, Nakamura H, Igarashi T, Ohashi Y, Iijima K, Japanese Study Group of Kidney Disease in Children (2015) A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int 87(1):225–232
Teeninga N, Kist-van Holthe JE, van Rijswijk N, de Mos NI, Hop WC, Wetzels JF, van der Heijden AJ, Nauta J (2013) Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol 24(1):149–159
Acknowledgments
The authors thank physicians at the departments in the central and northern region of Denmark for providing us with clinical information from the medical records of the included patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical considerations
The study and establishment of the clinical database were approved by the Danish Data Protection Agency (1-16-02-27-16). All patients and their parents were informed verbally and in writing. The patients were asked to provide written consent before initiation of any study-related procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Korsgaard, T., Andersen, R.F., Joshi, S. et al. Childhood onset steroid-sensitive nephrotic syndrome continues into adulthood. Pediatr Nephrol 34, 641–648 (2019). https://doi.org/10.1007/s00467-018-4119-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4119-8