Abstract
Background
Membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults, but is less frequent in children. Antibodies against four antigens leading to MN have been described in children: phospholipase A2 receptor 1 (PLA2R1), thrombospondin type-1 domain-containing 7A (THSD7A), neutral endopeptidase (NEP), and cationic bovine serum albumin (BSA).
Methods
Twelve children with MN were included in this study. Sera of all patients were analyzed for antibodies against PLA2R1, THSD7A, NEP, and BSA. All sera were also analyzed using Western blot with human glomerular extracts (HGE) under non reducing conditions. In 5 cases renal biopsies were analyzed for PLA2R1, THSD7A, NEP, BSA, and all IgG subclasses.
Results
Six patients were PLA2R1-antibody-positive, whereas THSD7A, NEP, and BSA antibodies were not found in any of our 12 patients. All sera were analyzed by Western blot using human glomerular extracts; however, no further potential antigens were found. Five kidney biopsies from 2 PLA2R1-antibody-positive and 3 PLA2R1-antibody-negative patients were available for additional analyses, confirming the diagnosis of PLA2R1-associated MN in 2 cases, whereas none of the biopsies revealed enhanced staining for THSD7A, NEP or BSA. IgG2 and IgG4 stainings were positive in both patients with PLA2R1-associated MN and negative in the other biopsies. During follow-up (median 24 months), 4 children with PLA2R1-associated MN went into remission, preceded by decline of PLA2R1 antibodies. Five of the 6 PLA2R1-antibody-negative children went into remission.
Conclusions
In children with MN, PLA2R1-associated MN appears to be common, whereas MN associated with THSD7A, NEP or BSA was not encountered. PLA2R1 antibody levels are closely associated with disease activity, whereas PLA2R1-antibody-negative patients often have a good prognosis. However, the pathophysiology of MN in a considerable number of children remains unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Membranous nephropathy (MN) is an immune complex disease usually presenting with nephrotic syndrome. The histological pattern of MN in kidney biopsies is characterized by a thickening of the glomerular basement membrane and immune deposits in the subepithelial space. Primary MN is an autoimmune disease caused by in situ binding of circulating antibodies to an endogenous podocytic antigen leading to the development of subepithelial immune deposits [1]. In some patients, MN develops secondary to other diseases, such as hepatitis B infection, lupus erythematosus, use of some medications, etc., and is referred to as secondary MN. The differentiation of primary from secondary MN is very important, as treatment and prognosis differ. Before the phospholipase A2 receptor 1 (PLA2R1) and thrombospondin type-1 domain-containing 7A (THSD7A) were characterized as potential antigens responsible for MN development in adults [2, 3], no reliable clinical marker was available for the differential diagnosis of MN.
In 80% of adult patients the target antigen is the PLA2R1. PLA2R1 antibodies can be detected in the serum of these patients using various methods such as Western blot, an indirect immunofluorescence test (IFT), and by ELISA [4]. Furthermore, immunohistochemical analyses of kidney biopsies show an enhanced staining for PLA2R1 in patients with PLA2R1-associated MN [5]. Staining for PLA2R1 can also be helpful for the differential diagnosis of MN in children [6]. There are substantial data showing a close association among the PLA2R1 antibody level, disease activity, and outcome in adult MN patients [7]. In contrast, only a few data exist concerning the role of PLA2R1 antibodies for the diagnosis and prognosis of MN in children and adolescents [8]. The second target antigen responsible for MN development in adult patients is THSD7A [3]. The prevalence of THSD7A-associated MN among adult MN patients is 2–3%. The rather limited clinical data, however, suggest that THSD7A antibody levels might also be associated with disease activity, similar to PLA2R1 antibodies [4]. Until now THSD7A antibodies have not been evaluated in patients younger than 18 years of age.
In children, two more pathogenic antigens responsible for MN development have been reported. The protein neutral endopeptidase (NEP) is targeted in fetomaternal alloimmune MN, whereas a cationic form of bovine serum albumin (BSA) is targeted in an early childhood form of MN [9, 10]. In contrast to the high prevalence in adults with nephrotic syndrome, MN in children is rare. Overall, the prevalence is estimated to be around 1–5% [11]. The lack of large multicenter studies hampers the possibility of studying the pathophysiology, treatment options, and clinical outcome of these patients in detail.
In this study we performed comprehensive serological and histological analyses in a cohort of children with MN, describing the appearance of the disease in children and investigating the role of autoantibodies and the clinical outcome of disease.
Materials and methods
Study population and design
In this study 12 children with biopsy-proven MN were included. Patients had to be younger than 18 years at the time of biopsy. Sera of all patients recruited in the study were analyzed for antibodies against PLA2R1, THSD7A, NEP, and BSA using Western blot. In 9 cases follow-up sera were collected every 3–6 months during the follow-up period. Sera were collected during clinical controls, which were routinely performed every 3–6 months. All sera were analyzed for PLA2R1 antibodies using IFT and an ELISA and for THSD7A antibodies using IFT. Patients with a known secondary cause of MN, such as hepatitis B or lupus erythematosus, were excluded from this study. This was a non-interventional study and following recruitment, the therapeutic strategy was decided by the treating pediatric nephrologists without any recommendations.
PLA2R1, THSD7A, NEP, and BSA antibody measurement
Western blot analyses for antibodies against PLA2R1, THSD7A, NEP, and BSA were performed as described in the original publications [2, 3, 9, 10]. Gel electrophoresis and protein transfer to polyvinylidene difluoride membranes were performed according to standard protocols. The sera were diluted at a 1:100 ratio. For specific detection of the antigens, the following commercially available antibodies were used: rabbit polyclonal antibody at a 1:1,000 dilution (Atlas Antibodies) for PLA2R1; rabbit polyclonal antibody at a 1:1,000 dilution (Atlas Antibodies) for THSD7A; goat polyclonal antibody at a 1:200 dilution (RnD Systems) for NEP, and rabbit polyclonal antibody at a 1:1,000 dilution (Sigma-Aldrich) for BSA. The Western blot analyses with human glomerular extracts were performed under non-reducing conditions, as described in the works identifying PLA2R1 and THSD7A as target antigens in MN [2, 3].
The IFT for the detection of PLA2R1 and THSD7A antibodies was performed as described before [4]. PLA2R1 antibodies were also measured by an ELISA [12]. According to the manufacturer, antibody levels were considered positive when >20 U/ml, negative when <14 U/ml, and “borderline” if the level was 14– 20 U/ml.
Definition of remission of proteinuria
Nephrotic range proteinuria was defined as proteinuria >40 mg/m2/h in children <10 years and protein/creatinine ratio in the urine >3.5 g/g in patients >10 years. Partial remission of proteinuria was defined as the reduction of proteinuria by at least 50% compared with the time of inclusion in the study and proteinuria <40 mg/m2/h for children <10 years and protein/creatinine ratio < 3.5 g/g for children >10 years. Complete remission of proteinuria was defined as proteinuria <4 mg/m2/h in children <10 years and protein/creatinine ratio < 0.5 g/g in children >10 years.
Immunohistochemistry for PLA2R1, THSD7A, NEP, and BSA
Slides were deparaffinized, pretreated in citrate buffer pH 6.2 (PLA2R1, THSD7A, NEP) or Dako Target Retrieval Solution (BSA, S1699) for 15 min at 120 °C and cooled down in iced water (10 min). After rinsing in 99% ethanol, slides were incubated for 10 min with normal serum (Vector S2000) followed by antibodies against PLA2R1 (Sigma, 1:5,000), THSD7A (Sigma, 1:3,000), NEP (novocastra 1:15,000) or BSA (Sigma, 1:50) overnight at 4 °C. The slides were then washed in PBS and for PLA2R1, THSD7A, and BSA detection incubated with polymer 1 (Zytomed Zytochem-Plus AP Polymer-Kit POLAP), rinsed in PBS, and incubated with polymer 2 (Zytomed Zytochem-Plus AP Polymer-Kit POLAP). For NEP antigen, detection was performed using VECTASTAIN ABC-AP Staining KIT AK-5000 and biotinylated horse anti-mouse IgG antibody BA-2000. After washing in PBS, slides were stained in new fuchsin naphthol As-Bi phosphate substrate mixture (30 min) followed by hydrochloric acid (1%, 15 min) and 1-min nuclear staining in hemalaun (Mayer).
Immunohistochemistry for IgG subclasses
After deparaffinization, slides were pretreated in protease (Proteinase, bacterial SIGMA-P8038) for 15 min at 40 °C, then rinsed in 99% ethanol followed by incubation with normal serum (Vector S2000) for 10 min. Primary antibodies (Southern Biotech IgG1 [1:100], IgG2 [1:40,000], IgG3 [1:100], IgG4 [1:4,000] in antibody diluent, Invitrogen) were added and incubated overnight at 4 °C. The slides were then washed in PBS and incubated with polymer 1 (Zytomed Zytochem-Plus AP Polymer-Kit POLAP), rinsed in PBS, and incubated with polymer 2 (Zytomed Zytochem-Plus AP Polymer-Kit POLAP). After washing in PBS, slides were stained with new fuchsin naphthol As-Bi phosphate substrate mixture (30 min). The slides were finally immersed in hydrochloric acid (1%, 15 min) followed by 1 min of nuclear staining in hemalaun (Mayer).
Results
Patient cohort
The patient cohort included 12 children with biopsy-proven MN, diagnosed between August 2010 and July 2016. At the time of renal biopsy, the median age of patients was 14 years (range: 3–17 years). Four (25%) patients were male. The median time between renal biopsy and collection of serum was 2 months. The first serum measurement was performed within 6 months from the time of renal biopsy in 8 patients and more than 6 months after renal biopsy in 4 patients (patients 3, 4, 10, 11, Table 1). Serum creatinine was within the normal range in all patients (median 0.47 mg/dl, range 0.30–0.91 mg/dl), and median eGFR, calculated using the Bedside Schwartz equation, was 135 ml/min/1.73 m2 (range: 78–177 ml/min/1.73 m2). One of the 12 children was younger than 10 years at the time of diagnosis; her proteinuria was 186 mg/m2/h. The remaining 11 patients were adolescents (range 10–17 years) and median proteinuria was 3.8 g/g creatinine (range: 0.5 to 11 g/g creatinine) at the time of the first serum collection. Median serum albumin was 28.2 g/l (range 18.6–43.0 g/l). Four patients had no immunosuppressive treatment before study inclusion, whereas 6 patients had been treated with steroids, 1 patient with cyclosporine A and steroids, and 1 patient with mycophenolate mofetil and steroids. Ten patients were treated with ACE inhibitors and 2 patients received AT1 receptor blockers. The median follow-up time was 24 months.
Serological and histological characterization of the study cohort
Western blot analyses
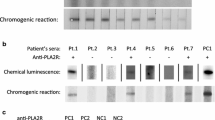
Sera of all 12 patients were analyzed by Western blot for the presence of antibodies against PLA2R1, THSD7A, NEP, and BSA (Fig. 1). All sera were negative for THSD7A, NEP, and BSA antibodies at the time of study inclusion, when the first serum collection was performed, and during the follow-up. Six patients (50.0%) were positive for PLA2R1 antibodies in the serum (patients 1–6, Table 1).
Measurement of antibodies against PLA2R1, THSD7A, NEP, and BSA by Western blot. Western blot analyses of patient sera for antibodies against a PLA2R1, b THSD7A, c NEP, and d BSA (D). The same amount of recombinant protein was loaded and then analyzed with specific commercially available antibodies against the respective protein (positive control), serum from a healthy donor (negative control), serum from patients 1–6, Table 1 (PLA2R1-antibody-positive), and serum from patient 7, Table 1 (PLA2R1-antibody-negative). All patients were negative for THSD7A, NEP, and BSA antibodies. The sera of all other PLA2R1-antibody-negative patients included in the study were analyzed using the same approach and procedures. Pos positive, Neg negative, NEP neutral endopeptidase, BSA bovine serum albumin
Next, we analyzed all sera using Western blot with human glomerular extracts (HGE) under nonreducing conditions (Fig. 2). All 6 PLA2R1-antibody-positive sera detected a 185-kDa band in the HGE, corresponding to PLA2R1. The reactivity of these sera with the PLA2R1 antigen was conformation-dependent and only seen when the Western blot was performed under nonreducing conditions, as described before [4]. On the other side, PLA2R1-antibody-negative sera showed no specific reaction with any proteins on HGE.
Western blot analyses of the sera on human glomerular extracts (HGE). a Western blot analyses of the sera from all PLA2R1-antibody-negative patients (patients 7–12, Table 1) on HGE. b, c Sera from patients 1–6 are positive for PLA2R1 antibodies and show a band corresponding to PLA2R1 on HGE only when analyzed under nonreducing conditions. Under reducing conditions, no PLA2R1 is detectable. The positive controls for PLA2R1 and THSD7A detection using specific commercially available antibodies are shown in d and e
Indirect immunofluorescence and ELISA analyses
All collected sera were further analyzed for THSD7A antibodies by IFT and for PLA2R1 antibodies by ELISA and IFT. In the IFT analyses, no THSD7A-antibody-positive serum could be identified at any time point, corresponding to the results of Western blot analyses. At study inclusion, PLA2R1 antibodies were detectable by IFT in all 6 patients, who were positive according to Western blot (patients 1–6, Table 1), but in none of the 6 patients who were negative according to Western blot (patients 7–12, Table 1). In ELISA analyses for PLA2R1 antibodies only 4 sera were positive at study start (patients 2–4, and 6); all 4 had been tested positive by IFT and Western blot. Two sera, which were tested positive for PLA2R1 antibodies using IFT and Western blot, were negative in the ELISA, with PLA2R1 antibody levels of 5 U/ml and 11 U/ml respectively (patients 1 and 5, Table 1). Interestingly, both patients became positive for PLA2R1 antibodies in the ELISA during further follow-up, with PLA2R1-antibody levels of 141 U/ml and 41 U/ml after 24 and 18 months respectively. In the sera from the remaining 6 patients, the negative results of the IFT and Western blot analyses were confirmed by ELISA (PLA2R1 antibody levels 0 U/ml to 10 U/ml).
Renal biopsy analyses
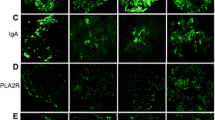
In 8 cases (patients 1–4 and 7–10), renal biopsies could be analyzed for the expression of PLA2R1. In 4 cases (patients 1–4), a clear PLA2R1 enhancement could be detected along the glomerular capillary wall, confirming the PLA2R1-associated nature of the disease (Fig. 3). In all 4 cases, PLA2R1 antibodies were detectable in the serum. In the other 4 cases (patients 7–10), the PLA2R1 staining showed only a faint positivity on the podocyte surface (Supplemental Fig. 1), similar to the negative control (Supplemental Fig. 2). PLA2R1 antibodies were not detectable in any of these 4 patients using any of the measurement methods.
Staining of the renal biopsy of patient 1 (PLA2R1-associated MN, Table 1) for PLA2R1, THSD7A, NEP, BSA, and IgG subtypes. Representative stainings for a PLA2R1, b THSD7A, c NEP, d BSA, and e–h IgG subtypes of the renal biopsy of patient 1 (Table 1). The granular positivity for PLA2R1 along the glomerular capillary is characteristic for a PLA2R1-associated MN. THSD7A and NEP stainings show only a faint positivity on the podocytes, as seen in the staining of a control biopsy (Supplemental Fig. 2). Staining for BSA was negative (d). The biopsy stained positive for all subclasses IgG1–IgG4. NEP neutral endopeptidase, BSA bovine serum albumin, MN membranous nephropathy
In addition, 2 biopsies from patients with PLA2R1-associated MN (patients 1 and 2) and 3 biopsies from patients with non-PLA2R1-associated MN (patients 7, 9, and 10) were stained for THSD7A, NEP, BSA, and all IgG subclasses. BSA could not be detected in any of the cases, whereas the THSD7A and NEP staining showed a weak positivity on the podocyte surface, similar to the negative control (Supplemental Fig. 2). The biopsies showed no specific granular positivity for THSD7A or NEP in the immune deposits along the glomerular capillary wall, as expected in positive cases.
Both renal biopsies from patients with PLA2R1-associated MN stained positive for IgG2 and IgG4, whereas biopsies from patients with non-PLA2R1-associated MN stained negative for both IgG2 and IgG4. IgG1 and IgG3 stained positive in all 5 biopsies, except for 1 patient with non-PLA2R1-associated MN (patient 9), who stained negative for IgG1. A representative staining for all IgG subclasses is shown in Fig. 3.
PLA2R1 antibody levels, renal function, and proteinuria during follow-up
Clinical follow-up data were available for all patients over a median follow-up time of 24 months (range: 6–72 months). Nine (75%) patients had remission of proteinuria (7 patients had complete remission, 2 patients had partial remission), whereas the remaining 3 patients had no remission of proteinuria during the follow-up. Serum creatinine levels remained stable within the normal range in all patients.
Five (83.3%) of the 6 PLA2R1-antibody-negative patients had remission of proteinuria (4 complete, 1 partial remission). Two patients were treated using cyclosporine A and steroids, 1 patient had been treated with mycophenolate mofetil and glucocorticoids before study inclusion and received no immunosuppression during the study follow-up, whereas the remaining 2 patients had spontaneous remission. One patient had no remission of proteinuria, despite treatment with mycophenolate mofetil.
Three of the 6 patients with PLA2R1-associated MN (50%) had complete remission of proteinuria. One patient (patient 2) was treated with tacrolimus und steroids, the PLA2R1 antibodies disappeared from the circulation after 12 months, and the patient had remission at the same time. Two years later, the PLA2R1 antibodies reappeared in the circulation. Initially, they were detectable only by Western blot and IFT and 3 months later also by ELISA. Proteinuria reappeared 3 months later than the detection of PLA2R1 antibodies. The patient was treated with rituximab, which led to the disappearance of the PLA2R1 antibodies within 3 months. The patient had remission of proteinuria 3 months later. The second patient (patient 5) had spontaneous remission of proteinuria. PLA2R1 antibodies spontaneously fell from 41 U/ml to 3 U/ml at the end of the follow-up. Remarkably, using the Western blot technique PLA2R1 antibodies could be detected throughout the follow-up period in this patient, which encompassed 72 months (Fig. 4). PLA2R1 antibodies were detectable by Western blot and IFT, even as they became negative according to ELISA during the time period between 24 months and 72 months after inclusion in the study. At the same time, the patient had complete remission of proteinuria. The third patient (patient 6) was treated with steroids and cyclosporine A, which was later switched to mycophenolate mofetil. The PLA2R1 antibodies disappeared after 15 months. The patient had complete remission of proteinuria at the same time.
Serial PLA2R1 antibody measurements of sera from patient 5 (Table 1) by ELISA, immunofluorescence test (IFT), and Western blot. In patient 5, PLA2R1 antibody levels measured by ELISA were negative during the time period between 24 and 72 months after study inclusion. In the IFT and Western blot analyses, however, PLA2R1 antibodies could be detected throughout the follow-up period. The patient had spontaneous remission of proteinuria during the follow-up. Detection of PLA2R1 antibodies by IFT and Western blot in the serum of a patient with high PLA2R1 antibodies (positive control), or healthy donor (negative control) are depicted in the last two columns on the right
One patient had a partial remission of proteinuria after treatment with steroids and cyclosporine A, which was later switched to tacrolimus, in addition to mycophenolate mofetil. The PLA2R1 antibody levels declined, but were still detectable by all measurement techniques.
The remaining 2 patients with PLA2R1-antibody-positive MN had no remission of proteinuria and PLA2R1 antibodies persisted during the follow-up. One patient was treated with steroids and cyclosporine A and the second patient was treated with steroids.
Discussion
The identification of PLA2R1 and THSD7A as target antigens in adult MN was pivotal to improving and personalizing the treatment of these patients [2, 3]. The differential diagnosis of MN and the treatment management have become more pathogenesis-specific [7]. MN is the most common cause of nephrotic syndrome in adult Caucasians; however, in children the disease is rare [11]. Two antigens have been identified only in pediatric forms of MN, NEP in a rare neonatal form of MN [9], and BSA in a few cases of MN in early childhood [10]. Only a few studies have investigated the detection of PLA2R1 antibodies in children, especially in adolescents with MN [6, 8]. To our knowledge, this is the first study to comprehensively analyze the pathogenesis of MN, including all known antigens, in a cohort of children with MN who were followed over a median period of 24 months.
This study offers a number of important findings in children with MN. Every second child with MN had a PLA2R1-associated form of disease, showing that immunization against PLA2R1 not only occurs at an older age, but also in a considerable number of children with MN, similar to the findings from Kumar et al. [8]. We did not find any child with THSD7A-associated MN. However, this could be related to the low prevalence of this form of disease (2–3% among all adult patients with MN) and the small study cohort. None of the patients included in our cohort was found to have a NEP-associated MN. As none of the patients in the study was younger than 3 years, this finding may suggest that except for newborns, NEP might not play a central role for MN in children. Also, BSA does not seem to be the major antigen causing MN, at least in our cohort of children with MN; however, our study cohort was older (median age 14 years) than the one in which the role of BSA was reported [10].
An interesting finding of our study was the fact that sera from the 6 PLA2R1-antibody-negative patients did not detect any antigen in the Western blot analyses using HGE under nonreducing conditions [2, 3]. This finding has several implications: it may be an indication that the antigen(s) leading to the development of MN in these patients is (are) not endogenously expressed on podocytes. Therefore, the relevant pathomechanisms of disease development in these cases may be different from those in PLA2R1- or THSD7A-associated MN. Different exogenous antigens, i.e., food or environmental antigens, may have led to the development of MN in these patients, as has been described for BSA-associated MN [10]. Five out of the six PLA2R1-antibody-negative patients had a remission of proteinuria, 2 of them spontaneously. It is therefore conceivable that in these cases an exogenous antigen (i.e., food) led to MN and upon its (random) cessation or a change in procession, the disease went into remission. The hypothesis that the pathomechanisms of disease in PLA2R1-antibody-negative patients are different from those in PLA2R1-associated MN is also supported by the results of the staining for IgG subclasses in renal biopsies. Both patients with PLA2R1-associated MN showed IgG2 and IgG4 positivity in the renal biopsies, whereas this was not the case in any of the patients with non-PLA2R1-associated MN. However, because of the small number of biopsies that could be studied, no definitive conclusions can be drawn and these results will have to be confirmed in larger cohorts of children with MN.
The absence of an identifiable antigen in the HGE and the high remission rate in the 6 PLA2R1-antibody-negative patients may also suggest that in these patients an immunological remission of disease might have occurred before study inclusion. We analyzed the renal biopsies of 4 PLA2R1-antibody-negative patients for PLA2R1 expression, 3 of these biopsies were also analyzed for all other antigens, excluding the possibility that these patients had MN associated with one of the antigens and were in immunological remission. Of note, we did not identify any histological (i.e., subendothelial immune deposits), serological (i.e., anti-dsDNA antibodies, or C3 and C4 depletion) or clinical findings suggesting that MN might be secondary to another disease (i.e., lupus erythematosus) in these cases.
In patients with PLA2R1-associated MN, we found a remarkable correlation of the PLA2R1 antibody levels to clinical outcome, as has been shown in adults and adolescents [12,13,14]. Disappearance of PLA2R1 antibodies preceded complete remission of proteinuria, whereas 2 of the 3 patients with persisting PLA2R1 antibodies had no remission of proteinuria and the third only partial remission. Moreover, a relapse of PLA2R1 antibodies was followed by a relapse of proteinuria.
As has been shown in adults, Western blot and IFT were more sensitive than the ELISA for the detection of PLA2R1 antibodies [4]. In 2 patients PLA2R1 antibodies were detectable by Western blot and IFT, but not by ELISA at the time of study inclusion. In both cases, PLA2R1 antibodies became detectable by ELISA during further follow-up. The seroconversion of initially PLA2R1-antibody-negative patients with an enhanced PLA2R1-staining in the biopsy has been shown in two previous studies [15, 16]. This finding was also observed in a patient with relapse of disease, in whom PLA2R1 antibodies were first detectable by Western blot and IFT and only 3 months later by ELISA. This finding is important, as the Western blot technique is very accurate, but also sophisticated, time- and personnel-consuming, and therefore not suitable for clinical routine. The IFT, on the other hand, offers high sensitivity and specificity for the detection of PLA2R1 antibodies and is easy to perform; however, its results are only semi-quantitative and observer-dependent. The ELISA offers a quantitative, fast, and observer-independent measurement of PLA2R1 antibodies. The sensitivity of the ELISA is somewhat lower, especially in patients with very low PLA2R1 antibody levels; however, the ELISA has been established to have a very good correlation with clinical outcome in patients with MN. We were particularly intrigued by the findings in one patient (patient 5, Table 1, Fig. 4). In this case, PLA2R1 antibodies became spontaneously negative in the ELISA (from 41 U/ml at 18 months to 3 U/ml at 72 months), but remained positive according to Western blotting and IFT (although at very low levels in both techniques) for a time period spanning at least 3 years. The patient had complete remission of proteinuria at this time. We can only speculate on the pathomechanisms behind this finding. The level of the persisting PLA2R1 antibodies may be so low, that the podocytes can clear the PLA2R1 antibodies and immune complexes binding to the glomeruli before the complement system is activated, therefore preventing renal damage. The PLA2R1 antibody levels have to reach a certain threshold to cause MN. Such a threshold may depend on a number of factors and be different between patients. Future experiments in animal models of PLA2R1-associated MN may shed more light on the pathomechanisms leading to these findings. Whether the antibodies can still be found in the kidney after the patient had complete remission of proteinuria, but persisting PLA2R1 antibodies in the blood, cannot be answered because performing a renal biopsy (with the respective risks) in a child in remission is not justifiable. In this case, we are following this patient closely to investigate whether in the long term the PLA2R1 antibodies will completely disappear, or if the patient will relapse. Such cases have important implications, because if PLA2R1 antibodies disappear, it would suggest that patients with extremely low PLA2R1 antibodies might be safely treated with supportive medication and closely monitored. However, if the patient relapses, particularly if renal function deteriorates over time, a more aggressive approach may be appropriate, suggesting treatment for every patient with detectable PLA2R1 antibodies with immunosuppression, regardless of proteinuria.
Because of the limited number of cases, this study has some limitations. Most patients had received glucocorticoids and 2 of them had additional immunosuppressive treatment before renal biopsy and study inclusion. We cannot exclude this treatment leading to changes in antibody findings. However, in 4 patients with non-PLA2R1-associated MN, staining of renal biopsies for PLA2R1 could be performed and confirm the diagnosis. In 2 PLA2R1-antibody-negative patients (patients 10 and 11) the first serum measurement was performed more than 6 months after renal biopsy. It is possible that in these cases, a remission of the autoantibodies had happened before serum analyses. Another limitation is that staining for IgG subclass and podocyte antigens was not available in all cases. Performing antigen staining in all biopsies may have led to the identification of more patients with PLA2R1- or THSD7A-associated MN. However, such cases of patients with PLA2R1- or THSD7A-associated MN showing enhanced antigen staining in the biopsy, but negative antibody findings in serum, are rare [4, 5]. As this was a non-interventional study, the therapeutic strategy was decided by the treating physicians without any recommendations. Because of the variability in the treatment for our 12-patient cohort, this study does not allow conclusions on the best immunosuppressive treatment in children with MN.
In conclusion, we described the appearance of MN in our cohort of children with detailed histological and serological analyses. PLA2R1-associated MN is a common form of MN in children, although its prevalence seems to be lower than in adults. PLA2R1 antibody levels are very closely associated with disease activity and outcome in children. The pathogenesis of disease in PLA2R1-antibody-negative patients remains unclear; however, these patients seem to have a good prognosis. The characterization of the responsible antigen(s) in these patients may lead to better differential diagnosis and treatment management.
References
Beck LH Jr, Salant DJ (2014) Membranous nephropathy: from models to man. J Clin Invest 124:2307–2314
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361:11–21
Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RA, Lambeau G (2014) Thromobospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371:2277–2287
Hoxha E, Beck LH Jr, Wiech T, Tomas NM, Probst C, Mindorf S, Meyer-Schwesinger C, Zahner G, Stahl PR, Schöpper R, Panzer U, Harendza S, Helmchen U, Salant DJ, Stahl RA (2017) An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol 28:520–531
Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA (2012) Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 82:797–804
Kanda S, Horita S, Yanagihara T, Shimizu A, Hattori M (2017) M-type phospholipase A2 receptor (PLA2R) glomerular staining in pediatric idiopathic membranous nephropathy. Pediatr Nephrol 32:713–717
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC (2017) A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28:421–430
Kumar V, Varma AK, Nada R, Ghosh RK, Suri D, Gupta A, Kumar V, Rathi M, Kohli HS, Jha V, Gupta KL, Ramachandran R (2017) Primary membranous nephropathy in adolescence a prospective study. Nephrology (Carlton) 22:678–683
Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM (2002) Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346:2053–2060
Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P (2011) Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 364:2101–2110
Menon S, Valentini RP (2010) Membranous nephropathy in children: clinical presentation and therapeutic approach. Pediatr Nephrol 25:1419–1428
Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, Hofstra JM, Hoxha E, Stahl RA, Lambeau G, Stöcker W, Schlumberger W (2013) Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421:213–218
Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA (2014) Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25:1357–1366
Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF (2012) Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23:1735–1743
Ramachandran R, Kumar V, Nada R, Jha V (2015) Serial monitoring of anti-PLA2R in initial PLA2R-negative patients with primary membranous nephropathy. Kidney Int 88:1198–1199
Van de Logt AE, Hofstra JM, Wetzels JF (2015) Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: seroconversion after prolonged follow-up. Kidney Int 87:1263–1264
Acknowledgements
We thank the German Association of Pediatric Nephrology (GPN) for the support and all members of the Pediatric MN Study Group, who participated in the recruitment of the patients (in alphabetical order): W Clasen, Münster; H Fehrenbach, Memmingen; B Friedrich, Leonberg; U Jacoby, Rostock; T Jungraithmayr, Memmingen, D Marx, Zurich; K Möller, Bremen; G Müller, Dresden; G Schalk, Zurich; H Staude, Rostock; J Urban, Augsburg; M Wallot, Moers; M Wigger, Rostock. We also thank Eugen Kinzler, Janine Eisenmann, Katharina Schulz, and Sonia Wulf for their excellent technical assistance.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
This work is supported by Grants from the “Deutsche Forschungsgemeinschaft” SFB 1192 (projects C1, B1 and B6 to RAKS, EH, and TW) and a grant of the “Else Kroener-Fresenius Stiftung” to EH. AD was supported by the Sonderforschungsbereich 1192 (project C1) and a grant of “Hamburg macht Kinder gesund, e.V.”.
The study was approved by the local ethics committee of the chamber of physicians in Hamburg and was conducted in accordance with the ethical principles stated by the Declaration of Helsinki. Informed consent was obtained from all participating patients and their parents (custodians).
Conflicts of interest
The authors declare that they have no conflicts of interest
Electronic supplementary material
Supplemental Fig. 1
Staining of the renal biopsy of Patient 7 (non-PLA2R1-associated MN) for PLA2R1, THSD7A, NEP, BSA, and IgG subtypes. Representative stainings for a PLA2R1, b THSD7A, c NEP, d BSA, and e–h IgG subtypes of the renal biopsy of patient 7 (Table 1). PLA2R1, THSD7A, and NEP stainings show only a faint positivity on the podocytes, as seen in the staining of a control biopsy (Supplemental Figure 2). Staining for BSA was negative (d). The biopsy stained positive for IgG1 and IgG3, but negative for IgG2 and IgG4 (DOCX 612 kb)
Supplemental Fig. 2
Positive and negative control stainings for PLA2R1, THSD7A, NEP, and BSA. a–d Positive controls: enhanced staining for a PLA2R1 and b THSD7A is shown in a patient with PLA2R1-associated MN (a) and a patient with THSD7A-associated MN respectively. c Brush borders of proximal tubules stain positive for NEP. d Bovine liver tissue stains positive for BSA. e–h Negative controls: a renal biopsy from a patient with no MN shows a faint positivity for e PLA2R1, f THSD7A, and g NEP on the podocytes; however, no granular positivity for any of the antigens is detectable along the glomerular capillary. h Staining for BSA was negative (DOCX 703 kb)
Rights and permissions
About this article
Cite this article
Dettmar, A.K., Wiech, T., Kemper, M.J. et al. Immunohistochemical and serological characterization of membranous nephropathy in children and adolescents. Pediatr Nephrol 33, 463–472 (2018). https://doi.org/10.1007/s00467-017-3817-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3817-y