Abstract
Background
Little is known about the associations between allergic disease, sleep-disordered breathing (SDB), and childhood nocturnal enuresis (NE). We examined whether allergic disease and SDB were associated with childhood NE.
Methods
Data were assessed from the 2007–2012 Taiwan National Health Insurance Research Database. We enrolled 4308 children aged 5–18 years having NE diagnosis and age- and sex-matched 4308 children as the control group. The odds ratios of NE were calculated to determine an association with preexisting allergic disease and SDB.
Results
A total of 8616 children were included in the analysis. Prevalence of allergic diseases and SDB was significantly higher for the NE group than the control group (all p < 0.001). After adjusting odds ratios for potential confounding factors, except asthma, children with allergic rhinitis, atopic dermatitis, allergic conjunctivitis, and obstructive sleep apnea (OSA) had significantly higher odds of NE compared with children never diagnosed. With stratification for sex, girls with allergic rhinitis, atopic dermatitis, allergic conjunctivitis, OSA, and snoring had significantly higher odds of NE, compared with girls never diagnosed. Only boys with allergic rhinitis and OSA were associated with increased odds of NE. With stratification for age, children aged 5–12 years with allergic rhinitis, atopic dermatitis, allergic conjunctivitis, and OSA had significantly higher odds of NE compared with those never diagnosed. Odds of NE increased with the number of comorbid allergic diseases.
Conclusions
Allergic diseases and SDB are associated with increased odds of childhood NE. The odds of NE increased with the number of comorbid allergic diseases present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nocturnal enuresis (NE) is involuntary loss of urine during sleep in children aged 5 years or older, the age at which bladder control usually occurs [1]. NE develops in 15–20% of 5-year-old children, and these children become continent at a rate of 15% per year [2]. It is the most common pediatric urologic complaint, and one of the most common pediatric healthcare issues [2]. Although the underlying mechanisms of NE are uncertain, the most predominant pathogenesis theories of NE include genetic factors, nocturnal detrusor overactivity, psycho-behavioral problems, sleep arousal dysfunction, bladder dynamics, and circadian rhythm of vasopressin [1, 2].
Childhood allergic diseases, including asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis, are common childhood inflammatory disorders with considerable impact on the lives of those affected and on healthcare [3, 4]. The development and phenotypic expression of allergic disease depends on a complex interaction between genetic and environmental factors, such as environmental exposure to food and inhalant allergens, and non-specific adjuvant factors (e.g., tobacco smoke, air pollution and infection) [3,4,5]. Previous studies have demonstrated an association between allergy and NE [6, 7] and suggested that the bladder acts as a target organ for inhaled or ingested allergens. Allergen-induced allergic reactions in the bladder may cause smooth muscle contraction and decrease functional bladder capacity by causing bladder allergic inflammation or bladder hypersensitivity [7, 8].
Sleep-disordered breathing (SDB) in children comprises a spectrum of sleep-related disorders from intermittent and habitual snoring to severe obstructive sleep apnea (OSA), with a prevalence of 1.2–5.7% [9, 10]; however, they are often overlooked and not recognized. An association between SDB and NE has been previously reported [11, 12]; furthermore, the prevalence of NE in SDB children is associated with the severity of obstruction of the upper airway [12, 13]. Recent studies have documented that nocturnal airway narrowing in asthmatic children is often associated with nocturnal SDB [14, 15], suggesting an association between asthma and NE [16].
Some studies have suggested that asthma and allergic rhinitis may be associated with NE [6, 16,17,18,19]; however, controversy regarding this still exists [20, 21]. Also, these reports have been conducted on subjects recruited from a single institute in which participants were based on local samples, with limited ability to examine a wide range of potential comorbidities [6, 16,17,18,19,20,21]. Little is known about whether allergic disease and SDB would be associated with childhood NE. This present study was conducted using a large, national population database to examine whether associations existed between allergic disease, SDB, and childhood NE.
Materials and methods
Data source
Taiwan launched a universal compulsory national health insurance program, as a single-payer, social insurance plan in March 1995. It is managed by Taiwan’s National Health Insurance Administration and covers over 99% of the country’s 23 million people. The National Health Insurance Research Database (NHIRD) is a universal health claim database in Taiwan and is maintained by the National Health Research Institute (NHRI). Because this study was focused on a disease more prevalent in childhood, we used the children’s database, which is a sub-database of NHIRD, to establish the research. The subjects in the children’s database were randomly selected from half of all insured children (age < 18 years old) from 1996 to 2012. This database includes the same claim data as the NHIRD, such as registry of beneficiary, disease history files (recorded as International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]), prescription records, and other medical services. The linkage of all datasets for the relevant variables used scrambled unique personal or medical institutional identification numbers, which were encrypted by the NHRI. To protect people’s privacy, only de-identified data were released by NHRI. The study protocol was approved by the hospital’s Institutional Review Board (CSMUH No: CS14019).
Study population
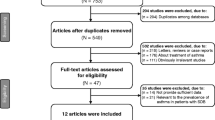
To interpret associations between allergic disease and NE, we built a retrospective population-based case-control study. Fig. 1 shows the flowchart for study population selection. The study comprised an NE group as the case group and a non-NE group as the control group. The NE group involved the newly diagnosed enuretic children (ICD-9-CM 307.6× and 788.36) aged 5–18 years from 2007 to 2012, with the diagnosed date set as the index date. Diagnosis of NE was based on at least three inpatient and/or outpatient department visits with diagnosis of NE in either primary or secondary coding fields. The control group consisted of randomly selected children without diagnosis of NE, and were frequency matched by age, sex, and index year in a 1:1 ratio. Diseases or conditions that might be related to NE were excluded, including children with history of infantile cerebral palsy and paralysis (ICD-9-CM 343.9× and 344.9×), neurogenic bladder (ICD-9-CM 596.xx), spinal abnormality (ICD-9-CM 741.4×), neurogenic abnormality (ICD-9-CM 742.xx), and urogenital anomaly (ICD-9-CM 752.xx).
Allergic diseases included asthma (ICD-9-CM 493.xx), allergic rhinitis (ICD-9-CM 477.xx), atopic dermatitis (ICD-9-CM 691.8×), and allergic conjunctivitis (ICD-9-CM 372.05, 372.10, and 372.14). Children with allergic diseases were defined as children with a disease history identified before the index date, and during a minimum of three medical visits where diagnosis appeared in either primary or secondary coding fields. The study also considered NE-related SDB, including snoring (ICD-9-CM 786.09 and 780.59) and OSA (ICD-9-CM 307.4×, 327.23, and 780.5×), and were defined under the same criteria as the allergic disease definition. The assessments of NE, allergic diseases and SDB were made by board-certified physicians at medical institutions.
Statistical analysis
The demographic characteristics, allergic diseases and SDB are displayed as number and percentage for each category of variables, and mean and standard deviation for continuous variables. The category variables, including age group, sex, allergic diseases, and SDB, were tested for distribution differences by chi-square tests, and the continuous variable (age) was assessed by t test. To measure the odds of NE for each variable, the crude and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were estimated by single variable and multivariable logistic regression models. All data processing and statistical analyses were performed in SAS 9.4 software (SAS Institute, Cary, NC, USA). All statistical tests were two-sided and p < 0.05 was considered statistically significant.
Results
Study population
Table 1 shows demographic characteristics and distribution of the cohort according to sex, age, occupation status, urbanization, and exposure status. The study consisted of 4308 children with NE and 4308 matched controls. Study subjects were more likely to have parents with a high socio-economic status. There was no significant difference between the two groups regarding sex, age, or urbanization.
Crude and adjusted analyses
The prevalence of allergic diseases and SDB was significantly higher for the NE group than the control group (all p < 0.001; Table 1). After adjusting for demographic data (age, sex, occupational status and urbanization level) and medical comorbidities (asthma, allergic rhinitis, atopic dermatitis, allergic conjunctivitis, OSA and snoring) in multivariate models, children with allergic rhinitis (aOR, 1.62; 95% CI, 1.48–1.78; p < 0.001), atopic dermatitis (aOR, 1.23; 95% CI, 1.05–1.43; p = 0.008), and allergic conjunctivitis (aOR, 1.15; 95% CI, 1.05–1.26; p = 0.004) had significantly higher odds of having NE compared with those without allergic diseases; while children with asthma (aOR, 1.02; 95% CI, 0.92–1.13; p = 0.69) were not associated with NE. For SDB, OSA (aOR, 2.19; 95% CI, 1.40–3.42; p < 0.001) was associated with significantly increased odds of NE, but not snoring (aOR, 1.48; 95% CI, 0.90–2.41; p = 0.12).
To examine the joint effect between allergic disease and SDB in association with NE in the study population, a subanalysis was made. We found that allergic disease, SDB and allergic disease in association with SDB had significantly increased odds of NE compared with children never diagnosed (Table 2). In particular, allergic disease in association with SDB had the greatest odds of NE (aOR, 3.16; 95% CI, 2.20–4.54; p < 0.001).
Stratified analyses for sex and age
To elucidate further the impact of sex and age, a stratified analysis was made. Table 3 shows associations between exposure status and childhood NE stratified by sex and age. With stratification for sex, girls with allergic rhinitis, atopic dermatitis, allergic conjunctivitis, OSA and snoring had significantly higher odds of NE compared with girls never diagnosed. Only boys with allergic rhinitis and OSA were associated with increased odds of NE. With stratification for age, children aged 5 to 12 years with allergic rhinitis, atopic dermatitis, allergic conjunctivitis, and OSA had significantly higher odds of NE, compared with those never diagnosed. However, history of allergic disease or SDB was not associated with increased odds of NE in children aged 13 to 18 years old.
Association between the number of allergic diseases and NE
The total number of allergic diseases was strongly associated with increased odds of NE. That is, as the number of comorbid allergic diseases increased, so the odds of NE increased from 1.44 to 2.19 (Fig. 2). A similar trend of odds for NE was shown in girls, boys, and children aged 5 to 12 years (all p for trend <0.001).
Adjusted odds ratios with 95% confidence intervals for the association of nocturnal enuresis (NE) with the number of allergic diseases in multivariate logistic regression models, according to sex and age. †Allergic diseases including asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis. ‡Model adjusted for sex, age, occupational status, urbanization, obstructive sleep apnea, and snoring
Discussion
Previous reports have suggested that history of asthma and allergic rhinitis was associated with childhood NE [16,17,18,19]. However, this is the first study to examine an association between history of allergic disease and childhood NE by using a large, national representative database that demonstrates increased odds of NE in children with allergic diseases, such as allergic rhinitis, atopic dermatitis, and allergic conjunctivitis. Further, an intriguing finding of this study is the increased odds of NE with the number of comorbid allergic diseases present, suggesting that increased number of allergic diseases may affect or worsen NE in children. We found that younger children aged 5 to 12 years with allergic diseases were more strongly associated with NE. This is similar to Dahan’s observation that the association between current asthma and current NE was stronger in younger children aged 6–10 years when compared with 11 to 14 years [16]. It may be related to spontaneous resolution of NE by age.
Our study shows that asthma was associated with increased odds of NE in the bivariate models, but not in multivariate models. In a demographic study, Rawashdeh et al. [19] described an association between NE and a history of asthma. Ozkaya et al. [17] recently reported that positive sensitization to pollens, high eosinophil count, and an additional allergic rhinitis diagnosis were risk factors for NE in asthmatic children. Dahan et al. [16] also observed associations between asthma and NE, especially between current asthma and current NE, in their cohort. Although previous pediatric epidemiologic studies found significant associations between asthma and NE, notably, these studies did not further control for confounding factors with multivariate models [16, 17, 19].
The precise pathophysiology and causal relationship between allergic disease and NE is unknown. Allergic diseases are a group of diseases linked by a shared underlying problem with the immune system [3, 5]. The early phase of an allergic reaction is mediated predominantly by mast cells and T helper type 2 (Th2) cytokines, in which interleukin (IL)-4, IL-6, and IL-13 drive immunoglobulin-E production, and a cascade of proinflammatory agents are also released sequentially at the site of an allergic reaction [3, 5, 22, 23]. The bladder may function as an allergic target organ like the nose, eye, lung, gastrointestinal tract and skin. Thus, after inhaled or ingested allergens reach the allergen-specific antibodies on mast cells or basophils, mediators like histamine, serotonin, and leukotriene are released, which then promote local edema, smooth muscle contraction, and mucus secretion, resulting in the symptoms of hypersensitivity in the target-organs [5, 22, 23]. That is, the hypothesis that certain foods or allergens might induce NE, and elimination of dietary allergens as well as environmental controls might lead to a decrease in frequency and intensity of both diseases. On the basis of this hypothesis, Bray [8] reported that avoiding foods and inhaled allergens could result in the cessation or alleviation of the allergic symptoms and NE in all of the allergic children. These findings imply that some allergen-induced allergic responses may lead to overactive bladder or decrease functional bladder capacity by causing bladder allergic inflammation [6]. Therefore, allergen-related detrusor instability and smooth muscle contraction may partly be explained in some allergic children with NE.
It has been suggested that hyperfunction of the parasympathetic nervous system causes increased detrusor muscle activity during sleep in enuretic children [24, 25]. This hypothesis has been further confirmed by the direct cytometric evidence that studies using ambulatory cystometry have demonstrated many enuretic children exhibit uninhibited detrusor contractions, that is, detrusor overactivity while asleep [26,27,28]. Recent studies have also found that the degree of parasympathetic nervous system overactivity was correlated with disease severity in children with allergy and NE [29,30,31]. Thus, the mechanism of autonomic nervous system dysfunction may explain a subset of allergic children with NE.
In this study, we found a significant association between allergic diseases and increased the odds of NE. Several hypotheses may explain this association between allergic diseases and NE. First, it has been suggested that allergic disease and the bladder may share a common allergic response pathway. The bladder acts as a target organ for allergens in triggering NE onset. Second, excessive parasympathetic nervous system activation after cycles of allergic responses would target the bladder and then lead to detrusor instability and overactive bladder. Further studies would be required to investigate these hypotheses and elucidate the definitive underlying mechanisms linking allergic diseases and NE.
Respiratory problems in children may affect or worsen NE [12, 15, 18]. Nocturnal nasal obstruction is the prominent symptom in childhood allergic rhinitis [18, 32]. Jesus et al. [32] recently demonstrated a high incidence (54.3%) of respiratory/ear-nose-throat problems (like symptomatic adenoid hypertrophy, mouth breathing, and asthma) with complicated bladder dysfunction in a pediatric cohort. Our results show that children with allergic rhinitis exhibited the highest odds (1.62-fold) of having NE diagnosis, consistent with previous reports [12, 15, 18, 32]. Our study affirms that children with OSA, a more severe form of SDB, were associated with increased odds of NE, which is in agreement with previous studies that obstructive airway problems are significant risk factors for enuretic children [12, 16, 18, 33,34,35,36,37]. Relief of upper airway obstruction is associated with significant improvement of NE in a high proportion, but not in all children with NE [12, 33,34,35,36,37]. Increased respiratory efforts against an obstructed airway in children with SDB may cause increased release of both brain and atrial natriuretic peptides from cardiac myocytes after cardiac wall distension owing to increased negative intrathoracic pressure. Release of this cardiac hormone will increase water and sodium excretion, and inhibit other hormones that regulate fluid homeostasis, like vasopressin and the renin-angiotension-aldosterone pathway [13, 38, 39]. These potential mechanisms may be an explanation in a specific subset of allergic children with NE. However, the NHIRD did not provide information regarding detailed anamnesis and laboratory testing for assessing disease severity of SDB and NE. Thus, we cannot stratify disease severity for further analysis.
Our results show that allergic disease or SDB itself can be a primary driver for childhood NE, and SDB in association with allergic disease may worsen NE. The mechanisms of association between allergic disease and childhood NE are multifactorial, including the effects of chronic allergic inflammation and sleep disturbance. We were unable to determine whether treatments of either allergy or NE could affect clinical outcomes of the other because the cross-sectional nature of this study precludes any conclusions about the directionality of the associations. While our study did not prove the mechanism of association between allergic disease and NE, our findings provide several important implications. First, it highlights a possible link between the two disorders, and that further studies are needed to determine the underlying mechanisms. Second, a history of NE may prognosticate those children with an increased number of comorbid allergic diseases or SDB severity. Finally, it suggests that NE may be a comorbidity of severe childhood allergic disease and SDB, which may require multidisciplinary management by primary care doctors, pediatricians, allergists, dermatologists, otolaryngologist, and urologists alike.
The study has several strengths. Data were retrieved from the NHIRD, a comprehensive, reliable, and powerful database that is highly representative of the general population. The subjects enrolled in our study had board-certified physicians’ diagnoses, yielding better diagnostic validity relative to the diagnoses by parents-reported questionnaires. To date, this study represents the largest population-based case-control study for examining the association between allergic diseases and childhood NE. This study included multiple allergic disorders, minimization of selection bias, and control for potential confounding variables in multivariate models. Moreover, we examined not only the association for each allergic disease but also the association between the number of comorbid allergic diseases present and NE, which has not been examined in previous studies [16,17,18,19,20,21].
The study has some inevitable limitations. First, case numbers of allergic diseases, SDB and NE may be underestimated because only patients who sought medical help over at least two visits were enrolled in the study, so, a problem of identification of the subjects may exist and influence the results. Second, the NHIRD did not provide detailed information, such as clinical conditions, disease severity, family history, environmental factors, and laboratory testing. Thus, we were unable to determine the influence of these factors. Finally, despite our meticulous control of measures for confounding factors, bias resulting from unknown confounders might have affected the results.
Conclusions
Our results suggest that allergic diseases and SDB are associated with increased odds of childhood NE. The odds of NE increased with the number of comorbid allergic diseases present. These findings would benefit from investigating the history and symptoms of allergic diseases and SDB when examining a child with NE, and make referrals if this is an issue. Further studies with larger cohorts and expanded diagnostic testing are needed to verify the direction of association between allergic diseases, SDB, and childhood NE.
References
Nevéus T (2011) Nocturnal enuresis-theoretic background and practical guidelines. Pediatr Nephrol 26:1207–1214
Nascimento Fagundes S, Azevedo Soster L, Lebl AS, Rodrigues Pereira RP, Tanaka C, Pereira RF, Ferreira de Mattos Silvares E, Koch VH (2016) Impact of a multidisciplinary evaluation in pediatric patients with nocturnal monosymptomatic enuresis. Pediatr Nephrol 31:1295–1303
Halken S (2004) Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 15(Suppl 16):9–32
Platts-Mills TA (2015) The allergy epidemics: 1870-2010. J Allergy Clin Immunol 136:3–13
Kay AB (2001) Allergy and allergic diseases. N Engl J Med 344:30–37
Mungan NA, Seckiner I, Yesilli C, Akduman B, Tekin IO (2005) Nocturnal enuresis and allergy. Scand J Urol Nephrol 39:237–241
Yamada T, Murayama T, Mita H, Akiyama K (2001) Bladder hypersensitivity of interstitial cystitis complicated by allergic diseases. Urology 57(6 Suppl 1):125
Bray GW (1931) Enuresis of allergic origin. Arch Dis Child 6:251–253
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Sheldon SH, Spruyt K, Ward SD, Lehmann C, Shiffman RN (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130:576–584
Witmans M, Young R (2011) Update on pediatric sleep-disordered breathing. Pediatr Clin N Am 58:571–589
Barone JG, Hanson C, DaJusta DG, Gioia K, England SJ, Schneider D (2009) Nocturnal enuresis and overweight are associated with obstructive sleep apnea. Pediatrics 124:e53–e59
Jeyakumar A, Rahman SI, Ambrecht ES, Mitchell R (2012) The association between sleep-disordered breathing and enuresis in children. Laryngoscope 122:1873–1877
Brooks LJ, Topol H (2003) Enuresis in children with sleep apnea. J Pediatr 142:515–518
Chan CS, Woolcock AJ, Sullivan CE (1988) Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis 137:1502–1504
Bohadana AB, Hannhart B, Teculescu DB (2002) Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma 39:85–100
Dahan P, de Bessa J Jr, de Oliveira DM, Gomes CC, Cardoso JC, Macedo IT, de Almeida Belo M, de Figueiredo AA, Netto JM (2016) Association between asthma and primary nocturnal enuresis in children. J Urol 195:1221–1226
Ozkaya E, Aydın SC, Yazıcı M, Dundaröz R (2016) Enuresis nocturna in children with asthma: prevalence and associated risk factors. Ital J Pediatr 42:59
Chimenz R, Manti S, Fede C, Stroscio G, Visalli C, Nicotera A, Di Rosa G, Romeo AC, Salpietro V, Cuppari C (2015) Primary nocturnal enuresis in children with allergic rhinitis and severe adenotonsillar hypertrophy: a single center pilot study. J Biol Regul Homeost Agents 29(2 Suppl 1):73–79
Rawashdeh YF, Hvistendahl GM, Kamperis K, Hansen MN, Djurhuus JC (2002) Demographics of enuresis attending a referral centre. Scand J Urol Nephrol 36:348–353
Kaplan GW, Wallace WW, Orgel HA, Miller JR (1997) Serum immunoglobulin E and incidence of allergy in group of enuretic children. Urology 10:428–430
Siegel S, Rawitt L, Sokoloff B, Siegel B (1976) Relationship of allergy, enuresis, and urinary infection in children 4 to 7 years of age. Pediatrics 57:526–528
Williams CM, Rahman S, Hubeau C, Ma HL (2012) Cytokine pathways in allergic disease. Toxicol Pathol 40:205–215
Wynn TA (2015) Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 15:271–282
Dhondt K, Van Herzeele C, Roels SP, Raes A, Groen LA, Hoebeke P, Walle JV (2015) Sleep fragmentation and periodic limb movements in children with monosymptomatic nocturnal enuresis and polyuria. Pediatr Nephrol 30:1157–1162
Fujiwara J, Kimura S, Tsukayama H, Nakahara S, Haibara S, Fujita M, Isobe N, Tamura K (2001) Evaluation of the autonomic nervous system function in children with primary monosymptomatic nocturnal enuresis--power spectrum analysis of heart rate variability using 24-hour Holter electrocardiograms. Scand J Urol Nephrol 35:350–356
Ryu DS, Lee HW, Kwak KW, Park KH, Baek M (2014) Role of urodynamic study in nocturnal enuresis: urodynamic findings and treatment outcome correlation in children with pharmacotherapy-resistant monosymptomatic nocturnal enuresis or severe non-monosymptomatic nocturnal enuresis. Low Urin Tract Symptoms 6:88–93
Yeung CK, Sit FK, To LK, Chiu HN, Sihoe JD, Lee E, Wong C (2002) Reduction in nocturnal functional bladder capacity is a common factor in the pathogenesis of refractory nocturnal enuresis. BJU Int 90:302–307
Yeung CK, Chiu HN, Sit FK (1999) Bladder dysfunction in children with refractory monosymptomatic primary nocturnal enuresis. J Urol 162:1049–1055
Emin O, Esra G, Aysegül D, Ufuk E, Ayhan S, Rusen DM (2012) Autonomic nervous system dysfunction and their relationship with disease severity in children with atopic asthma. Respir Physiol Neurobiol 183:206–210
Tascilar E, Yokusoglu M, Dundaroz R, Baysan O, Ozturk S, Yozgat Y, Kilic A (2009) Cardiac autonomic imbalance in children with allergic rhinitis. Tohoku J Exp Med 219:187–191
Unalacak M, Aydin M, Ermis B, Ozeren A, Sogut A, Demirel F, Unluoglu I (2004) Assessment of cardiac autonomic regulation in children with monosymptomatic nocturnal enuresis by analysis of heart rate variability. Tohoku J Exp Med 204:63–69
Jesus LE, Tomé A, Cobe D, Camelier P (2016) Psychosocial and respiratory disease related to severe bladder dysfunction and non-monosymptomatic enuresis. J Pediatr Urol 12:126.e1–126.e6
Firoozi F, Batniji R, Aslan AR, Longhurst PA, Kogan BA (2006) Resolution of diurnal incontinence and nocturnal enuresis after adenotonsillectomy in children. J Urol 175:1885–1888
Cinar U, Vural C, Cakir B, Topuz E, Karaman MI, Turgut S (2001) Nocturnal enuresis and upper airway obstruction. Int J Pediatr Otorhinolaryngol 59:115–118
Kovacevic L, Wolfe-Christensen C, Lu H, Toton M, Mirkovic J, Thottam PJ, Abdulhamid I, Madgy D, Lakshmanan Y (2014) Why does adenotonsillectomy not correct enuresis in all children with sleep-disordered breathing? J Urol 191:1592–1596
Aydil U, Işeri E, Kizil Y, Bodur S, Ceylan A, Uslu S (2008) Obstructive upper airway problems and primary enuresis nocturna relationship in pediatric patients: reciprocal study. J Otolaryngol Head Neck Surg 37:235–239
Zaffanello M, Piacentini G, Lippi G, Fanos V, Gasperi E, Nosetti (2017) Obstructive sleep-disordered breathing, enuresis and combined disorders in children: chance or related association? Swiss Med Wkly 147:w14400
Sans Capdevila O, Crabtree VM, Kheirandish-Gozal L, Gozal D (2008) Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: a community-based study. Pediatrics 121:e1208–e1214
Kaditis AG, Alexopoulos EI, Hatzi F, Kostadima E, Kiaffas M, Zakynthinos E, Gourgoulianis K (2006) Overnight change in brain natriuretic peptide levels in children with sleep-disordered breathing. Chest 130:1377–1384
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Ethics Committee at the Chung Shan Medical University Hospital (# CS14019).
Conflict of interest
The authors declare no conflicts of interest.
Funding/support
This study was supported in part by Chung Shan Medical University Hospital (CSH-2016-a-018), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113,004), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 105–2325-B-039-003), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Rights and permissions
About this article
Cite this article
Tsai, JD., Chen, HJ., Ku, MS. et al. Association between allergic disease, sleep-disordered breathing, and childhood nocturnal enuresis: a population-based case-control study. Pediatr Nephrol 32, 2293–2301 (2017). https://doi.org/10.1007/s00467-017-3750-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3750-0