Abstract
Background
The aim of this study was to identify predictors of ‘intrauterine fetal renal failure’ in fetuses with severe congenital lower urinary tract obstruction (LUTO).
Methods
We undertook a retrospective study of 31 consecutive fetuses with a diagnosis of LUTO in a tertiary Fetal Center between April 2013 and April 2015. Predictors of ‘intrauterine fetal renal failure’ were evaluated in those infants with severe LUTO who had either a primary composite outcome measure of neonatal death in the first 24 h of life due to severe pulmonary hypoplasia or a need for renal replacement therapy within 7 days of life. The following variables were analyzed: fetal bladder re-expansion 48 h after vesicocentesis, fetal renal ultrasound characteristics, fetal urinary indices, and amniotic fluid volume.
Results
Of the 31 fetuses included in the study, eight met the criteria for ‘intrauterine fetal renal failure’. All of the latter had composite poor postnatal outcomes based on death within 24 h of life (n = 6) or need for dialysis within 1 week of life (n = 2). The percentage of fetal bladder refilling after vesicocentesis at time of initial evaluation was the only predictor of ‘intrauterine fetal renal failure’ (cut-off <27 %, area under the time–concentration curve 0.86, 95 % confidence interval 0.68–0.99; p = 0.009).
Conclusion
We propose the concept of ‘intrauterine fetal renal failure’ in fetuses with the most severe forms of LUTO. Fetal bladder refilling can be used to reliably predict ‘intrauterine fetal renal failure’, which is associated with severe pulmonary hypoplasia or the need for dialysis within a few days of life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fetal lower urinary tract obstruction (LUTO) or fetal bladder outlet obstruction is a spectrum of congenital anomalies characterized by a dilated fetal bladder, a dilated proximal urethra, and bilateral hydronephrosis consistent with obstruction in the urethra [1–4]. Its incidence is approximately 2.2 per 10,000 births, and it is commonly diagnosed during the late first or early second trimester of pregnancy [1–4]. There are different etiologies for LUTO, including posterior urethral valves, anterior urethral valves, urethral atresia, urethral stenosis, and prune-belly syndrome [5–7]. Complete bladder outlet obstruction (severe LUTO) is associated with high perinatal mortality due to pulmonary hypoplasia and severe renal impairment/damage [5–8]. Fetal intervention is usually performed for severe LUTO (presence of enlarged fetal bladder with severe bilateral hydronephrosis and oligohydramnios/anhydramnios) with ‘favorable’ renal indices, such as the absence of ultrasonographic aspects compatible with renal dysplasia and good fetal urinary biochemistry (urinary sodium <100 mEq/L, chloride <90 mEq/L, osmolarity <200 mOsm/L and β2-microglobulin <6 mg/L) [2, 7–24]. Fetuses with ‘unfavorable’ renal indices seem not to benefit from prenatal intervention [2, 3, 7, 8, 10, 25].
Later in pregnancy, some fetuses develop ultrasound (US) findings suggestive of renal dysplasia associated with either a mildly distended bladder with thick bladder wall or progressively decreasing amniotic fluid levels. These findings suggest that the fetal kidneys are producing little urine (oliguria/anuria). In this situation, perinatal mortality is extremely high due to severe pulmonary hypoplasia and neonatal renal failure in the first days of life.
In the study reported here, we propose the concept of ‘intrauterine fetal renal failure’ based on a series of fetuses with LUTO followed in our tertiary referral Fetal Center. In addition, we investigated potential predictors of ‘intrauterine fetal renal failure’ at the time of the prenatal diagnosis.
Methods
Study design
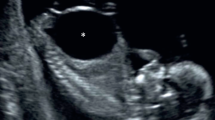
Between April 2013 and April 2015, we reviewed a cohort of consecutive fetuses with isolated LUTO that were referred to the Baylor College of Medicine/Texas Children’s Fetal Center. LUTO was diagnosed based on the ultrasonographic confirmation of a dilated bladder and proximal urethra associated with bilateral hydroureternephrosis [8, 26]. Isolated LUTO was considered if there were no associated fetal structural malformations on comprehensive fetal US examination fetal echocardiography and normal karyotype. The diagnosis of ‘intrauterine fetal renal failure’ was considered when (1) a fetus with LUTO presented with US signs suggestive of renal dysplasia (hyperechogenic cystic kidneys with no cortical-medullary differentiation) (Fig. 1a) associated with a mildly distended bladder with a thickened wall (Fig. 1b) and (2) progressively decreasing amniotic fluid levels after fetal vesicoamniotic shunt placement.
Perinatal management
All patients were evaluated according to a prenatal multidisciplinary LUTO management protocol that included: (1) an initial comprehensive ultrasonographic evaluation of fetal anatomy; (2) fetal echocardiogram; (3) prenatal genetic counseling and fetal karyotyping; (4) prenatal consultation with maternal–fetal (fetal intervention) specialists, pediatric nephrologists, and pediatric urologists. Amniotic fluid was collected during the amniocentesis and cordocentesis for the fetal fluorescence in-situ hybridization test and karyotyping (or chromosomal microarray), and at the same time an US-guided vesicocentesis was performed for investigating fetal urinary biochemistry. ‘Favorable’ urinary biochemistry was defined using standard criteria: urinary sodium of <100 mEq/L; chloride of <90 mEq/L; osmolality of >200 mOsm/L; beta2-microglobulin of <6 mg/L, at between 18 and 30 weeks of gestation [27, 28]. We performed a second evaluation 48 h after the initial evaluation, including a repeat fetal US examination and a second fetal vesicocentesis (in the case of ‘unfavorable’ urinary biochemistry observed during the initial evaluation). Fetal bladder volume was measured during the first and second US examinations using SonoAVC software (Automatic Volume Calculation; GE Medical Systems, Waukesha, WI) and a Voluson E8 ultrasound machine (GE Medical Systems) (Fig. 2a, b). The percentage of fetal bladder refilling 48 h after the first fetal vesicocentesis was calculated by the following equation: (Last fetal bladder volume − initial fetal bladder volume)/(initial fetal bladder volume) × 100.
US-guided percutaneous fetal vesicoamniotic shunt placement was offered in cases of severe LUTO associated with oligohydramnios/anhydramnios with either (1) ‘favorable’ renal function in the first sample or (2) improvement in biochemistries in the second sample and no signs on the US image suggestive of renal dysplasia. Patients were followed once a week, and a repeated vesicoamniotic shunting was offered in the case of shunt dislodgement or obstruction.
Delivery timing and route were based on standard obstetric principles. After delivery, all surviving neonates were evaluated and followed by pediatric specialists, including pediatric urologists, nephrologists, and neonatologists. After birth, a voiding cystourethrogram and cystoscopy were performed to evaluate the bladder and the urethra in all cases. Autopsy was offered if fetal or neonatal demise occurred.
Statistical analysis
The primary composite postnatal outcome was death within the first 24 h of life due to severe pulmonary hypoplasia or the need for dialysis within the first week of life.
The following data were analyzed: (1) gestational age at diagnosis; (2) US findings during the first examination at our center, including severity of hydronephrosis according to the Grignon’s classification [26]; hyperechogenicity of the kidneys (renal tissue with similar echogenicity to the bones); presence of renal cortical cysts; renal dysplasia (enlarged hyperechogenic cystic kidneys with no corticomedullary differentiation); presence of hydroureter; (3) severe oligohydramnios (amniotic fluid index < 5th percentile for gestational age or presence of anhydramnios); (4) fetal urinary biochemistry (favorable vs. unfavorable) [27, 28]; (5) percentage of fetal bladder refilling 48 h after the first fetal vesicocentesis; (6) fetal intervention versus no fetal intervention; (7) gestational age at diagnosis of ‘intrauterine fetal renal failure’; (8) gestational age at delivery, and (9) newborn weight.
Continuous variables were expressed as the mean and standard deviation or as the median and range, as appropriate, based on the distribution of the data. Categorical variables were expressed as absolute values or as percentages. Data were evaluated using the Fisher’s exact test, Mann–Whitney U test, and two-tailed Student’s t-test as appropriate. Receiver operating characteristic (ROC) curve analysis was used to select cutoff-values for the percentage of fetal bladder refilling to predict ‘intrauterine fetal renal failure’ (highest sensitivity and specificity) (IBM SPSS Statistics for Windows ver. 20; IBM Corp., Armonk, NY). A p value of <0.05 was considered to be significant.
Results
During the study period, a total of 31 consecutive fetuses with a diagnosis of primary LUTO were evaluated at our center. Four patients elected to terminate the pregnancy, one fetus died after the first US examination at our center, and one patient was lost to follow-up. These six fetuses were excluded from the analysis, and therefore, the evaluation of ‘intrauterine fetal renal failure’ was investigated in 25 patients. Among these, eight (32.0 %) fetuses developed ‘intrauterine fetal renal failure’ later during pregnancy (Table 1). All of them met the primary composite postnatal outcome of either death within 24 h of life (n = 6) or the need for dialysis within the first week of life (n = 2). Table 1 shows the characteristics, prenatal US findings at initial evaluation and at diagnosis of ‘intrauterine fetal renal failure’, and clinical outcomes. Cases #1, 2, 3, and 5 were considered to have ‘unfavorable’ fetal urinary biochemistry and no fetal intervention was offered. All of those infants died immediately after birth due to severe pulmonary hypoplasia.
Case #4 was a male fetus diagnosed with severe LUTO and ‘favorable’ renal function at gestational age (GA) 17 weeks with severe oligohydramnios. A fetal vesicoamniotic shunt (VAS) was placed at GA 18 weeks and was confirmed to be working at GA 19 weeks. However, at GA 20 weeks the shunt dislodged into the amniotic cavity, and another shunt had to be placed, with success. Weekly US follow-up revealed that the shunt was working, as the bladder was decompressed and the amniotic fluid volume was normal. At GA 26 weeks, however, the amniotic fluid index dropped from 12 to 6 cm and the fetal bladder remained empty; the kidneys became more hyperechogenic and developed signs of renal dysplasia. At GA 28 weeks, there was anhydramnios. The diagnosis of ‘intrauterine fetal renal failure’ was discussed with the family. At GA 34 weeks, a 2000-g male newborn was born by spontaneous vaginal delivery with mild to moderate pulmonary hypoplasia, as the infant required mechanical support for the first 3 days of life. He also required peritoneal dialysis. Postnatal diagnosis of urethra atresia was confirmed by cystoscopy.
Case #6 was a male fetus with a prenatal diagnosis of severe LUTO at GA 20 weeks associated with anhydramnios. At our initial evaluation, favorable urinary analysis was observed at GA 21 weeks and a VAS was placed at GA 22 weeks. At GA 23 weeks, US examination confirmed that the shunt was in the correct place with an empty fetal bladder and normal amount of amniotic fluid. At GA 24 weeks, the shunt was dislodged into the amniotic cavity, and a large fetal bladder with associated oligohydramnios was identified on US examination. A second VAS was then placed the next day. At GA 26 weeks, fetal US revealed oligohydramnios (amniotic fluid index 6 cm), empty fetal bladder with the shunt in the correct position, and bilateral hyperechoic kidneys with renal cysts. At GA 27 weeks, we observed anhydramnios, empty fetal bladder with the shunt in the correct position, and signs of renal dysplasia. The diagnosis of ‘intrauterine fetal renal failure’ with extremely high likelihood of postnatal end-stage renal disease (ESRD) was considered and discussed with the parents. The 2081-g baby boy was born at 33 weeks with minimal respiratory distress. However, the infant required peritoneal dialysis within the first week of life. Urethra atresia was diagnosed by postnatal cystoscopy.
Case #7 was a male fetus referred to our center at GA 19 weeks due to a diagnosis of severe LUTO and anhydramnios. A VAS was placed the same week after a complete multidisciplinary evaluation. Initially, the fetal urinary analysis was considered ‘favorable’. However, 2 weeks later US examination revealed oligohydramnios with a small fetal bladder and the shunt in the correct position. At GA 24 weeks, signs of ‘intrauterine fetal renal failure’ were observed. A 2664-g baby boy was born, but died within the first day of life due to severe pulmonary hypoplasia.
Case #8 was a female fetus referred to our Fetal Center because of extremely large kidneys and a cystic lesion close to the fetal bladder neck, with possible diagnosis of cloacal anomaly, in association with oligohydramnios. At our center, we confirmed enlarged bladder (15 mL), extremely large kidneys (right kidney 85 mL; left kidney 45 mL) due to severe urinoma (Fig. 3a) associated with a cystic lesion inside the bladder that was more likely to be a prolapsed ureterocele causing urethral obstruction (Fig. 3b). Fetal echocardiogram revealed signs of cardiac failure due to compression caused by the extremely dilated kidneys. Bilateral US-guided fetal nephrocentesis and vesicocentesis were performed. After the procedure, fetal magnetic resonance imaging confirmed the findings but suggested that there were large pericapsular urinomas with small kidneys. The results of the fetal urinary biochemistry suggested a borderline renal function (urinary sodium 115 mEq/L; chloride 90 mEq/L; osmolarity 250 mOsm/L; beta2-microglobulin 4 mg/L). After multidisciplinary evaluation, we proposed a fetal cystoscopy with potential laser ablation of the ureterocele and a vesicoamniotic shunt. The family elected to go ahead with the procedure. The Fetal Therapy Board approved the request. At GA 20 weeks, we attempted entry into the bladder with fetoscope without success due to a small bladder. We placed a VAS (Harrison fetal shunt) after amnioinfusion and injection of sterile saline into the fetal bladder, but the shunt migrated 2 days later. The fetal bladder remained small during the following days with associated oligohydramnios. No more fetal intervention was indicated. There was a progressive reduction in the size of the bladder and the bilateral pericapsular urinomas. At GA 25 weeks, fetal bladder volume was minimal (7 mL), and the pericapsular urinomas were respectively 5 and 12 mL (much smaller than before) associated with anhydramnios (‘intrauterine fetal renal failure’). A 1636-g female baby was born at GA 31 weeks, with signs of moderate pulmonary hypoplasia, extremely small kidneys, and anuria. The newborn died in the first day of life.
Seventeen fetuses did not have signs of ‘intrauterine renal failure’. None of these met the primary composite postnatal outcome of either death within 24 h of life or the need for dialysis within the first week of life. Two of them had a normal amount of amniotic fluid during the entire pregnancy and did not undergo fetal intervention; all of them survived with normal renal function (6 month follow-up). Fetal intervention was performed in 13 fetuses with severe LUTO and oligohydramios (9 of whom had severe oligohydramios/anhydramnios); 11 (84.6 %) of these infants survived, of whom eight (61.5 %) had normal renal function (2 infants needed dialysis) at 6 months of life. The other two infants did not undergo fetal intervention, survived but needed dialysis that started after the first month of life. The overall survival in this group of patients was 88.2 % (15/17), with four (29.4 %) infants needing dialysis after the first month of life (at 6-month follow-up).
Table 2 shows the prenatal US characteristics in fetuses with ‘intrauterine fetal renal failure’ in comparison to those born without severe pulmonary hypoplasia or the need for dialysis in the first week of life. Fetuses that progressed with ‘intrauterine fetal renal failure’ later in pregnancy had less severe bilateral hydronephrosis (p = 0.02) and reduced percentage of bladder refilling 48 h after the first vesicocentesis (p = 0.008) during their initial evaluation. In addition, there was a tendency of fetuses with ‘intrauterine fetal renal failure’ to present with initial US findings suggestive of ‘renal dysplasia’ when compared to fetuses without ‘intrauterine fetal renal failure’ (p = 0.08). The other factors were not associated with the prenatal diagnosis of ‘intrauterine fetal renal failure’.
The ROC curve analysis provided the value −27 % of the variable ‘percentage of fetal bladder refilling’ as the best cut-off to predict the condition ‘intrauterine fetal renal failure’ (sensitivity 80 %, specificity 75 %, area under the time–concentration curve 0.86, 95 % confidence interval 0.68–0.99; p = 0.009; Fig. 4).
The perinatal mortality in the group with ‘intrauterine fetal renal failure’ was 75 % (6/8), and the two surviving infants had fetal VAS placements and required dialysis in the first weeks of life.
Discussion
In the study reported here we describe the concept and the clinical findings related to intrauterine renal failure in fetuses with severe LUTO. This situation is characterized by the appearance of hyperechogenic kidneys consistent with renal dysplasia and anuria on US images. Fetal anuria can be diagnosed when there is a small bladder with a thick wall associated with anhydramnios. Intrauterine fetal renal failure can also occur despite fetal intervention (e.g., VAS placement). In our experience, intrauterine fetal renal failure occurred even after fetal intervention, but later in pregnancy and despite the initially ‘favorable’ urinary indices. Postnatally these infants have either severe pulmonary hypoplasia (depending on the GA that anhydramnios occurred) or ESRD within first days of life. The ‘intrauterine fetal renal failure’ is clearly the most severe form of renal disease in LUTO.
Our study suggests that ‘intrauterine fetal renal failure’ can be predicted by evaluating bladder refilling after US-guided vesicocentesis. In our experience, we observed that those fetuses with severe forms of LUTO and poor renal function did not produce adequate urine to refill the bladder within 2 days. We objectively evaluated bladder refilling by measuring the fetal bladder volumes before fetal vesicocentesis and 48 h after the fetal bladder was drained. We estimated the percentage of bladder refilling, which was the only factor we found to be associated statistically with the diagnosis of ‘intrauterine fetal renal failure’. Based on the ROC curve analysis, if the fetal bladder refilling within 48 h is <27 %, there is a high probability that this fetus will progress to ‘intrauterine fetal renal failure’.
Once the diagnosis of ‘intrauterine renal failure’ is established, the prognosis is very poor. At the present time, these cases are not considered to be candidates for fetal intervention (VAS or fetal cystoscopy) due to the extremely high mortality and morbidity regardless of intervention. It has been suggested that a therapeutic option for fetuses with severe LUTO and anhydramnious could be serial amnioinfusions. Bienstock et al. [29] reported a case of bilateral renal agenesis treated with serial amnioinfusion in which the newborn survived the newborn period and was able to undergo peritoneal dialysis as a bridge to planned renal transplantation. It is possible that these same principles can be applied to fetuses with severe LUTO and ‘intrauterine fetal renal failure’. The objective of serial amnioinfusion for those fetuses would be to reduce the severity of pulmonary hypoplasia and therefore increase the chance that the newborn survives to begin peritoneal dialysis. This hypothesis has not been confirmed and needs further investigation.
In conclusion, we have characterized a subset of fetuses with severe LUTO who do not produce sufficient urine in utero due to ‘intrauterine renal failure’. This situation is associated with severe pulmonary hypoplasia and ESRD within the first few days of life. While the present study has some limitations, including the retrospective design and the small cohort size, it appears that the percentage of fetal bladder refilling 48 h after fetal vesicocentesis can be used to clinically predict the ‘intrauterine fetal renal failure’.
References
Clayton DB, Brock JW 3rd (2014) Lower urinary tract obstruction in the fetus and neonate. Clin Perinatol 41:643–659
Tonni G, Vito I, Ventura A, Grisolia G, De Felice C (2013) Fetal lower urinary tract obstruction and its management. Arch Gynecol Obstet 287:187–194
Wu S, Johnson MP (2009) Fetal lower urinary tract obstruction. Clin Perinatol 36:377–390
Lissauer D, Morris RK, Kilby MD (2007) Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med 12:464–470
Robyr R, Benachi A, Daikha-Dahmane F, Martinovich J, Dumez Y, Ville Y (2005) Correlation between ultrasound and anatomical findings in fetuses with lower urinary tract obstruction in the first half of pregnancy. Ultrasound Obstet Gynecol 25:478–482
Bernardes LS, Aksnes G, Saada J, Masse V, Elie C, Dumez Y, Lortat-Jacob SL, Benachi A (2009) Keyhole sign: how specific is it for the diagnosis of posterior urethral valves? Ultrasound Obstet Gynecol 34:419–423
Ruano R, Sananes N, Sangi-Haghpeykar H, Hernandez-Ruano S, Moog R, Becmeur F, Zaloszyc A, Giron AM, Morin B, Favre R (2015) Fetal intervention for severe lower urinary tract obstruction: a multicenter case-control study comparing fetal cystoscopy with vesicoamniotic shunting. Ultrasound Obstet Gynecol 45:452–458
Ruano R (2011) Fetal surgery for severe lower urinary tract obstruction. Prenat Diagn 31:667–674
Sananes N, Favre R, Koh CJ, Zaloszyc A, Braun MC, Roth DR, Moog R, Becmeur F, Belfort MA, Ruano R (2015) Urological fistulas after fetal cystoscopic laser ablation of posterior urethral valves - surgical technical aspects. Ultrasound Obstet Gynecol 45:183–189
Ruano R, Yoshizaki CT, Giron AM, Srougi M, Zugaib M (2014) Fetal cystoscopic placement of transurethral stent in a fetus with urethral stenosis. Ultrasound Obstet Gynecol 44:238–240
Morris RK, Malin GL, Quinlan-Jones E, Middleton LJ, Hemming K, Burke D, Daniels JP, Khan KS, Deeks J, Kilby MD, Percutaneous vesicoamniotic shunting in Lower Urinary Tract Obstruction (PLUTO) Collaborative Group (2013) Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet 382:1496–1506
Ethun CG, Zamora IJ, Roth DR, Kale A, Cisek L, Belfort MA, Haeri S, Ruano R, Welty SE, Cassady CI, Olutoye OO, Cass DL (2013) Outcomes of fetuses with lower urinary tract obstruction treated with vesicoamniotic shunt: a single-institution experience. J Pediatr Surg 48:956–962
Ruano R, Yoshisaki CT, Salustiano EM, Giron AM, Srougi M, Zugaib M (2011) Early fetal cystoscopy for first-trimester severe megacystis. Ultrasound Obstet Gynecol 37:696–701
Morris RK, Ruano R, Kilby MD (2011) Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol 37:629–637
Ruano R, Duarte S, Bunduki V, Giron AM, Srougi M, Zugaib M (2010) Fetal cystoscopy for severe lower urinary tract obstruction--initial experience of a single center. Prenat Diagn 30:30–39
Brown C, Morris RK, Daniels J, Khan KS, Lilford RJ, Kilby MD (2010) Effectiveness of percutaneous vesico-amniotic shunting in congenital lower urinary tract obstruction: divergence in prior beliefs among specialist groups. Eur J Obstet Gynecol Reprod Biol 152:25–29
Clifton MS, Harrison MR, Ball R, Lee H (2008) Fetoscopic transuterine release of posterior urethral valves: a new technique. Fetal Diagn Ther 23:89–94
Hofmann R, Becker T, Meyer-Wittkopf M, Tekesin I, Sierra F, Schmidt S (2004) Fetoscopic placement of a transurethral stent for intrauterine obstructive uropathy. J Urol 171:384–386
Welsh A, Agarwal S, Kumar S, Smith RP, Fisk NM (2003) Fetal cystoscopy in the management of fetal obstructive uropathy: experience in a single European centre. Prenat Diagn 23:1033–1041
Quintero RA, Homsy Y, Bornick PW, Allen M, Johnson PK (2001) In-utero treatment of fetal bladder-outlet obstruction by a ureterocele. Lancet 357:1947–1948
Holmes N, Harrison MR, Baskin LS (2001) Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics 108:E7
Agarwal SK, Fisk NM (2001) In utero therapy for lower urinary tract obstruction. Prenat Diagn 21:970–976
Quintero RA, Shukla AR, Homsy YL, Bukkapatnam R (2000) Successful in utero endoscopic ablation of posterior urethral valves: a new dimension in fetal urology. Urology 55:774
Quintero RA, Hume R, Smith C, Johnson MP, Cotton DB, Romero R, Evans MI (1995) Percutaneous fetal cystoscopy and endoscopic fulguration of posterior urethral valves. Am J Obstet Gynecol 172:206–209
Biard JM, Johnson MP, Carr MC, Wilson RD, Hedrick HL, Pavlock C, Adzick NS (2005) Long-term outcomes in children treated by prenatal vesicoamniotic shunting for lower urinary tract obstruction. Obstet Gynecol 106:503–508
Grignon A, Filion R, Filiatrault D, Robitaille P, Homsy Y, Boutin H, Leblond R (1986) Urinary tract dilatation in utero: classification and clinical applications. Radiology 160:645–647
Muller F, Dommergues M, Mandelbrot L, Aubry MC, Nihoul-Fekete C, Dumez Y (1993) Fetal urinary biochemistry predicts postnatal renal function in children with bilateral obstructive uropathies. Obstet Gynecol 82:813–820
Nicolini U, Fisk NM, Rodeck CH, Beacham J (1992) Fetal urine biochemistry: an index of renal maturation and dysfunction. Br J Obstet Gynaecol 99:46–50
Bienstock JL, Birsner ML, Coleman F, Hueppchen NA (2014) Successful in utero intervention for bilateral renal agenesis. Obstet Gynecol 124:413–415
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Ethics statement
Local institutional review board approval was received for this study. Due to the retrospective nature of the study, parental consent was not required.
Additional information
All authors contributed to the present study
Rights and permissions
About this article
Cite this article
Ruano, R., Safdar, A., Au, J. et al. Defining and predicting ‘intrauterine fetal renal failure’ in congenital lower urinary tract obstruction. Pediatr Nephrol 31, 605–612 (2016). https://doi.org/10.1007/s00467-015-3246-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3246-8