Abstract
Background
Peritoneal dialysis is the preferred mode of renal replacement therapy in infants with end-stage renal disease (ESRD). Hemodialysis (HD) is seldom used in neonates and infants due to the risk of major complications in the very young.
Methods
Demographic, clinical, laboratory, and imaging data on all infants younger than 12 months with ESRD who received HD in our Pediatric Dialysis Unit between January 1997 and June 2013 were analyzed.
Results
Eighteen infants (n = 6 male) with ESRD (median age 3 months; median weight 4.06 kg) received HD through a central venous catheter (CVC) for a total of 543 months (median duration per infant 16 months). Seven of the infants (39 %) were neonates, and five (28 %) had serious comorbidities. There were five episodes of CVC infection, which is a rate of 0.3/1000 CVC days. Median catheter survival time was 320 days. Most infants had good oral intake, and only four (22 %) required a gastric tube; 14 (78 %) infants displayed normal growth. Fourteen (78 %) infants had hypertension, of whom four (22 %) had severe cardiac complications; eight (44 %) showed delayed psychomotor development. Eleven (61 %) of the infants, including six (86 %) of the neonates, survived. Five (28 %) infants underwent renal transplantation; 10-year graft survival was 80 %.
Conclusions
Based on these results, long-term HD in neonates and infants with ESRD is technically feasible, can be implemented without major complications, carries a very low rate of CVC infection and malfunction, and results in adequate nutrition, good growth, as well as good kidney graft and patient survivals. Future efforts should aim to prevent hypertension and its cardiac sequelae, improve neurodevelopmental outcome, and lower mortality rate in these infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The care for infants with end-stage renal disease (ESRD) poses one of the most challenging tasks in pediatric nephrology. The need to maintain fluid balance and metabolic homeostasis, to enable adequate growth, and to promote cognitive development at early age in the face of uremia make this small but unique group of children extremely difficult to treat [1–4]. In particular, renal replacement therapy (RRT) in these infants, which is their mainstay of therapy while awaiting sufficient growth for renal transplantation, is a very demanding and complex procedure to perform.
Traditionally, peritoneal dialysis (PD) has been the preferred mode of RRT in infants with ESRD [3–11]. The 2011 annual data report of the U.S. Renal Data System [12] reports that of the children with ESRD who were less than 2 years of age in 2011, 92.5 % were receiving PD compared with 7.5 % who were receiving hemodialysis (HD). There has been reluctance to use HD in infants because of the technical difficulties and the major complications potentially associated with this procedure in the very young patient. These include difficulties related to vascular access, HD catheter infections, hemodynamic instability, rapid fluctuations in infant’s volume status, bleeding episodes, the need for blood priming of circuits resulting in potential sensitization against potential donors for transplantation, and other complications [13, 14]. However, HD has several advantages over PD, including a fast and very efficient correction of fluid and metabolic abnormalities and avoidance of potential PD-related abdominal fullness and satiety.
These factors along with the significant improvement in HD equipment and technology offered to small children and infants have led to a recent increase in the use of HD in very young patients [1, 12]. Nevertheless, data on the use and outcome of HD in infants are scarce. The few studies on small numbers of infants treated with this mode of RRT describe a successfully implemented procedure albeit with major HD-related complications and relatively high mortality rate [13, 15–18] (Table 1).
The aim of the present study was to retrospectively summarize our experience with long-term HD in infants under the age of 12 months with ESRD in a pediatric dialysis unit in northern Israel, and to describe the care and outcome of these infants.
Methods
The data of all infants younger than 12 months of age with ESRD who received HD therapy in the Pediatric Dialysis Unit at Ruth Rappaport Children’s Hospital in Haifa between January 1997 and June 2013 were analyzed. This tertiary care hospital serves a population of approximately half a million children of various ethnic groups in northern Israel. Demographic, clinical, laboratory, and imaging data on all infants who were under the age of 12 months at the initiation of RRT were obtained and analyzed. We analyzed all patients grouped together and infants who initiated RRT at less than 1 month of age or at age 1–12 months separately.
Long-term HD was performed through tunneled, double-cuffed central venous catheters (CVC) (Medcomp, 8 F, 12-cm length; Medical Components, Inc., Harleysville, PA) inserted by the angiographic route by an invasive radiologist or by the surgical route. HD was performed by highly qualified pediatric dialysis nursing staff under the supervision of experienced pediatric nephrologists. The procedure was carried out with AK200s® dialysis machines (Gambro, Lund, Sweden). Between 2004–2006 we used Fresenius polysulfone® F3 dialysis filters (surface area 0.4 m2; Fresenius SE & Co., Bad Homburg, Germany), and from 2007 onward we used FXPaed® filters (surface area 0.2 m2; Fresenius). We used infant HD tubes (Fresenius) with a total extracorporeal volume (filter + tubing) of 58 ml when F3 filters were used, and 48 ml for dialysis with FXPaed. G-204 Acidic Component solutions (Teva Medical, Petach Tikva, Israel) with low calcium concentration (1.25 mmol/L) were used. When the extracorporeal volume was >10 % of the infant’s blood volume (80 ml/kg body weight), the system was primed with packed red blood cells. HD was performed 5–6 times per week for 3–4 h per treatment (total weekly dialysis time 18–24 h) under close monitoring of clinical status, heart rate, and blood pressure. The effectiveness of HD was assessed in all infants by determining urea reduction rate and Kt/V; the target values were >65 % and >1.3, respectively.
Our hospital charges the third party payers the sum of $458 per HD treatment performed in the Pediatric Dialysis Unit per child (or, with a monthly average of 22 treatments per child $10,076 per month per child).
Our routine management of HD-CVCs is described in a previous publication [19]. Briefly, it includes a strictly sterile use and care of catheters performed on a daily basis: in the hospital on dialysis days by the nursing staff and at home by all appropriately trained parents. Our meticulous care of HD catheters makes no use of prophylactic (topical, catheter lock, or systemic) antibiotic therapy. Between dialysis sessions, both CVC lumens are filled with a concentrated (5000 u/ml) heparin solution [19].
The ESRD therapeutic regimen included, in addition to the intensive HD therapy, appropriate nutrition, vitamin supplements, correction of acid–base and electrolyte abnormalities, control of mineral balance, treatment of bone disease, control of blood pressure, correction of anemia, and promotion of cognitive development, according to established standards [20].
All infants received physiotherapy and occupational therapy on a regular basis, and parents were instructed to provide these therapies also in the home setting. Hearing test [brainstem evoked response audiometry (BERA)] was performed on all infants before the age of 12 months or when indicated.
Results
Patients
During the 16-year study period, 18 infants younger than 12 months with ESRD received long-term HD therapy (Tables 1 and 2). Of these 18 infants, seven (39 %) (group 1) started RRT before the age of 1 month and 11 (61 %) (group 2) between 1 and 12 months of age (Table 2). Six (33 %) of the patients started PD therapy which was subsequently (1–3 months later) changed to HD (see below). The median age and weight of patients at insertion of the first RRT catheter were 3 months and 4.06 kg, respectively. The median individual duration of RRT for the 18 infants in the study was 12 months (PD 4 months, HD 16 months) (Table 2). The cumulative duration of RRT in all infants was 645 months, with PD accounting for 102 (16 %) months and HD accounting for 543 (84%) months (Tables 1 and 2). Twelve infants were girls, and six were boys; all were of Caucasian origin. Six (33 %) of the infants were born to consanguineous parents. The etiologies of ESRD included primary hyperoxaluria (6 infants), congenital anomalies of the kidney and urinary tract (CAKUT; 4 infants), congenital nephrotic syndrome (3 infants), autosomal recessive polycystic kidney disease (ARPKD; 2 infants), acute kidney injury resulting in irreversible cortical necrosis (2 infants, 1 of whom also had tuberous sclerosis), and atypical hemolytic uremic syndrome (1 infant).
Five (28 %) of the infants had serious comorbidities (Table 2), including brain damage secondary to tuberous sclerosis, hepatic fibrosis due to ARPKD, hemolytic anemia due to neonatal autoimmune antibodies, hypothyroidism due to congenital nephrotic syndrome, and VACTERL association.
HD catheters
In total, 38 CVCs were inserted in these 18 infants during the 16-year study period (Table 2). Four (10 %) of these were inserted by the surgical route into the subclavian vein (all of them before January 2005), and 34 (90 %) were inserted by the angiographic route into the internal jugular vein by the invasive radiology team. Median age and weight of the infants in group 1 at insertion of the HD catheter was 3 days and 3.1 kg, respectively; in the infants in group 2, these were 137 days and 5.3 kg, respectively (Table 2).
During the study period, there were five episodes of catheter infection (Tables 1 and 2). CVC infection rate for all HD catheters was 0.3/1000 CVC days or one infection per 9 CVC years. The infection rates for the infants in groups 1 and 2 were 0.56/1000 and 0.1/1000 CVC days, respectively (Table 2).
CVC survival times during the study period are given in Tables 1 and 2. Of the 38 catheters inserted, 19 (50 %) were removed because of malfunction, including obstruction, catheter tear, and (in 4 infants) extrusion of the catheter at the exit site due to the infant’s growth. No catheter was removed due to infection. In 12 (32 %) of the CVCs, a properly functioning catheter was removed because of successful transplantation (5 infants) or patient death (7 infants). Seven (18 %) of the catheters inserted were in place at the end of the study. The median CVC survival time for the whole group of infants during the study period was 320 days (group 1 infants 391 days; group 2 infants 283 days).
Initiation of RRT
In six of the patients (4 in group 1, 2 in group 2), PD was selected as the initial mode of RRT. For this purpose, a Tenckhoff PD catheter was inserted surgically into the abdomen at an age ranging from 1 day to 2 months. Major PD-related complications necessitated PD catheter removal within 1–3 months from start of PD and the change to HD therapy in all six infants.
In 12 of the patients, HD was the initial mode of RRT. HD was selected as the initial mode in all infants with hereditary hyperoxaluria not only to treat the ESRD but also to use the well-known superiority of HD over PD in removing oxalate loads from the circulation [21]. Severe malnutrition/hypoalbuminemia (2 infants), severe volume overload (3 infants), and inadequate family/home environment for PD (2 infants) served as other indications for initiating HD.
In eight of the 18 patients in the study, HD was initiated using an acute HD catheter that was subsequently (4 days to 7 months later) replaced by the angiographic route to a permanent HD-CVC.
Hypertension
Of the 18 infants, 14 (78 %) had long-standing hypertension {systolic and/or diastolic blood pressure values above 2 standard deviations (SD) of the mean for height [22]} during the time period of HD therapy (Table 2). The hypertension was treated with volume removal by HD, dietary salt restriction, and treatment with various combinations of antihypertensive drugs. Four (22 %) of all infants received two drugs, three (16.6 %) received three drugs, and five (31.2 %) received four or more drugs to treat their hypertension.
Echocardiograms showed different degrees of left ventricular hypertrophy in all infants with hypertension. Two of these infants also had left ventricular dilatation associated with poor contraction of the ventricular wall caused by the hypertension. Two infants developed hypertension-induced aneurysms of the ascending aorta which have remained stable during aggressive antihypertensive therapy.
Overall, although renal transplantation has resulted in a marked decrease in blood pressure values, most transplanted patients have continued to show mild to moderate hypertension necessitating drug therapy.
Nutrition and growth
Median weight SD score (SDS) for all infants at initiation of their RRT was −2.9 (Fig. 1); the body weight of ten of all infants (55.5 %) was below −2SDS. The median height SDS for all infants at initiation of RRT was −1.9 SDS; the height of three infants (17 %) was below −2SDS.
We observed a markedly better appetite and oral food intake in infants receiving HD therapy compared with infants receiving PD. This improvement may be attributable to HD not being associated with the appetite-suppressing effect of abdominal fullness and glucose absorption into the circulation which is typical of PD. As a result, only four (22 %) of the infants in our study required gastrostomy tube placement for proper feeding and nighttime supplemental nutrition. One infant received growth hormone therapy.
As depicted in Fig. 1, median weight and height SDS for all infants at the end of their study period were −0.8 and −.65 SDS, respectively. Median change (∆) of the SDS in the whole group of infants at the end of their study when compared with its beginning was +2.0 for weight and +0.25 for height. As indicated in Tables 1 and 2, at the end of the study, the SDS for weight and height was below −2 for only two (11 %) and four (22 %) of the infants, with the latter group of 4 infants including the former two infants.
Psychomotor development
Of the 18 infants, eight (44 %; 5 in group 1, 3 in group 2) displayed various degrees of delay in psychomotor development as assessed by Alberta Infant Motor Scale (AIMS) [23] (Tables 1 and 2). Four of these infants also had recurrent seizure episodes (including the patient with tuberous sclerosis, 2 infants who required resuscitation in the neonatal period, and 1 infant who had prenatal sonographic undefined brain lesions). Three of the infants (who received a variety of ototoxic drugs during the course of their kidney disease) had sensorineural deafness demonstrated by abnormal BERA; two subsequently underwent cochlear implants which resulted in a marked improvement in hearing.
Outcome of patients
Transplantation
Five of the 18 infants (28 %) underwent renal transplantation at a median age of 4 (range 3–4.9) years and a median weight 14.8 (range 14–19) kg (Tables 1 and 2). All but one of the infants transplanted received a deceased donor kidney. Two patients with primary hyperoxaluria were transplanted; one underwent sequential deceased donor liver/living-related kidney transplantation, and one had liver transplantation only. Four (80 %) of the infants who underwent kidney transplantation had a properly functioning kidney 12 years following transplantation. One transplanted patient died 4.5 years following transplantation as a result of noncompliance with immunosuppressive therapy.
Patient survival
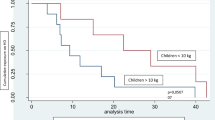
The 5- and 10-year patient survival for the whole group of infants was 66 and 61 %, respectively (Fig. 2). Whereas the infants in group 2 had 5- and 10-year survival rates of 64 and 55 %, respectively, it is remarkable that those in group 1 had 5- and 9-year survival rates of 85 % (Fig. 2).
Of the 18 infants, seven (39 %) died (1 in group 1, 6 in group 2) (Tables 1 and 2). The median age of death was 2.9 years (range 6 months to 8 years). Six infants who succumbed to their disease died while receiving HD therapy, and one died after receiving kidney transplantation. The causes of death in those infants on HD therapy were uncontrolled infection (2 two infants: fungal sepsis), uncontrolled hemorrhage post nephrectomy (1 infant), surgical complications following liver transplantation (1 infant), and unknown cause (death at home; 2 infants. The one infant who died after undergoing a kidney transplant suffered a cardiac arrest.
Eleven (61 %) of the infants were alive at the end of the study (median age 4 years, range: two months to 8.5 years). Seven of these were receiving HD therapy, and four had a functioning kidney graft (see above).
Discussion
Whereas ample data exists on outcome of infants treated with long-term PD [3–11, 24, 25], very little is known about the outcome of infants receiving HD therapy. The present study, which summarize 543 months of HD therapy in infants, is to the best of our knowledge the largest and the most comprehensive study on the use of long-term HD in infants in general and in neonates in particular (Table 1).
Our Division of Pediatric Nephrology serves a large multi-ethnic population characterized by a high rate of parental consanguinity and genetic/hereditary diseases, deeply rooted religious and cultural traditions, and a low rate of pregnancy terminations even in the face of a fetal disease diagnosed in utero. These social and medical circumstances have contributed to the establishment of several unique features of our pediatric ESRD program:
-
1.
The proportion of neonates and infants to the total number of children receiving chronic dialysis in our program is exceptionally high (40–45 %).
-
2.
There is a very high rate (60–70 %) of genetic/hereditary diseases (most with systemic involvement) leading to ESRD in early life, with hyperoxaluria leading the list.
-
3.
There is a high rate of serious comorbidities accompanying the ESRD in infants.
-
4.
The vast majority of infants and children with ESRD, even in those with severe/life-threatening comorbidities, are given RRT. Motivated by religious/cultural considerations, parents almost always reject the option that is thoroughly discussed with and offered to them—that of withholding dialysis, when indicated [26–28].
-
5.
Social/familial circumstances and considerations as well as the high rate of hyperoxaluria in our ESRD patient population dictate the use of HD rather than PD in most infants and children with ESRD in our program.
-
6,
There is a low rate of both living related and cadaver kidney transplantations (see discussion in subsequent text).
Whereas HD-CVCs are efficient in delivering adequate dialysis in infants, accumulating data have shown a high rate of catheter- related blood stream infections and short survival times for these catheters [13, 15–18, 29] (Table 1). In contrast to these data, our findings (Tables 1 and 2) show a markedly low CVC infection rate (0.3/1000 catheter days) and prolonged catheter survival time (median 320 days) in the population of infants included in the study. Of note is the higher median catheter survival time (391 days) in the very young infants (group 1) when compared with the catheter survival time (283 days) in the older infants (group 2) (Table 2). We believe that it is our routine, meticulous, and very strict care of HD catheters (as outlined in [19]) which results in our remarkably low catheter infection rates and prolonged catheter survival times.
Of the 18 infants in our study, 14 (78 %) had long-standing hypertension, including six of the seven neonates (group 1) (Table 2). Hypertension (mainly due to fluid overload) is a well-known complication in children receiving long-term dialysis therapy and may be present in up to 80 % of patients [11, 30–32]. Risk factors for uncontrolled hypertension include, among others, HD therapy and young age (at which fluid restriction is limited by the dependence of the young on formula feeding) [11, 30, 31].
Cardiovascular disease is increasingly recognized as a significant health problem in young patients with kidney disease in general and in children receiving dialysis therapy in particular [4, 20, 33, 34]. Arrhythmias, valvular disease, cardiomyopathy, and death from cardiac arrest have been reported in pediatric dialysis patients [4, 20, 33]. Noteworthy in this regard are the hypertension- induced echocardiographic findings in all hypertensive infants in our study and the severe cardiac complications in four of them (2 infants with left ventricular dilatation, 2 infants with aneurysms of the ascending aorta; see Results). These findings highlight the importance of careful monitoring and aggressive management of hypertension in infants receiving HD therapy, including dietary salt restriction, more frequent HD therapies, more aggressive fluid removal during dialysis, the development of reliable techniques to monitor extracellular/intravascular volume during HD, and full compliance with the appropriate drug therapy.
Promoting adequate growth is one of the major challenges faced by the medical staff caring for infants with ESRD. While several studies have reported that an early and more intensive approach to feeding improves weight and height SDS in infants receiving PD [4, 6, 7, 35, 36], a recent multi-continental study demonstrated growth retardation in 63 % of neonates with ESRD, 2 years after the start of PD therapy [11]. Al Hermi et al. [17] and Quinlan et al. [18] reported that 90 and 33 %, respectively, of the infants receiving long-term HD in their studies were growth-retarded at the end of their studies (Table 1). In contrast, the infants in our study displayed improved growth SDS, in particular for weight, during the study period (Fig. 1). There was a very limited use of growth hormone in the infants in our study. It is possible that a more widespread provision of growth hormone to these infants could have resulted in even more favorable growth rates.
Infants and children with ESRD receiving dialysis therapy are at risk for neurocognitive and neurodevelopmental delay [4, 14, 20]. Whereas some studies [37, 38] have reported relatively mild neurocognitive delays in infants receiving long-term PD or transplanted in early childhood, other studies [39–41] have found impaired cognitive and educational attainments in patients with ESRD since infancy. Among our 18 infants who received HD, eight (44 %) demonstrated psychomotor retardation and four (22 %) had seizures; these values are in line with the data cited above [39–41] as well as with previous studies on infants receiving HD demonstrating psychomotor impairments in 45 % [13] and 50 % [17] of these infants (Table 1).
The relative roles of very young age, perinatal events, presence of comorbidities and, finally, the use of HD, in the pathogenesis of these neurocognitive impairments should be explored on larger numbers of infants. It is plausible, however, that the modern, significantly upgraded HD equipment and the continuously improving RRT methods in infants [42] will minimize the potential adverse effects of HD-induced hemodynamic fluctuations on brain structure and development.
During the study period, five (28 %) of the infants received kidney transplantation at a median age of 4 years. These are relatively low transplantation rates and late transplantation times when compared with data reported in previous studies showing 39–80 % of the infants treated with PD receiving a kidney transplantation [7, 8, 10] and 20–80 % of neonates treated with PD being transplanted at the age of 1–3 years [8, 9, 11]. Transplantation rates varying between 40 and 89 % have been reported in infants receiving HD [13, 15–18] (Table 1).
Several factors contributed to the low transplantation rate and late transplantation time in our study. These include the high rate of infants with primary hyperoxaluria under our care (mandating a liver transplantation prior to kidney transplantation), the cultural/ religious objections to living-related kidney transplantation within the population we serve, and, finally, the national shortage of organs for transplantation. The high graft survival rate of 80 % in the small number of infants in our study, which was achieved despite the relatively long time these infants needed to be maintained on dialysis, should not be interpreted as endorsing long-term dialysis for infants. Early transplantation is the goal in infants with ESRD.
One of the main challenges of the care of infants with ESRD is to improve their relatively low survival rate. Overall, reported mortality rates in infants treated with PD have varied between 9.5 and 48 %, with some studies reporting risk of death in these infants to be three- to fourfold higher than those in older children [2, 3, 7, 10, 43, 44]. In addition, 5- to 10-year infant survival rates in infants have varied between 50 and 76 % [2, 9–11], which are markedly lower than those in older children. The three main factors that have been found to be the best predictors of poor outcome in these studies include very young age at dialysis initiation, the presence of comorbidities, and the absence of a renal transplant. Of note, however, are the findings from recent studies demonstrating no difference in mortality rates between neonates receiving PD therapy and older infants [5, 8] and 5- to 10-year survival rates in infants undergoing PD of around 80 % [6]. The reported mortality rates in infants receiving HD have varied between 18 and 50 % [13, 15–18] (Table 1).
The overall survival rate of the whole group of infants in our study was 61 % (Tables 1 and 2), with 5- and 10-year survival rates of 66 and 61 %, respectively (Fig. 2). These survival rates, which are in agreement with the data in most of the studies cited above, have been achieved despite the presence of serious comorbidities in a large proportion of our infants, as well as the relatively low rate and late age of kidney transplantation. The very high overall survival rate of 86 % (Table 1) and the 5- and 9-year survival rates of 85 % (Fig. 2) in the seven neonates in our study, which stand in contrast to reports of the adverse effect of very young age on the survival of dialyzed infants [2, 3, 7–10, 15, 44], may be secondary to the small cohort. These findings should be the subject of future studies on larger numbers of infants.
Our study is limited by it being a single-center study in which a relatively small number of patients were enrolled. In addition, the study period was relatively long (16 years), and dialysis techniques in the young evolved during this time. These limitations, however, stem from the nature of this study, the subject of which is a very challenging, and the relative newness of the therapeutic options offered to this very small and specific group of patients in selected pediatric dialysis centers.
In conclusion, long-term HD in neonates and infants with ESRD, when performed by skilled and experienced personnel in the appropriate setting, is technically feasible and can be implemented without major complications. HD therapy in neonates and infants, even in the most challenging cases, carries a very low rate of CVC infection and malfunction and results in adequate nutrition, good growth, as well as good kidney graft and patient survivals. As such, it represents a valid alternative to PD. Future efforts should aim to prevent hypertension and its cardiac sequelae, improve neurodevelopmental outcome, and lower mortality rate in infants receiving HD therapy.
References
Rees L (2013) Infant dialysis-what makes it special? Nat Rev Nephrol 9:15–17
McDonald SP, Craig JC, for the Australian and New Zealand Pediatric Nephrology Association (2004) Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662
Wood EG, Hand M, Briscoe DM, Donaldson LA, Yiu V, Harley FL, Warady BA, Ellis EN, for the North American Pediatric Renal Transplant Cooperative Study (2001) Risk factors for mortality in infants and young children on dialysis. Am J Kidney Dis 37:573–579
Shroff R, Ledermann SE (2009) Long-term outcome of chronic dialysis in children. Pediatr Nephrol 24:463–474
Wedekin M, Ehrich JH, Offner G, Pape L (2010) Renal replacement therapy in infants with chronic renal failure in the first year of life. Clin J Am Soc Nephrol 5:18–23
Mekahli D, Shaw V, Ledermann SE, Rees L (2010) Long-term outcome of infants with severe chronic kidney disease. Clin J Am Soc Nephrol 5:10–17
Vidal E, Edefonti A, Murer L, Gianoglio B, Maringhini S, Pecoraro C, Sorino P, Leozappa G, Lavoratti G, Ratsch IM, Chimenz R, Verrina E, on behalf of Italian Registry of Paediatric Chronic Dialysis (2012) Peritoneal dialysis in infants: the experience of the Italian Registry of Paediatric Chronic Dialysis. Nephrol Dial Transplant 27:388–395
Carey WA, Talley LI, Shering SA, Jaskula JM, Mathias RS (2007) Outcomes of dialysis initiated during the neonatal period for treatment of end stage renal disease: a North American Pediatric Renal Trials and Collaborative Studies Special Analysis. Pediatrics 119:e468–e473
Rheault MN, Rajpal J, Chavers B, Nevins TE (2009) Outcomes of infants <28 days old treated with peritoneal dialysis for end-stage renal disease. Pediatr Nephrol 24:2035–2039
Alexander T, Foster BJ, Tonelli MA, Soo A, Nettel-Augirre A, Hemmelgarn BR, Samuel SM, of the Pediatric Renal Outcomes Group Canada (2012) Survival and transplantation outcomes of children less than 2 years of age with end-stage renal disease. Pediatr Nephrol 27:1975–1983
Van Stralen KJ, Borzych-Duzalka HH, Kennedy SE, Jager KJ, Verrina E, Inward C, Rönnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P, for the ESPN/ERA-EDTA, IPPN, ANZDATA and Japanese RRT registries (2014) Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86:168–174
Anonymous (2012) Excerpts from the United States Renal Data System 2011 Annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59:e257–e266
Shroff R, Wright E, Ledermann S, Hutchinson C, Rees L (2003) Chronic hemodialysis in infants and children under 2 years of age. Pediatr Nephrol 18:378–383
Zaritsky J, Warady BA (2011) Peritoneal dialysis in infants and young children. Semin Nephrol 31:213–224
Feinstein S, Rinat C, Becker-Cohen R, Ben-Shalom E, Schwartz SB, Frishberg Y (2008) The outcome of chronic dialysis in infants and toddlers-advantages and drawbacks of haemodialysis. Nephrol Dial Transplant 23:1336–1345
Kovalski Y, Cleper R, Krause I, Davidovits M (2007) Hemodialysis in children weighing less than 15 kg: a single-center experience. Pediatr Nephrol 22:2105–2110
Al-Hermi BE, Al-Saran K, Secker D (1999) Hemodialysis for end-stage renal disease in children weighing less than 10 kg. Pediatr Nephrol 13:401–403
Quinlan C, Bates M, Sheils A, Dolan N, Riordan M, Awan A (2013) Chronic hemodialysis in children weighing less than 10 kg. Pediatr Nephrol 28:803–809
Eisenstein I, Tarabeih M, Magen D, Pollack S, Kassis I, Ofer A, Engel A, Zelikovic I (2011) Low infection rates and prolonged survival times of hemodialysis catheters in infants and children. Clin J Am Soc Nephrol 6:793–798
Warady BA, Neu AM, Schaefer F (2014) Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis 64:128–142
Cochat P, Rumsby G (2013) Primary hyperoxaluria. N Engl J Med 369(7):649–658
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Darrah J, Bartlett D, Maguire TO, Avison WR, Lacaze-Masmonteil T (2014) Have infant gross motor abilities changed in 20 years? Are-evaluation of the Alberta Infant Motor Scale normative values. Dev Med Child Neurol 9:877–881
Zurowska AM, Fischbach M, Watson AR, Edefonti A, Stefanidis CJ, on behalf of the European Pediatric Dialysis Working Group (2013) Clinical practice recommendations for the care of infants with stage 5 chronic kidney disease (CKD5). Pediatr Nephrol 28:1739–1748
Neu AM, Sander A, Borzych-Duzatka D, Watson AR, Valles PG, Soo Ha IS, Patel H, Askenazi D, Balasz-Chmielewska I, Lauronen J, Groothoff JW, Feber J, Schaefer F, Warady BA, on behalf of the IPPN investigators (2012) Comorbidities in chronic pediatric dialysis patients: a report of the International Pediatric Peritoneal Dialysis network. Perit Dial Int 32:410–418
Shooter M, Watson A (2000) The ethics of withholding and withdrawing dialysis therapy in infants. Pediatr Nephrol 14:347–351
Lantos JD, Warady BA (2013) The evolving ethics of infant dialysis. Pediatr Nephrol 28:1943–1947
Rees L (2014) The dilemmas surrounding the decision to start chronic dialysis in the neonate. Kidney Int 86:18–20
Hayes WN, Watson AR, Callaghan N, Wright E, Stefanidis CJ, European Pediatric Dialysis Working Group (2012) Vascular access: choice and complications in European paediatric haemodialysis units. Pediatr Nephrol 27:999–1004
Mitsnefes M, Stablein D (2005) Hypertension in pediatric patients on long-term dialysis: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Am J Kidney Dis 45:309–315
Chavers BM, Solid CA, Daniels FX, Chen SC, Collins AJ, Frankenfield DL, Herzog CA (2009) Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol 4:1363–1369
Halbach SM, Martz K, Mattoo T, Flynn J (2012) Predictors of blood pressure and its control in pediatric patients receiving dialysis. J Pediatr 160:621–625
Chavers BM, Li S, Collins AJ, Herzog CA (2002) Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int 62:648–653
Mitsnefes MM (2008) Cardiovascular complication of pediatric chronic kidney dialysis. Pediatr Nephrol 23:27–39
Rees L, Azocar M, Borzych D, Watson AR, Buscher A, Edefonti A, Bilge I, Askenazi D, Leozappa G, Gonzales C, van Hoeck K, Secker D, Zurowska A, Rönnholm K, Bouts AH, Stewart H, Ariceta G, Ranchin B, Warady BA, Schaefer F (2011) Growth in very young children undergoing chronic peritoneal dialysis. J Am Soc Nephrol 22:2303–2312
Kari JA, Gonzalez C, Ledermann SE, Shaw V, Rees L (2000) Outcome and growth of infants with severe chronic renal failure. Kidney Int 57:1681–1687
Warady BA, Belden B, Kohaut E (1999) Neurodevelopmental outcome of children initiating peritoneal dialysis in early infancy. Pediatr Nephrol 13:759–765
Qvist E, Pihko H, Fagerudd P, Valanne L, Lamminranta S, Karikoski J, Sainio K, Rönnholm K, Jalanko H, Holmberg C (2002) Neurodevelopmental outcome in high-risk patients after renal transplantation in early childhood. Pediatr Transplant 6:53–62
Groothoff JW, Grootenhuis M, Dommerholt A, Gruppen MP, Offringa M, Heymans HAS (2002) Impaired cognition and schooling in adults with end stage renal disease since childhood. Arch Dis Child 87:380–385
Laakkonen H, Lonnqvist T, Valanne L, Karikoski J, Holmberg C, Ronnholm K (2011) Neurological development in 21 children on peritoneal dialysis in infancy. Pediatr Nephrol 26:1863–1871
Johnson RJ, Warady BA (2013) Long-term neurocognitive outcomes of patients with end-stage renal disease during infancy. Pediatr Nephrol 28:1283–1291
Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL (2014) Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturized machine (CARPEDIEM). Lancet 383:1807–1813
Coulthard MG, Crosier J, on behalf of the British Association for Pediatric Nephrology (2002) Outcome of reaching end stage renal failure in children under 2 years of age. Arch Dis Child 87:511–517
Shroff R, Rees L, Trompeter R, Hutchinson C, Ledermann S (2006) Long-term outcome of chronic dialysis in children. Pediatr Nephrol 21:257–264
Acknowledgments
We gratefully acknowledge the dedication and assistance of our Pediatric Dialysis Unit staff and the medical and nursing staff of the Pediatric Intensive Care Unit and Ruth Rappaport Children’s Hospital throughout the years of the study.
Conflict of interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pollack, S., Eisenstein, I., Tarabeih, M. et al. Long-term hemodialysis therapy in neonates and infants with end-stage renal disease: a 16-year experience and outcome. Pediatr Nephrol 31, 305–313 (2016). https://doi.org/10.1007/s00467-015-3214-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3214-3