Abstract
Neonatal renal vascular thrombosis is rare but has devastating sequelae. The renal vein is more commonly affected than the renal artery. Most neonates with renal vein thrombosis present with at least one of the three cardinal signs, namely, abdominal mass, macroscopic hematuria and thrombocytopenia, while unilateral renal artery thrombosis presents with transient hypertension. Contrast angiography is the gold standard for diagnosis but because of exposure to radiation and contrast agents, Doppler ultrasound scan is widely used instead. Baseline laboratory tests for platelet count, prothrombin time, activated partial thromboplastin time and fibrinogen concentration are essential before therapy is initiated. Maternal blood is tested for lupus anticoagulant and anticardiolipin antibody. Evaluation for prothrombotic disorders is warranted when thrombosis is clinically significant, recurrent or spontaneous. Management should involve a multidisciplinary team that includes neonatologists, radiologists, pediatric hematologists and nephrologists. In addition to supportive therapy, recent guidelines recommend at least prophylactic heparin therapy in the majority of cases to prevent thrombus extension. Thrombolytic therapy is reserved for bilateral thrombosis compromising kidney function. Long-term sequelae, such as kidney atrophy, systemic hypertension and chronic kidney disease, are common, and follow-up by pediatric nephrologists is recommended for monitoring of kidney function, early detection and management of hypertension and chronic kidney disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thromboembolic complications involving the renal vasculature are rare but serious, and are potentially fatal in children. The neonatal period carries the greatest risk for thromboembolic complications owing in part to the many unique features of the neonatal hemostatic system, in particular the tightly regulated balance between the variable concentrations and reduced activity of pro-coagulant and anticoagulant proteins involved in preventing either hemorrhage or thrombosis under physiologic conditions. Slight perturbations, such as hypoxia, dehydration, hypotension and infection, to name a few, can easily tip over this delicate balance, resulting in either bleeding or thrombosis. More importantly, what makes the neonatal kidney especially susceptible to thrombotic complications is the low renal blood flow coupled with small vessel diameter, as well as the enhanced renal vasoconstrictive and angioproliferative effects associated with high catecholamine levels, endothelin and angiotensin II [1, 2].
Developmental hemostasis
The sequential and dynamic changes in the coagulation, fibrinolytic and inhibitor systems are the key to understanding the natural history and response to therapy, as well as to preventing thromboembolic disease in the newborn. The neonatal hemostatic system is largely influenced by the age of gestation and postnatal age. By the tenth week of gestation, most hemostatic factors have already been synthesized, predominantly by the liver, and their levels in the plasma continue to increase with gestational age. At birth, the components of these systems are similar to those in older children and adults, but their plasma concentration and activity are markedly different, reflecting the likely differences in rates of protein synthesis, secretion and turnover. Thus, the overall pattern of hematologic systems in preterm and term infants is similar, with only minor differences in values [3–5]. However, it has been shown that postnatal maturation is generally accelerated in preterm compared to term neonates, although by the sixth month both show equivalent levels for all but four components of the coagulation system. Development continues through the first year of postnatal life until the plasma activity of most coagulation factors reaches levels comparable with those of adults [6]. As shown in Table 1, the concentrations of the vitamin K-dependent coagulation factors (II, VII, IX, X) and contact factors (XI, XII, prekallikrein, high-molecular-weight kininogen) at birth are 25–70 % of adult values [3–6]. These values increase rapidly after birth, with most components reaching adult levels by age 6 months. The concentrations of factors V, VIII and XIII, von Willebrand’s factor and fibrinogen at birth are at least 70–140 % of adult values. Coagulation inhibitors (antithrombin, heparin cofactor II, protein C, protein S) at birth are approximately 50 % of adult levels, except for the concentration of alpha-2 macroglobulin which is twofold greater in newborns than adults. The rate of thrombin generation in newborn plasma is 30–50 % of adult values [5, 7]. Concentrations of the fibrinolytic factors plasminogen and alpha-1-antiplasmin are lower in newborns than adult values, but the levels of tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 are higher. Infants born prematurely have even lower levels of vitamin K-dependent clotting factors than those born at term and also lower values for inhibitors of coagulation, including antithrombin and protein C. Because of their immature coagulation system, premature infants are particularly at risk for developing bleeding or thrombotic complications in response to perinatal risk factors or iatrogenic events [3].
Epidemiology of renal vascular thrombosis
Because renal vascular thrombosis is an infrequently reported condition, its exact incidence is unknown. Renal vein thrombosis (RVT) is by far the most common manifestation of neonatal venous thrombosis and much more common in the neonatal population than renal artery thrombosis (RAT). Data from a 1995 Canadian and international registry showed that 21 of the 97 reported cases of neonatal thrombosis had spontaneous RVT at a median age of 2 days, while a German registry reported the incidence of symptomatic RVT in neonates to be at least 2.2 per 100,000 live births [8–10]. RAT is even more uncommon in neonates. Retrospective and prospective data are mostly from catheter-related RAT, and the reported incidence varies according to the method used to diagnose the event. The reported incidence based on clinical signs ranges from 1 to 3 %, that based on ultrasound (US)-based studies varies from 14 to 35 % and the highest incidence of RAT has been reported in ion angiographic investigations at 64 % [11, 12]. With the increased use of central catheters for invasive therapeutic interventions, especially umbilical venous and umbilical artery catheters (UACs), as well as femoral catheters, the incidence of renal vascular thrombosis is expected to be much higher.
How do we identify the infant at risk of renal vascular thrombosis?
Both RVT and RAT have been associated with inherent fetal, neonatal and maternal risk factors (Fig. 1). Inherited thrombophilia, prematurity, polycythemia, congenital heart disease, respiratory distress syndrome, asphyxia, hypertonic dehydration, acute blood loss, in utero death of a twin, sepsis and prolonged central venous cannulation are documented fetal and neonatal risk factors for RVT [13, 14]. Maternal factors associated with RVT include infertility, oligohydramnios, thrombotic states, preeclampsia, autoimmunity (especially anti-phospholipid syndrome), diabetes and chorioamnionitis [13, 14]. Fetal and neonatal risk factors associated with RAT include prematurity, low birth weight, prolonged arterial cannula in situ, sepsis, retinopathy of prematurity [11], relatively small caliber of the renal vessels, vascular damage during insertion of catheter, type of substance infused (calcium-containing substance and hypertonic solutions), catheter type and the location of the catheter. A higher incidence of RAT has been reported to be associated with femoral artery cannulation compared to umbilical artery cannulation. A prospective study on neonates with RAT found that there were no identifiable maternal risk factors associated with RAT [11].
The incidence of genetic thrombophilia in newborns with thrombosis is not known, and the contribution of the prothrombotic state to the pathogenesis of neonatal thrombosis is uncertain. Of the inherited thrombophilias, deficiencies of protein C, protein S and antithrombin, the factor V Leiden mutation and the prothrombin gene mutation have been shown to have clear pathogenic links to thrombosis [15, 16]. However, in some studies recurrent thrombosis did not appear to be associated with hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T (MTHFR667) gene mutations [17]. Other thrombophilias which are less well characterized, and not necessarily genetically determined, include elevated lipoprotein(a), dysfibrinogenemias and increased levels of factors VIII, IX and XI [15]. In one study on infants with RVT in whom prothrombotic factors were investigated, 53 % had at least one risk factor identified [14]. Prothrombotic states are associated with the recurrence of thrombosis, with the exception of factor V Leiden mutation and elevated apolipoprotein A. In a study involving 59 newborns with RVT followed up for a median time of 4 years (range 0.6–15 years), 6.8 % had a second thrombotic event occurring during puberty. All of these patients with recurrence had at least one prothrombotic risk factor [14].
How do we recognize renal vascular thrombosis in the newborn?
The clinical features of renal vascular thrombosis are extremely variable and largely dependent on the location (unilateral or bilateral) and degree of involvement (from mild to massive thrombus). Presentation can vary from discrete signs and symptoms (flank mass, hematuria, proteinuria, elevated blood pressure) to asymptomatic (incidental finding from imaging of other abdominal pathologies) to life-threatening (e.g. overt kidney failure) conditions. In RVT, most patients will present with at least one of the three cardinal signs of RVT, namely, macroscopic hematuria (56.2 %), palpable flank mass (45.4 %) and thrombocytopenia (47.5 %). Only 22 % of neonates will manifest with the triad at presentation [1]. Males are more commonly affected than females. In a large meta-analysis of case series of RVT reported between 1992 and 2006 involving 271 neonates from 13 case studies, 67.2 % of the patients were male, 71 % of whom were born at term [14]. Most cases of RVT were found to occur within 3 days of birth (67 %), with 25.6 % occurring at >3 days after birth, while in utero occurrence was rare (7.3 %). RVT in neonates is often unilateral in 70 % of cases, occurring primarily in the left kidney and extending to the inferior vena cava in 52–60 % of cases. However, the incidence of bilateral RVT in neonates can 25 %. Accompanying adrenal hemorrhage occurs in 14.8 % of neonates. In addition, patients presenting with thrombocytopenia that cannot be explained by other conditions should be evaluated for thromboembolic disease.
On the other hand, diagnosis of spontaneous RAT in the newborn is often suspected based on the presence of risk factors, as many infants only present with transient hypertension [12, 18]. Acute renal insufficiency is rare, unless there is extension of thrombus in the aorta to occlude both renal arteries. Most cases of RAT are diagnosed commonly in association with indwelling intravascular catheters. A study conducted on immature non-human primates with umbilical catheters demonstrated that the probability of developing aortic thrombus in situ with a UAC in place increases in proportion to the duration of the placement, with the risk for thrombosis reaching as high as 80 % if the catheter is left in place for ≥21 days [19]. A similar study conducted in newborns found the incidence of thrombotic lesions to be 16 % within 1 day, 32 % within 7 days, 56 % within 14 days and 80 % within 21 days of UAC placement [20].
How do we work-up the neonate with renal vascular thrombosis?
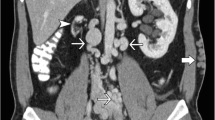
A diagnosis of renal vascular thrombosis is confirmed by imaging. Contrast angiography, although regarded as the gold standard for the diagnosis of vascular thrombosis, exposes the newborn to radiation and contrast agent and is rarely used because newborns with thrombosis are usually severely ill. US is the most commonly applied diagnostic modality to confirm a clinical suspicion of renal vascular thrombosis or to screen babies for clinically silent disease. US findings in RVT include enlarged and echogenic kidneys with attenuation or loss of corticomedullary differentiation. Calcification and thrombus may be seen extending outside the kidneys to the IVC. Doppler studies are particularly useful for detecting the resistance or absence of flow in renal venous branches and collateral vessels. Although the blood flow in the main renal vein and its branches may be normal, there may be an increase in resistance in the renal arteries caused by thrombosis in the small intrarenal veins. The accuracy of US may be reduced by the presence of a catheter because reduced compressibility of the vessel lumen by the US probe (a sign of thrombosis) is difficult to assess. US is also not very useful in low-birth-weight neonates due to the inaccessibility of adequate windows and the small catheter caliber. A 27-gauge catheter, the smallest commercially available catheter for neonates, is not consistently visualized during US examinations [21]. Interpretation may also be limited by the low pulse pressure in preterm and sick newborns. Despite its advantages as point of care testing (minimal invasiveness and absence of radiation), the overall performance of US to detect thrombi is poor. Renal scarring and atrophy are well-recognized features in the RVT-affected kidneys, and these can also be assessed by a radionuclide scan. An isotope renogram may be useful to measure relative perfusion of the kidneys.
In addition to diagnostic imaging, baseline laboratory tests in the neonate before initiation of any therapy should include the platelet count, prothrombin time, activated partial thromboplastin time (aPTT) and fibrinogen concentration. Maternal blood should be tested for lupus anticoagulant and anticardiolipin antibody. Evaluation for prothrombotic disorders should be conducted in newborns with thrombosis which is clinically significant, recurrent or spontaneous following the guidelines set by the Subcommittee for Perinatal and Pediatric Thrombosis of the Scientific and Standardization Committee of the International Society of Thrombosis and Hemostasis, but the timing of this evaluation may better be left until the acute clinical event has resolved, and the results must always be interpreted in the light of age-appropriate normal ranges and laboratory-specific reference ranges [22]. Whether newborns with catheter-related thrombosis require these studies is uncertain. Testing can be deferred if blood sampling is difficult because the results will not affect therapy, although they may affect the risk of recurrent thrombosis. Alternatively, these conditions can be excluded by testing the parents. Tests that are abnormal in the newborn should be repeated within 6–8 weeks. Both parents should be tested for the prothrombotic state if the results of the newborn’s tests are abnormal, as this will help to distinguish acquired from congenital deficiencies.
How do we treat the neonate with renal vascular thrombosis?
As is true with most therapeutic agents intended for neonates, evidence-based recommendations on prophylaxis and treatment of neonatal vascular thrombosis are not available. Current recommendations are purely expert opinion guidelines mostly based on extrapolation from adults, data from registries, case studies and knowledge of current common clinical practice [23]. Current guidelines recommend that any management protocol should involve a multidisciplinary team that includes neonatologists, radiologists, pediatric hematologists with experience in managing thromboembolism and pediatric nephrologists. The management of neonates with vascular thrombosis must take into account that neonatal hemostasis is a dynamic and complex system. The rapid physiologic changes occurring during the perinatal and early postnatal periods markedly affect the pharmacokinetic profile of drugs. As such, individual response in terms of efficacy, safety and toxicity is highly variable, and treatment should therefore be individualized. Early recognition is necessary to avoid morbidity and mortality, and the risk/benefit ratio of each individual must be carefully considered. Potential benefits of anticoagulation, such as the restoration of tissue perfusion to prevent renal failure, hypertension and renal shrinkage, must be weighed against the risk of massive and life-threatening hemorrhagic events, especially in premature babies at high risk for intracranial bleeding [18]. Treatment options available for neonatal renal vascular thrombosis include supportive therapy (“wait and see”), anticoagulation and thrombolysis. Surgical removal of a clot or embolectomy has been reported in neonates but in sites other than the renal vasculature and therefore beyond the scope of this article.
Careful attention must be paid to the fluid and electrolyte status, acid–base balance and nutrition of all newborns with renal vascular thrombosis. Neonates who are in acute renal failure and require renal replacement therapies (RRTs) must be promptly referred to the pediatric nephrologist. Radiologic monitoring for extension of thrombus or anticoagulation with unfractionated (UFH) or low-molecular-weight heparin (LMWH) in therapeutic doses is recommended for spontaneous unilateral RVT in the absence of renal impairment or extension [23]. Anticoagulation with UFH/LMWH or LMWH in therapeutic doses is recommended for spontaneous unilateral RVT associated with renal impairment and unilateral RVT not associated with renal impairment but with extension to the IVC [23]. For spontaneous bilateral RVT with evidence of renal impairment, anticoagulation with UFH or LMWH or initial thrombolytic therapy with tPA followed by anticoagulation with UFH or LMWH is suggested [23]. Current guidelines recommend continuous monitoring of the thrombus by US and continuing therapy until resolution of the clot for a total duration of between 6 weeks and 3 months. A summary of the dosages of the recommended anticoagulants is given in Table 2.
It is well-recognized that central lines are one of the major risk factors for renal vascular thrombosis. Thus, measures to prevent catheter-related thrombosis are paramount in the management of newborns with catheters in the umbilical artery, femoral artery and femoral vein [24]. Depending on the indication and the contemplated duration of use, maintaining the patency of the line and thrombus prevention can be achieved through heparin infusion (Table 2).
For neonates with UACs, catheter-tip placement and end-hole single-lumen construction minimize the occurrence of UAC-related thrombosis [25]. A high-level (T6–T9 thoracic vertebral bodies) rather than a low-level (L3–L4 lumbar vertebral bodies) UAC tip position placement is preferred as this is placed above the celiac axis, superior mesenteric artery and renal arteries, resulting in a lower incidence of clinical ischemic events, such as necrotizing enterocolitis, hypertension and hematuria. Prophylaxis for neonates with UAC involves continuous infusion with a fluid containing UFH (Table 2). Urgent removal of UAC is indicated when the catheter is no longer needed. Ideally, the UAC should not be left in place for >5 days. Some studies favor conservative treatment and the avoidance of antithrombotic and/or thrombolytic medications for asymptomatic UAC-related thrombus [12, 20]. For neonates and children with a symptomatic peripheral arterial catheter-related thromboembolism, in particular femoral artery catheter thrombosis, UFH anticoagulation with or without thrombolysis or surgical thrombectomy and microvascular repair with subsequent heparin therapy is recommended [23].
Standard heparin or UFH is still widely used in the treatment of neonatal thrombosis. The low incidence of heparin-induced thrombocytopenia, the availability of protamine for rapid reversal of UFH action and the low cost support the use of UFH in the treatment of preterm and term neonates [18]. The downside of UFH is its unpredictable pharmacokinetic response and resultant requirement for frequent monitoring. In addition, the infusion of UFH in newborns requires a dedicated intravenous catheter in order to avoid any interruption of anticoagulation therapy while at the same time minimizing the risk of inadvertent flushing of the catheter that may lead to excessive anticoagulation. The efficacy and safety of UFH is ensured by monitoring the activity of anti-factor Xa and aPTT. The results of aPTT testing can be used to facilitate dose adjustments, but only after establishing the aPTT range that corresponds to the target anti-factor Xa levels in an individual patient. This differs from the approach to dose adjustment in adults, in whom the aPTT range can be used alone because it generally corresponds to an anti-factor Xa activity of 0.35–0.7 U/mL. The additional monitoring for neonates is necessary because they have a higher clearance rate of heparin than do older children or adults. In addition, the efficacy of heparin may be reduced in newborns because the physiologic plasma concentration of antithrombin is low. It is advised to discontinue infusion and to administer protamine immediately if bleeding occurs and when the anti-factor Xa level exceeds 0.8 U/mL.
LMWH has widely replaced UFH in the treatment of adult patients with thromboembolism. In recent years, neonatal intensive care units have shown an increasing trend to use LMWH for the post-acute treatment of venous and arterial thromboembolism, as well as in the prevention of thrombosis in high-risk patients. Its greater bioavailability when given by subcutaneous injection, longer duration of anticoagulant effect and longer, dose-independent clearance time, resulting in a more predictable response, are some of its advantages over UFH. Although LMWH requires less laboratory monitoring, a feature useful in neonates with poor venous access, daily testing of anti-factor Xa levels is often necessary until therapeutic levels are obtained. An added feature of LMWH that makes it favorable for neonatal treatment of thrombosis is its reduced risk of immune-mediated thrombocytopenia [26–28]. Of the LMWHs available in the market, enoxaparin is widely used in the treatment of neonatal thromboembolism. Data on the use of other LMWH in neonates, such as dalteparin and tinzaparin, are still limited. Complications from LMWH therapy include soreness from the injection itself, bruising and, rarely, bleeding (5 %) [29, 30].
Thrombolytic therapy for neonatal thrombosis is generally not recommended but rather reserved only for cases in which there is a critical compromise of life, organs or limbs. In renal vascular thrombosis, thrombolysis is indicated for bilateral vessel involvement causing kidney failure. The thrombolytic drugs currently used are intravenously infused plasminogen activators which promote the conversion of plasminogen to plasmin, with subsequent cleavage of fibrin, fibrinogen and factors V and VIII, resulting in breakdown of the clot [31]. However, the thrombolytic activity of these agents may be reduced in newborns due to a decreased plasminogen concentration, resulting in a decreased generation of plasmin. Supplementation with plasminogen by administration of 10–15 ml/kg of fresh frozen plasma may improve fibrinolytic activity and is usually indicated prior to initiating therapy. In cases requiring thrombolysis, thrombocytopenia (platelet count of <100× 109/L), low fibrinogen concentration (<1 g/dL) and severe deficiency of coagulation factors should be corrected before treatment is initiated. Recombinant tissue-type PA (rtPA) is the thrombolytic agent of choice due to its high specificity for fibrin with poor activation of free plasmin, a lower risk of hypersensitivity and short half-life (4 min in plasma and 45 min for thrombolytic effects) (Table 3) [18]. Thrombolysis using rtPA should be guided by radiological imaging and meticulous monitoring of hematological parameters in conjunction with regular clinical assessment (Table 3) [23, 32]. If bleeding occurs as a result of treatment, the rtPA infusion should be stopped and fresh frozen plasma and/or cryoprecipitate administered. Alternatively, thrombolysis with urokinase is a safe and effective treatment for neonatal thrombosis. Thrombolytic therapy is contraindicated with major surgery, hemorrhage within the previous 10 days, neurosurgery within 3 weeks, a severe asphyxial event within 7 days, an invasive procedure within the previous 3 days, seizures within 48 h, systemic septicemia, active bleeding or the inability to maintain platelets at >100 × 109/L or fibrinogen at >1 g/dL.
What are the long-term outcomes of neonatal renal vascular thrombosis?
Renal vascular thrombosis is associated with low mortality, but long-term kidney dysfunction is common [14, 33, 34]. Kidney atrophy, hypertension and chronic kidney disease are the most common long-term outcomes of renal vascular thrombosis. In a review of RVT in neonates, kidney atrophy was seen in 70.6 % of participating neonates, hypertension in 20 % and chronic kidney disease requiring RRT in 3 % (most of the latter cases were sequelae of bilateral RVT) [14]. Kidney atrophy and irreversible damage were observed regardless of the treatment received (anticoagulant therapy vs. supportive therapy). In addition, the incidence of hypertension was 18.9 % in those with unilateral involvement and 21.7 % in neonates with bilateral RVT.
Neonates with renal vascular thrombosis will therefore require long-term follow-up for early detection and timely intervention of chronic kidney disease. Regular monitoring for proteinuria and hypertension should be performed at every clinic visit, and renal function assessment may be required annually, especially in those with bilateral renal vascular involvement.
Key summary points
-
1.
Management of renal vascular thrombosis should involve a multidisciplinary team that includes neonatologists, radiologists, hematologists and nephrologists.
-
2.
During the acute phase, supportive management is vital to stabilize patients. Neonates who are in acute renal failure requiring RRTs necessitate prompt referral to the pediatric nephrologist.
-
3.
The unique nature of the neonatal hemostasis requires individualization of treatment.
-
4.
Benefits of anticoagulation and thrombolytic therapy must be weighed against the risks.
-
5.
Close follow-up for long-term renal complications such as hypertension, kidney atrophy, functional loss and chronic renal insufficiency is necessary.
Multiple choice questions (answers are provided following the References)
-
1.
Which of the following statements is correct about the neonatal hemostatic system?
-
a.
The neonatal hemostatic system is largely influenced by the age of gestation and postnatal age.
-
b.
Concentrations of the vitamin K-dependent coagulation factors (II, VII, IX, X) are higher at birth.
-
c.
Concentrations of the contact factors (XI, XII, prekallikrein, high molecular weight kininogen) at birth are similar to adult values.
-
d.
Concentrations of the fibrinolytic factors plasminogen and alpha-1-antiplasmin are higher than adult values.
-
e.
Infants born prematurely have similar levels of vitamin K-dependent clotting factors as those born at term.
-
a.
-
2.
Which of the following is a risk factor for renal vein thrombosis:
-
a.
Maternal polyhydramnios
-
b.
Maternal smoking
-
c.
Pre-eclampsia
-
d.
Twin delivery
-
e.
Neonatal thrombocytopenia
-
a.
-
3.
Which of the following investigations is most useful in the diagnosis of renal vascular thrombosis?
-
a.
2D-echocardiogram
-
b.
Doppler ultrasound
-
c.
MAG3 radioisotope scan
-
d.
Abdominal CT-scan
-
e.
Contrast angiography
-
a.
-
4.
Which of the following is true of LMWH:
-
a.
Less monitoring is required compared to UFH.
-
b.
Its effect can be measured using the aPTT ratio.
-
c.
Dosage per kilogram is the same for all age groups.
-
d.
Overdose can be reversed with protamine.
-
e.
It has been documented to cause major bleeding in premature babies.
-
a.
-
5.
According to current guidelines on the management of babies with spontaneous unilateral renal venous thrombosis with normal kidney function:
-
a.
Only supportive treatment with radiologic monitoring for extension of thrombus is recommended.
-
b.
UFH is contraindicated in neonates.
-
c.
Use of LMWH is associated with lower risk of immune-mediated thrombocytopenia.
-
d.
LMWH is recommended to prevent extension of the thrombus.
-
e.
rtPA is recommended for thrombolytic therapy.
-
a.
References
Winyard PJD (2006) Perinatal renal venous thrombosis: presenting renal length predicts outcome. Arch Dis Child Fetal Neonatal Ed 91:F273–F278
DiBona GF (2000) Nervous kidney. Interaction between renal sympathetic nerves and the renin–angiotensin system in the control of renal function. Hypertension 36:1083–1088
Andrew M, Paes B, Milner R, Johnson M, Mitchell L, Tollefsen DM, Castle V, Powers P (1988) Development of the human coagulation system in the healthy premature infant. Blood 72:1651–1657
Andrew M, Paes B, Milner R, Johnson M, Mitchell L, Tollefsen DM, Powers P (1988) Development of the human coagulation system in the full-term infant. Blood 70:165–172
Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, De Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S (2006) Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost 95:362–372
Sharathkumar AA, Pipe SW (2008) Bleeding disorders. Pediatr Rev 24:121–130
Andrew M, Schmidt B, Mitchell L, Paes B, Ofosu F (1990) Thrombin generation in newborn plasma is critically dependent on the concentration of prothrombin. Thromb Haemost 63:27–30
Bokenkamp A, von Kries R, Nowak-Gottl U, Gobel U, Hoyer PF (2000) Neonatal renal venous thrombosis in Germany between 1992 and 1994: epidemiology, treatment and outcome. Eur J Pediatr 159:44–48
Nowak-Göttl U, von Kries R, Göbel U (1997) Neonatal symptomatic thromboembolism in Germany: two year survey. Arch Dis Child 76:F163–F167
Schmidt B, Andrew M (1995) Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics 96:939–943
Cohen RS, Ramachandran P, Kim EH, Glasscock GF (1995) Retrospective analysis of risks associated with an umbilical artery catheter system for continuous monitoring of arterial oxygen tension. J Perinatol 15:195–198
Ergaz Z, Simanovsky N, Rozovsky K, Leil SA, Ofek-Shlomai N, Revel-Vilk S, Bar-Oz B (2012) Clinical outcome of umbilical artery catheter-related thrombosis—a cohort study. J Perinatol 32:933–940
Kosch A, Kuwertz-Broking E, Heller C, Kurnik K, Schobess R, Nowak-Gottl U (2004) Renal venous thrombosis in neonates: prothrombotic risk factors and long-term follow-up. Blood 104:1356–1360
Lau KK, Stoffman JM, Williams S, McCusker P, Brandao L, Patel S, Chan AK (2007) Neonatal renal vein thrombosis: review of the English-language literature between 1992 and 2006. Pediatrics 120:e1278–e1284
Raffini L (2008) Thrombophilia in children: who to test, how, when, and why. Hematology Am Soc Hematol Educ Program 2008:228–235
Young G, Albisetti M, Bonduel M, Brandao L, Chan A, Friedrichs F, Goldenberg NA, Grabowski E, Heller C, Journeycake J, Kenet G, Krumpel A, Kurnik K, Lubetsky A, Male C, Manco-Johnson M, Mathew P, Monagle P, van Ommen H, Simioni P, Svirin P, Tormene D, Nowak-Gottl U (2008) Impact of inherited thrombophilia on venous thromboembolism in children: a systematic review and meta-analysis of observational studies. Circulation 118:1373–1382
Joachim E, Goldenberg NA, Bernard TJ, Armstrong-Wells J, Stabler S, Manco-Johnson MJ (2013) The methylenetetrahydrofolate reductase polymorphism (MTHFR c.677C>T) and elevated plasma homocysteine levels in a U.S. pediatric population with incident thromboembolism. Thromb Res 132:170–174
Veldman A, Nold MF, Michel-Behnke I (2008) Thrombosis in the critically ill neonate: incidence, diagnosis, and management. Vasc Health Risk Manag 6:1337–1348
McAdams RM, Winter VT, McCurnin DC, Coalson JJ (2009) Complications of umbilical artery catheterization in a model of extreme prematurity. J Perinatol 29:685–692
Boo NY, Wong NC, Zulkifli SS, Lye MS (1999) Risk factors associated with umbilical vascular catheter-associated thrombosis in newborn infants. J Paediatr Child Health 35:460–465
Park CK, Paes BA, Nagel K, Chan AK, Murthy P (2014) Neonatal central venous catheter thrombosis. Blood Coagul Fibrinolysis 25:97–106
Manco-Johnson MJ, Grabowski EF, Hellgreen M, Kemahli AS, Massicotte MP, Muntean W, Peters M, Schlegel N, Wang M, Nowak-Göttl U (2002) Laboratory testing for thrombophilia in pediatric patients. On behalf of the Subcommittee for Perinatal and Pediatric Thrombosis of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis (ISTH). Thromb Haemost 88:155–156
Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Vesely SK (2012) Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:e737S–e801S
Revel-Vilk S, Ergaz Z (2011) Diagnosis and management of central-line-associated thrombosis in newborns and infants. Semin Fetal Neonatal Med 16:340–344
Barrington KJ (2000) Umbilical artery catheters in the newborn: effects of catheter design (end vs side hole). Cochrane Database Syst Rev 2:CD000508
Obeng EA, Harney KM, Moniz T, Arnold A, Neufeld EJ, Trenor CC 3rd (2015) Pediatric heparin-induced thrombocytopenia: prevalence, thrombotic risk, and application of the 4Ts scoring system. J Pediatr 166:144–150
Ranze O, Ranze P, Magnani HN, Greinacher A (1999) Heparin-induced thrombocytopenia in paediatric patients—a review of the literature and a new case treated with danaparoid sodium. Eur J Pediatr 158[Suppl 3]:S130
Spadone D, Clark F, James E, Laster J, Hoch J, Silver D (1992) Heparin-induced thrombocytopenia in the newborn. J Vasc Surg 15:306–311
van Elteren HA, Te Pas AB, Kollen WJ, Walther FJ, Lopriore E (2011) Severe hemorrhage after low-molecular-weight heparin treatment in a preterm neonate. Neonatology 99:247–249
van Elteren HA, Veldt HS, Te Pas AB, Roest AA, Smiers FJ, Kollen WJ, Sramek A, Walther FJ, Lopriore E (2011) Management and outcome in 32 neonates with thrombotic events. Int J Pediatr 2011:217564
Andrew ME, Monagle P, deVeber G, Chan AK (2001) Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program 2001:358–374
Manco-Johnson MJ, Grabowski EF, Hellgreen M, Kemahli AS, Massicotte MP, Muntean W, Peters M, Schlegel N, Wang M, Nowak-Göttl U (2002) Recommendations for tPA thrombolysis in children. On behalf of the Scientific Subcommittee on Perinatal and Pediatric Thrombosis of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. Thromb Haemost 88:157–158
Keidan I, Lotan D, Gazit G, Boichis H, Reichman B, Linder N (1994) Early neonatal renal venous thrombosis: long-term outcome. Acta Paediatr 83:1225–1227
Messinger Y, Sheaffer JW, Mrozek J, Smith CM, Sinaiko AR (2006) Renal outcome of neonatal renal venous thrombosis: review of 28 patients and effectiveness of fibrinolytics and heparin in 10 patients. Pediatrics 118:e1478–e1484
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Answers
1. a
2. c
3. e
4. a
5. c
Rights and permissions
About this article
Cite this article
Resontoc, L.P.R., Yap, HK. Renal vascular thrombosis in the newborn. Pediatr Nephrol 31, 907–915 (2016). https://doi.org/10.1007/s00467-015-3160-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3160-0