Abstract
Background
Intermittent hemodialysis (IHD) is the most efficient form of renal replacement therapy (RRT) for removing toxic substances from patients’ bodies. However, the efficacy and safety of IHD in infants and young children with inborn errors of metabolism are still not clear.
Methods
This retrospective study included patients with urea cycle disorders, maple syrup urine disease, and methylmalonic acidemia who received IHD or non-IHD RRT at our hospital between 2001 and 2012 to remove ammonia, leucine, or methylmalonic acid. Both the efficacy and safety of the RRT were evaluated.
Results
Thirty-five courses of RRT, including 25 courses of IHD and ten courses of non-IHD RRT, for 15 patients were included in the analysis. Before 2006, non-IHD RRT procedures, including peritoneal dialysis (PD) and continuous venous-venous hemofiltration (CVVH), were the most often used; from 2006 onwards IHD was used. There was one procedure-unrelated death. Catheter penetration occurred in one course of IHD. The efficacy data revealed that both the median duration of dialysis and the median 50 % toxin reduction time were shorter in IHD than in non-IHD RRT.
Conclusions
In infants and young children with inborn errors of metabolism, IHD is safe and more efficient than non-IHD RRT at removing toxins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute metabolic decompensation in patients with inborn errors of metabolism (IEM) is a medical emergency that requires immediate renal replacement therapy (RRT) to remove the toxic substances from the body to prevent death or permanent brain damage [1–3]. The common forms of RRT include peritoneal dialysis (PD), continuous venous-venous hemofiltration (CVVH), continuous arteriovenous hemofiltration (CAVH), and intermittent hemodialysis (IHD). Although IHD is the most efficient form of RRT to remove toxic substances from the body [4, 5], and infants and young children are more susceptible than adult patients to damage due to IEM, due to the difficulties in vascular access and hemodynamic instability in infants and young children, PD and CVVH have been the most commonly used forms of RRT in these patients [6–8].
IHD performed in infants and young children has been described in a number of studies, but in most of these, the case numbers were small, and the safety and efficacy data were not complete [4, 9–11]. Experiences in Asian countries are especially lacking. To improve the treatment of acute metabolic decompensation in IEM, we initiated IHD for infants and young children in 2006. In the study reported here, we compared IHD and non-IHD RRT with regard to the safety and efficacy for these patients.
Materials and methods
Data from RRT performed for acute metabolic decompensation in patients with urea cycle disorders (UCDs), maple syrup urine disease (MSUD), and methylmalonic acidemia during the period from 2001 to 2012 were analyzed (Table 1). The indication for RRT was hyperammonemia of >500 μM or persistent hyperammonemia of >300 μM despite medical treatment for UCDs, intractable metabolic acidosis for methylmalonic acidemia, and consciousness change in MSUD. Patients described in one of our previous publications were also included in this current study when biomarker data were available [6]. Before 2006, non-IHD RRT was the main form of treatment, except for one patient (patient No. 1) with MSUD who received PD at ages 3 and 4 years due to the parents’ preference. The procedures for CVVH have been described previously [6]. Briefly, a Gambro FH22/FH66 hemofilter (polyamide; surface area 0.2/0.6 m2; priming volume 12/43 mL, respectively; Gambro, Lund, Sweden) was utilized with an Alaris Imed Gemini model PC-2TX infusion pump (Soma Technology, Bloomfield, CT) and Lifecare Abbot Mocri Macro XL infusion pump (Abbot Laboratories, Abbott Park, North Chicago, IL) with the priming volume of blood tubing according to the selected tubing volume. The blood flow was continuously controlled by a Gambro AK-10 monitor (Gambro). CAVH was applied in some patients, and it was also achieved by a 0.2-m2 polyamide hemofilter (Gambro FH22) without rolling pumps. The hemofiltration replacement fluid was a combination of two solutions with a final composition of 142 mEq/L sodium, 2.6 mEq/L calcium, 1.4 mEq/L magnesium, 113 mEq/L chloride, and 33 mEq/L bicarbonate. Blood flow rate was 5–7 mL/kg/h, and the replacement fluid flow rate was 35–50 mL/kg/h. Heparin was applied with a loading dose of 10–20 U/kg and a maintenance dose of 10–20 U/Kg/h. PD was also used in the treatment course for 24 cycles daily in some patients. The intraperitoneal dwell volume was set as 30 mL/kg with a 30-min dwell time. The glucose concentration of the PD solution (Baxter Inc., Singapore) was 4.25 %.
After 2006, IHD for infants and young children with IEM was developed at our hospital and became the main form of RRT, except for one patient with argininosuccinate synthase (ASS) deficiency who experienced a vascular access failure and CVVH was applied. For the IHD procedures, a double-lumen catheter of the appropriate size was first inserted through a right internal jugular vein puncture performed by a cardiac surgeon. The dialysis machine used was a Fresenius 4008B (Fresenius Medical Care AG & Co., Bad Homburg vor der Höhe, Germany) with a targeted blood flow rate of 7–10 mL/kg/min and a dialysate (hemodialysis concentrate containing sodium, calcium, potassium, bicarbonate, but not phosphate) flow rate of 500 mL/min. An appropriate dialysis filter was chosen to have a surface area (SA) approximating the patient’s estimated body surface area. A typical setting for babies who weighed <5 kg was FR 7 double-lumen catheters (Medcomp, Harleysville, PA) coupled with an FX Paed dialyzer (SA 0.2 m2; Fresenius Medical Care AG & Co.) and AV-set PAED/BABY bloodlines (Fresenius Medical Care AG & Co.). The priming volume was 18 mL for the FX Paed dialyzer and 56 mL for the AV-set PAED/BABY bloodlines. For patients who weighed <10 kg, priming with blood was necessary for both CVVH and IHD. Supportive care, including caloric support and protein restriction, was provided for all patients. Intravenous l-carnitine was prescribed for methylmalonic acidemia, and oral sodium phenylacetate and sodium benzoate were prescribed for patients with UCD.
Each course of IHD was 4 h, and the definition of one course for non-IHD RRT was from the start of the procedure until either recovery or death of the patients (usually for 1–3 days). Endpoints for non-IHD RRT were an ammonia level of ≤200 μL in patients with UCD, or a significant improvement in consciousness and metabolic acidosis in patients with other diseases. Biomarkers used in this study were blood ammonia level (monitored every 2 h for patients with UCD), blood spot methylmalonic acid (MMA) level (monitored every 4 h for patients with methylmalonic acidemia), and plasma leucine level (monitored every 4 h for patients with MSUD). The “50 % toxin reduction time (TRT)” was defined as the time used to achieve a toxin concentration that was 50 % of the initial level, based on calculation of the first and the last measurement of that course. Data are expressed as the median values (range). Continuous variables were analyzed using the Mann–Whitney U test. The level of statistical significance was set at p < 0.05. This study was approved by the institutional review board (no. 201303020RINC).
Results
A search of medical records led to the identification of 19 patients with IEM who received 41 courses of RRT during the study period. Four courses of continuous arteriovenous hemodialysis (CAVHD) were given to three patients with methylmalonic acidemia in the early stage, and the MMA levels were not measured during that period. Two newborns, one with ornithine transcarbamylase (OTC) deficiency and the other with HMG CoA lyase deficiency, presented at our hospital in late stages, with attacks in association with arrhythmia and pulmonary hemorrhage. IHD and extracorporeal membrane oxygenation pump (ECMO)/CAVH were applied, but their neurologic outcomes of these two patients were poor, and the families declined further treatment. The data from these two cases were excluded from this study.
Thirty-five courses of RRT, including 25 courses of IHD and ten courses of non-IHD RRT, performed in 15 patients (7 male and 8 female patients) were analyzed in this study. There were five patients with UCDs [2 with OTC deficiency, 2 with ASS deficiency, and 1 with carbamoyl phosphate synthetase (CPS) deficiency], six patients with methylmalonic acidemia, and four patients with MSUD (Table 1). One OTC deficiency patient (patient 8) decided to have RRT but the condition was complicated with cardiac perforation, and the RRT was started while the ammonia level was 182 μM [12]. Of the 15 patients, seven received one course of RRT and eight received two or more courses. One patient with MSUD received 11 courses of IHD up to age 3.5 years.
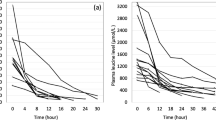
The median age and body weight of the patients at the time of dialysis and the initial toxin levels did not differ between the IHD and non-IHD RRT groups (Table 2). The smallest patients in the two groups both weighed 2.3 kg. However, the median duration of dialysis was significantly shorter in the IHD courses than in the non-IHD RRT courses (p = 0.001), and the median 50 % TRT was also significantly shorter (p = 0.001) in the IHD courses. This difference remained significant (p = 0.002) with the exclusion of PD from the non-IHD RRT group. When individual diseases were examined, the 50 % TRT of the IHD courses for MSUD patients (3.34 h) was significantly shorter than that for the non-IHD RRT courses (11.6 h) (p = 0.036). When we correlated initial toxin level to the 50 % TRT, we determined that IHD was effective at all initial toxin levels, while non-IHD lost its effect at high toxin levels (Fig. 1a). Non-IHD was also less effective in patients who were relatively heavier (Fig. 1b). Only two courses of IHD had a 50 % TRT of >4 h: one (9.69 h) was in a patient with a low initial leucine level (247.6 μM, normal <188 μM), and the other (7.8 h) was in a patient with clotting circuits (twice). We had a good opportunity to compare different RRT models in the same patient. One MSUD patient received four courses of different types of RRT (2 courses of IHD, 1 course of PD, and 1 course of CVVH) at different ages. Figure 2 shows that IHD removed leucine faster than CVVH and PD.
Comparison of 50 % toxin reducing time (TRT) between intermittent hemodialysis (IHD) and non-IHD, including continuous venous-venous hemofiltration (CVVH) and two courses of peritoneal dialysis (PD). a IHD was effective in reducing overall initial toxin levels as evidenced by the very short 50 % TRT. Non-IHD was less effective than IHD, especially when the initial toxin levels were high. b IHD was effective regardless of the body weight (BW) of the patients. Non-IHD was less effective than IHD, especially when the patients were relatively heavier. The initial toxin level is expressed as a fold change of the upper limit of the normal range (180 μM for leucine, 100 μM for ammonia in newborns, 35 μM for ammonia in older infants and children, 1 μM for methylmalonic acid)
A comparison of the efficacy of different forms of renal replacement therapy (RRT) in one patient with maple syrup urine disease. The results reveal that intermittent hemodialysis (IHD) decreased plasma leucine levels much faster than either continuous venous-venous hemofiltration (CVVH) or peritoneal dialysis (PD). The two courses of IHD were performed at different ages
Although IHD removes toxins quickly, a rebound of toxin levels can occur because protein catabolism has not been completely suppressed in such patients. For example, a 1-year-old patient with ASS deficiency came to our hospital because of intermittent hyperammonemia over a 1-month period. His ammonia level decreased rapidly after the first course of IHD, but the level rose a few hours later despite the administration of sodium phenylbutyrate and calories. A repeat IHD course was performed immediately, and the patient required still another course of IHD a few days later (Fig. 3).
The clinical course of one patient with a urea cycle disorder. The first course of intermittent hemodialysis (IHD) rapidly lowered the initial ammonia (NH3) level. The ammonia level increased (arrow) a few hours later, and a second course of IHD was performed. The ammonia level rebounded yet again a few days later (arrowhead) but was successfully managed by the third course of IHD
A transient drop in blood pressure occurred in both IHD and CVVH when the dialysis was started, but none of these episodes needed to be managed by vasoactive agents. No symptomatic hypokalemia, hypophosphatemia, or significant bleeding occurred, although circuit clotting was a common problem in both IHD and CVVH. On average, two sets of tubing were required for each course of IHD and even more sets were needed for CVVH. Cardiac perforation following catheter penetration occurred during the insertion of a non-cuffed double-lumen central venous catheter for IHD in one patient [12]. The penetration hole was repaired rapidly by surgery, and there were no further issues. One patient with CPS deficiency received CVVH for 8.5 h without a satisfactory reduction in the ammonia level (from 1,070 to 861 μM). After shifting to IHD, the ammonia level of this patient dropped from 476 to 199 μM in 2.5 h. Unfortunately, the patient died several hours later; however, his death was not attributed to either CVVH or IHD. There was no complication in the PD.
The safety and efficacy of IHD can be best demonstrated in one MSUD patient, now 3.5 years old, who has received 11 courses of IHD to date. This patient was diagnosed through newborn screening at the age of 7 days. She already had decreased activity when she was admitted to the hospital for the confirmatory tests, and the first IHD was performed at a body weight of 3.2 kg. The patient was then put on a low branched-chain amino acid diet and calorie supplementation, but frequent metabolic decompensation still occurred. A brain magnetic resonance imaging study at 3 years of age did show some high T2-weighted signals over both sections of the globus pallidus. However, the patient has shown normal mental development, and her gross motor developmental quotients for gross motor (score = 83) and fine motor (score = 73) skills are very close to normal values.
The long-term outcome of each patient is given in Table 1. Of the nine patients who had non-IHD as the first RRT for metabolic decompensation, six patients had mental retardation, one CPS deficiency patient died after one attack (as described above), one girl with OTC deficiency received a liver transplant after the attack, and one patient had a stroke due to methylmalonic acidemia. Of the four patients who had IHD as the first RRT for metabolic decompensation, one methylmalonic acidemia patient had developmental delays and died of Pneumocystis jiroveci pneumonia 4 months after liver transplantation. Another methylmalonic acidemia patient had hypoxia-ischemic encephalopathy as a complication of the transplantation.
Discussion
Most of the severe forms of IEM have symptom onset during infancy or the early childhood period. Recurrent metabolic decompensation is also frequent in these patients because of the difficulties encountered in controlling the diseases. However, the brains of infants and young children are highly vulnerable to hyperammonemia, metabolic acidosis, or other metabolic insults; consequently, neurological sequelae, developmental delays, and mental retardation are common. Therefore, the management of acute metabolic decompensation in infants and young children with IEM constitutes a medical emergency.
In our patient cohort, IHD removed toxins more rapidly than PD or CVVH, but currently sufficient data are not available for a direct comparison between IHD and/or high-volume CVVH/continuous veno-venous hemodialysis (CVVHD). In previous studies for patients with UCD, the 50 % TRT was reported to be longer for CVVHD than for IHD [11]. Patients with MSUD can have a 50 % TRT of <4 h both with IHD and CVVH [11, 13, 14], but CVVH usually gives more variable results [15]. The 50 % TRT of CVVHD reported in one study [16] is longer than that of IHD in our current study. Nevertheless, a rebound of the toxin levels may occur before catabolism has been controlled. The rebound episodes can be managed by promptly resuming IHD, as shown in our cases. Either high-volume CVVH [6] or CVVHD may be used to prevent the rebound of toxin levels after IHD.
The mortality rate in patients with inborn errors of metabolism on continuous RRT can be high [17]. IHD in infants and young children has been thought to be excessively risky because of the lack of proper circuits, difficulties with vascular access, and hemodynamic instability, among other concerns. Based on our experience, however, IHD in infants and young children can be performed safely in the presence of proper equipment (circuits) and by a team that includes experienced cardiac surgeons, nephrologists, and medical geneticists. Our study reveals that among our patient cohort the risks associated with IHD did not exceed those associated with CVVH, which is consistent with the opinion of Walters et al. [18]. IHD can also be used in patients with an unstable hemodynamic status by including an ECMO to support blood pressure [19]. In fact, a prompt decision to initiate IHD, rather than waiting for further symptoms or signs, increases the chance that patients will receive IHD before hemodynamic instability develops.
The long-term benefit of IHD regarding neurological outcomes is difficult to evaluate based on our results because individual differences in disease severity, time to the start of the intervention, and pre-existing neurological status all affect the outcomes. For example, one of our previous studies revealed that even mild non-life-threatening hyperammonemia in young patients with methylmalonic acidemia caused significant brain damage [20]. Hopefully, after early disease detection by the expanded newborn screening and prompt treatments, including IHD, we can improve the long-term outcomes of patients with IEM in the future.
There were several limitations to our study. First, these disorders are rare and, consequently, the number of patients is usually too small to run randomized trials. Second, IHD is a more recent treatment, and the availability of new medications, such as intravenous l-carnitine or sodium phenylbutyrate, may have helped to stabilize the patients. Therefore, the effectiveness of IHD may be at least partially attributable to the detoxifying medication, and not only to the different RRT regimen. However, in our practice intravenous sodium phenylbutyrate has only become available very recently and it was not a contributory factor in our study, Further studies will be necessary to demonstrate the long-term benefits of IHD treatment for infants and small children with IEM, and data comparing the effectiveness of different RRT regimens with the same detoxifying medications are urgently needed.
In conclusion, IHD in infants and young children with IEM is a safe and efficient treatment to remove toxins at any levels. IHD should be used to quickly decrease the toxin levels.
References
Surtees RA, Matthews EE, Leonard JV (1992) Neurologic outcome of propionic acidemia. Pediatr Neurol 8:333–337
Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED (1984) Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med 310:1500–1505
Hilliges C, Awiszus D, Wendel U (1993) Intellectual performance of children with maple syrup urine disease. Eur J Pediatr 152:144–147
McBryde KD, Kershaw DB, Bunchman TE, Maxvold NJ, Mottes TA, Kudelka TL, Brophy PD (2006) Renal replacement therapy in the treatment of confirmed or suspected inborn errors of metabolism. J Pediatr 148:770–778
Donn SM, Swartz RD, Thoene JG (1979) Comparison of exchange transfusion, peritoneal dialysis, and hemodialysis for the treatment of hyperammonemia in an anuric newborn infant. J Pediatr 95:67–70
Lai YC, Huang HP, Tsai IJ, Tsau YK (2007) High-volume continuous venovenous hemofiltration as an effective therapy for acute management of inborn errors of metabolism in young children. Blood Purif 25:303–308
Wong KY, Wong SN, Lam SY, Tam S, Tsoi NS (1998) Ammonia clearance by peritoneal dialysis and continuous arteriovenous hemodiafiltration. Pediatr Nephrol 12:589–591
Pela I, Seracini D, Donati MA, Lavoratti G, Pasquini E, Materassi M (2008) Peritoneal dialysis in neonates with inborn errors of metabolism: is it really out of date? Pediatr Nephrol 23:163–168
Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, Sabetta G, Rizzoni G, Bartuli A (2001) Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol 16:862–867
Rajpoot DK, Gargus JJ (2004) Acute hemodialysis for hyperammonemia in small neonates. Pediatr Nephrol 19:390–395
Schaefer F, Straube E, Oh J, Mehls O, Mayatepek E (1999) Dialysis in neonates with inborn errors of metabolism. Nephrol Dial Transplant 14:910–918
Wang CC, Chen YW, Wu ET, Chien YH, Hwu WL, Ko WJ, Huang SC (2007) Identification and management of cardiac perforation from a double lumen catheter in an infant. Paediatr Anaesth 17:500–501
Rutledge SL, Havens PL, Haymond MW, McLean RH, Kan JS, Brusilow SW (1990) Neonatal hemodialysis: effective therapy for the encephalopathy of inborn errors of metabolism. J Pediatr 116:125–128
Puliyanda DP, Harmon WE, Peterschmitt MJ, Irons M, Somers MJ (2002) Utility of hemodialysis in maple syrup urine disease. Pediatr Nephrol 17:239–242
Jouvet P, Poggi F, Rabier D, Michel JL, Hubert P, Sposito M, Saudubray JM, Man NK (1997) Continuous venovenous haemodiafiltration in the acute phase of neonatal maple syrup urine disease. J Inherit Metab Dis 20:463–472
Arbeiter AK, Kranz B, Wingen AM, Bonzel KE, Dohna-Schwake C, Hanssler L, Neudorf U, Hoyer PF, Buscher R (2010) Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant 25:1257–1265
Fleming GM, Walters S, Goldstein SL, Alexander SR, Baum MA, Blowey DL, Bunchman TE, Chua AN, Fletcher SA, Flores FX, Fortenberry JD, Hackbarth R, McBryde K, Somers MJ, Symons JM, Brophy PD (2012) Nonrenal indications for continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Pediatr Crit Care Med 13:e299–304
Walters S, Porter C, Brophy PD (2009) Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol 24:37–48
Summar M, Pietsch J, Deshpande J, Schulman G (1996) Effective hemodialysis and hemofiltration driven by an extracorporeal membrane oxygenation pump in infants with hyperammonemia. J Pediatr 128:379–382
Lee NC, Chien YH, Peng SF, Huang AC, Liu TT, Wu AS, Chen LC, Hsu LW, Tseng SC, Hwu WL (2008) Brain damage by mild metabolic derangements in methylmalonic acidemia. Pediatr Neurol 39:325–329
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, IJ., Hwu, WL., Huang, SC. et al. Efficacy and safety of intermittent hemodialysis in infants and young children with inborn errors of metabolism. Pediatr Nephrol 29, 111–116 (2014). https://doi.org/10.1007/s00467-013-2609-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2609-2