Abstract
Background
Minimal change disease (MCD) is the most common cause of nephrotic syndrome in children and is associated with the expression of CD80 in podocytes and the increased excretion of CD80 in urine. We hypothesized that serum from patients with MCD might stimulate CD80 expression in cultured podocytes.
Methods
Sera and peripheral blood mononuclear cells (PBMCs) were collected from subjects with MCD in relapse and remission and from normal controls. Immortalized human podocytes were incubated with culture media containing patient sera or supernatants from patient and control PBMC cultures. CD80 expression was measured by quantitative PCR and western blot analysis.
Results
Sera collected from patients with MCD in relapse, but not in remission, significantly increased CD80 expression (mean ± standard deviation: 1.8 ± 0.7 vs. 0.8 ± 0.2; p < 0.004) and CD80 protein secretion by podocytes (p < 0.05 between relapse and normal controls). No such CD80 increase was observed when podocytes were incubated with supernatants of PBMC cultures from patients in relapse.
Conclusions
Sera from MCD patients in relapse, but not in remission, stimulated CD80 expression in cultured podocytes. Identifying this factor in sera could provide insights into the pathogenesis of this disorder. No role in CD80 expression by podocytes was found for cytokines released by PBMCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimal change disease (MCD) is the most common nephrotic syndrome in childhood [1]. In 1974, Shalhoub proposed that proteinuria in these patients was due to a circulating factor secreted by the patient’s lymphocytes and suggested that MCD was a disorder of T-cell function [2]. Support for this hypothesis was provided by Koyama et al. [3] who immortalized T-cells from patients with MCD in relapse and healthy controls and injected supernatants of these T-cells into rats. The injection of supernatants from the T-cell hybridoma from patients in relapse caused immediate proteinuria and glomerular podocyte foot process fusion, whereas no changes were noted when the T-cell hybridoma supernatants from healthy controls were infused [3]. Proteinuria was also reported when supernatants from cultures of peripheral blood mononuclear cells (PBMCs) from MCD patients in relapse were infused into rats [4]. However, despite numerous efforts over the last 30 years, the pathogenic cytokine has not been identified and the mechanism of proteinuria in these patients has not been elucidated.
CD80 or B7-1 is a dendrite-associated receptor that mediates co-stimulatory signaling of the T-cell [5]. In the kidney, CD80 is a transmembrane glycoprotein belonging to the immunoglobulin superfamily and is present in many cell types, including podocytes. CD80 has been found to be expressed by podocytes in several experimental models of glomerular disease associated with nephrotic syndrome [6]. We have recently reported that CD80 is expressed by the podocytes but not the tubules of patients with MCD in association with increased urinary excretion of CD80 [7, 8].
CD80 has been suggested as a mediator of proteinuria. Reiser et al. have shown that mice administered lipopolysaccharide (LPS) developed CD80 expression in podocytes and proteinuria [6]. However, when LPS was administered to CD80−/− knockout mouse, no increase in urinary protein excretion was observed [6]. Because CD80 is expressed by podocytes in patients with MCD in relapse but not in remission [7, 8], we have hypothesized that CD80 could play a role in the proteinuria of MCD-associated nephrotic syndrome [9].
The aim of this study was to evaluate whether serum and/or supernatants from PBMC cultures from MCD patients induce podocyte CD80 expression in vitro. If true, this finding would support Shalhoub’s hypothesis of a circulating factor in MCD nephrotic syndrome.
Patients and methods
Patients
Sixteen patients (8 females, 8 males) with biopsy-proven MCD, as defined by the International Study of Kidney Disease in Children [10], ranging in age from 3 to 21 years, were included in the study. Patients were considered in relapse if they had edema, a serum albumin level of <3.0 g/dl, and a urinary protein/creatinine (mg/mg) ratio of >2.0. Patients were considered in remission if they had no edema and their urinary protein/creatinine ratio was <0.40 in a random urine sample. Two patients were included in the MCD remission group although their urinary protein/creatinine ratio was >0.4, as they were both considered in early remission. One patient showed an increment in the serum albumin from 1.8 to 2.6 g/dl at the time the blood sample was obtained and 4.4 g/dl 2 weeks later. Patient proteinuria improved from 300 mg/dl to 1.43 (urinary protein/creatinine ratio) at the time that the blood sample was obtained, and 0.5 2 weeks later. The second patient had an improved urinary protein/creatinine ratio from 3.1 to 1 at the time the blood sample was obtained, and 0.1 2 weeks later.
Four subjects aged 12–18 years, one female and three males, with nocturnal enuresis without systemic diseases or urinary congenital anomalies served as controls. The study was approved by the Institutional Review Board of the University of Florida.
All patients had a normal serum creatinine for age at the time of study.
Methods
Serum collection
Blood from MCD patients in relapse and in remission, respectively, and normal controls was centrifuged and serum collected and stored at −80 °C.
PBMC cultures
Peripheral blood mononuclear cells were isolated by density centrifugation on a Ficoll–Hypaque gradient as previously described [11] and cultured in 5 ml of RPMI (Grand Island Biological, Grand Island, NY) supplemented with 10 % fetal bovine serum (FBS) in petri dishes for 72 h at 35 °C, at a concentration of 1 × 106 cells/ml [4]. At the end of the incubation period supernatants were collected and concentrated 1.5- to 9-fold.
Podocyte cultures
Immortalized human podocytes [12] were obtained from Dr. Moin Saleem at the University of Bristol and maintained in RPMI-1640 containing 10 % FBS, 1 % insulin–transferrin–selenium A supplement (Invitrogen, Carlsbad, CA), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were grown at 33 °C in 95 % air, 5 % CO2 and then converted to differentiated cells by incubation at 37 °C for 10–14 days. Differentiated cells were kept in growth media supplemented with 1 % FBS for 16 h and then incubated with 15 % serum and 15 % supernatant from cultured PBMCs collected from MCD patients in relapse or in remission, from normal controls, or with 1 % FBS growth media. Serum and supernatants were filtered through a 0.22-μm syringe filter (Millipore Cork, Ireland) prior to the stimulation, and RNA was extracted at 6 h after the stimulation. To examine the effect of interleukin-8 (IL-8) on podocytes, differentiated podocytes were kept in RPMI media containing 1 % FBS for 16 h and then incubated with or without IL-8 (R&D, Minneapolis, MN) at concentrations of 0.5 or 1.0 ng/ml. RNA was extracted at 6 h after the stimulation.
RNA extraction, reverse transcription and quantitative reverse transcription-PCR
Total RNA was extracted using RNeasy (Qiagen, Valencia, CA). A 1-μg sample of each total RNA was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Quantitative real-time PCR analysis for human CD80 was performed using the iCycler thermal cycler (Bio-Rad). Each reaction was carried out in a total volume of 25 μl containing 12.5 μl of 2× iQ SYBR Green supermix (Bio-Rad), forward and backward primers (100 nM), and 2 μl of complementary DNA (cDNA). Amplification was performed for one cycle at 95 °C for 3 min and for 50 cycles at 95 °C for 30 s, the designated annealing temperature for 30 s, and 72 °C for 30 s. The results were normalized using human glyceraldehyde-3- phosphate dehydrogenase (GAPDH) expression, and the ratio to non-stimulated control (1 % FBS growth media) was calculated. Primer sequences and annealing temperatures were: human CD80 (forward, 5′-TTTGACCCTAAGCATCTGAAGC-3′; reverse, 5′-ACCAGCCAGCACCAAGAG-3′, 60 °C) and human GAPDH ( forward, 5′-CGCTGAGTACGTCGTGGAGT-3′; reverse, 5′-AGAGGGGGCAGAGATGATG-3′, 64 °C).
Western blot analysis
Cell protein lysates were prepared from confluent podocyte cultures as described above. Sample protein content was determined by the BCA protein assay (Cat#23225, Thermo Scientific Pierce, Rockford, IL). A 50-μg sample of total protein was loaded per lane for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10 % wt/vol) analysis. The electrophoretic products were transferred to a PVDF membrane (Bio-Rad), and the membrane was blocked with 5 % non-fat milk and then incubated overnight with primary CD80 antibodies (Cat#AF140, R&D System). After washing, the membrane was incubated with a mouse anti-goat horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. The blot was visualized using GE Healthcare Amersham ECL Western Blotting Detection Reagents (cat#RPN2109; GE Healthcare, Piscataway Township, NJ). For a loading control, the same membrane was stripped and reimmunoblotted with a mouse GAPDH antibody (Cat#NB300-221, Novus Biologicals, Littleton, CO) for 1 h at room temperature and with an anti-mouse second antibody (Cat#sc-2314, Santa Cruz Biotechnology) for 1 h. The Alpha Innetch FluorChem gel image program was used to measure band density. CD80 band density data were normalized with GAPDH data to obtain the final results.
Enzyme-linked immunosorbent assay
Serum IL-13 was measured using a commercially available enzyme-linked immunosorbent assay kit from eBioscience (San Diego, CA).
Serum creatinine
Serum creatinine was measured on an autoanalyzer.
Statistical analysis
Data graphics and statistical analysis were performed using Prism 5 software (GraphPad, San Diego, CA). Kruskal–Wallis tests and Mann–Whitney tests were applied to evaluate differences between the groups, and the Spearman correlation coefficient was calculated between CD80 expression and prednisone dose. P < 0.05 was regarded as statistically significant.
Results
Serum from MCD patients was studied at the time of relapse and at remission (Table 1). Mean serum albumin was 2.3 ± 0.5 g/dl at relapse and 3.6 ± 1.2 g/dl at remission. The median urinary protein/creatinine ratio was 5.9 (mg/mg) at relapse and <0.3 (mg/mg) at remission.
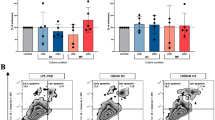
Human podocytes incubated with serum from MCD patients in relapse showed a statistically significant increase in CD80 expression compared to those incubated with serum from MCD patients in remission [mean ± standard deviation (SD) 1.8 ± 0.7 vs. 0.8 ± 0.2; p = 0.004) (Fig. 1a).
Serum increases CD80 expression in podocytes but supernatant does not. Human (Hu) podocytes were incubated for 6 h with 15 % serum (a) or 15 % concentrated supernatants (b) of peripheral blood mononuclear cell (PBMC) cultures from minimal change disease (MCD) patients in relapse or in remission and from normal subjects. RNA was extracted and real-time PCR for human CD80 was performed. Human glyceraldehyde-3- phosphate dehydrogenase (GAPDH) was used as an internal control. Number in columns Number of subjects, horizontal line above columns respective p values for significant difference. Data are presented as the mean ± standard error of the mean (SEM)
In these patients, those in relapse received a lower dose of prednisone than those in remission (0.16 ± 0.31 vs. 0.84 ± 0.76 mg/kg/day; p = 0.02). However, there was no correlation between CD80 expression and the dose of prednisone for either patients in relapse or those in remission (Fig. 2a, b). Finally, CD80 expression in podocytes stimulated with serum from MCD patients in remission receiving low-dose prednisone (0.1 ± 0.05 mg/kg/day) was not significantly different from that in MCD patients receiving a higher dose of prednisone (1.39 ± 0.46 mg/kg/day; p = 0.8) (Fig. 3), although the differences between the doses were significantly different (p = 0.05).
There was no significant correlation between prednisone dose and CD80 expression in podocytes cultured with serum from MCD patients. Human podocytes were incubated for 6 h with 15 % serum from MCD patients in relapse (a) or in remission (b). RNA was extracted and real-time PCR for human CD80 was performed. Human GAPDH was used as an internal control. Spearman correlation is presented
Prednisone does not decrease CD80 expression in podocytes cultured with serum from MCD patients in remission. Human podocytes were incubated for 6 h with 15 % serum from MCD patients in remission. RNA was extracted and real-time PCR for human CD80 was performed. Human GAPDH was used as an internal control. Data are presented as the mean ± SEM
Supernatant was obtained from PBMC cultures from eight MCD patients in relapse, seven patients with MCD in remission, and four normal subjects who served as controls (Table 2). Mean serum albumin was 1.9 ± 0.6 g/dl at relapse and 4.3 ± 0.5 g/dl at remission. The median urinary protein/creatinine ratio was 5.3 (mg/mg) at relapse and 0.1 (mg/mg) at remission.
When podocytes were incubated with two- to ninefold concentrated supernatants from PBMC cultures from MCD patients in relapse or in remission and from normal subjects, no statistically significant differences in CD80 expression among groups was observed (Fig. 1b). There was also no difference in CD80 expression by podocytes between supernatants from PBMC cultures from patients in relapse or in remission concentrated by 1.5- to fourfold and those concentrated four- to ninefold (Fig. 4a, b).
Highly concentrated supernatants of PBMC cultures from MCD patients did not increase CD80 expression in human podocytes. Human podocytes were incubated for 6 h with 15 % concentrated supernatants (1.5- to 4-fold vs. 4- to 9-fold) of PBMCs from MCD patients in relapse (a) and in remission (b), cultured with fetal bovine serum (FBS). RNA was extracted and real-time PCR for human CD80 was performed. Human GAPDH was used as an internal control. Data are presented as the mean ± SEM
Supernatants of PBMC cultures from patients in remission and in relapse were collected. In these patients, the dose of prednisone in the latter was not different than that in the former (0.47 ± 0.6 vs. 0.49 ± 0.5 mg/kg/d, respectively; p = 0.77).
There was no correlation between prednisone dose and CD80 podocyte expression in supernatants of PBMC cultures from patients in relapse (r = −0.07, p = 0.88). Moreover, although there was a significant difference in the prednisone dose of MCD patients in relapse who received a high dose of prednisone (1.09 ± 0.57 mg/kg/day) and those on a low dose of steroids (0.1 ± 0.1 mg/kg/day) (p = 0.03), there was no difference in podocyte CD80 expression between these two groups (p = 0.78).
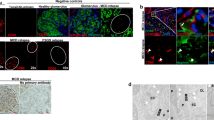
Cellular podocyte extracts were probed with anti-CD80 antibody to quantify CD80 protein level after podocyte culture stimulation with sera from MCD patients in relapse or in remission and in normal controls (Fig. 5). A significant increase (p < 0.05) in CD80 protein was observed when podocytes were incubated with serum from MCD patients in relapse in comparison to those stimulated with sera from normal controls.
Cellular podocyte extracts were probed with anti-CD80 antibody to quantify CD80 protein level after podocyte culture stimulation with sera from 5 MCD patients in relapse, 2 MCD patients in remission, and 3 normal controls. Western blot bands from patients and normal controls (a) were quantified by densitometry and the results normalized using GADPH (p < 0.05 between relapse and normal controls) ( b). c Range of densitometry CD80 results. d CD80 in the human tonsil. CD80 is expressed (red stain) in normal human tonsil using the same CD80 goat antibody (R&D Systems) as in the western blots. CD80 and anti-CD80 complexes were visualized as previously described [7]
We also examined the effect of IL-8 on CD80 expression in human podocytes. When podocytes were incubated with growth media containing recombinant IL-8, no statistically significant differences in CD80 expression among groups were observed [CD80/GAPDH ratio: control, 1.00 ± 0.36; IL-8 (0.5 ng/ml), 0.83 ± 0.39; IL-8 (1.0 ng/ml), 0.46 ± 0.03; n = 3; no significant difference among groups].
IL-13 was measured in the serum and supernatants used in the study (Table 3). In all sera from patients in relapse or in remission IL-13 was below the limit of detection (<10 pg/ml). IL-3 was detected in only one supernatant of PBMC from an MCD patient in relapse (80.6 pg/ml) and in only one supernatant from an MCD patient in remission (161.8 pg/ml).
Discussion
The pathogenesis of proteinuria in MCD has not yet been elucidated. Shalhoub proposed in 1974 that a circulating factor secreted by T-cells might be responsible for the increased glomerular permeability to plasma proteins in patients with this disease, further suggesting that MCD was a disorder of T-cell function [2]. Shalhoub based his hypothesis on the absence of immune complexes in the glomeruli of MCD patients, the rapid response of MCD to steroids, the association of MCD with Hodgkin’s disease, and the observation that MCD often remits subsequent measles infection (the latter is known to suppress cell-mediated immunity) [2].
Despite numerous attempts, the pathogenic cytokine responsible for MCD has not been identified nor has the mechanism of the proteinuria in MCD patients been elucidated. Different hypotheses have been postulated to explain the mechanism of proteinuria in MCD patients. One of the first was a role for sialoprotein in coating the glomerular capillary wall [13]. This was followed by the proposal that the defect could be attributed to a decrease in the heparan sulfate proteoglycans of the glomerular basement membrane (GBM) [14]. At the present time, MCD is thought to be due to podocyte dysfunction. Changes in podocyte attachment to the GBM and/or podocyte cytoskeleton could lead to increased glomerular permeability to plasma proteins [15].
The role of podocytes in MCD is supported by the seminal studies on experimental animal models of proteinuria by Reiser et al. [6]. These researchers found an increased expression of CD80 by podocytes in association with LPS-induced proteinuria and a histological lesion that resembled MCD. Proteinuria was not seen in LPS-treated CD80 knockout mice, suggesting a pivotal role for this molecule in the development of proteinuria [6]. Following the lead of these tantalizing results, we proceeded to study CD80 in MCD patients and found increased CD80 podocyte expression and increased CD80 urinary excretion in these patients [8]. These results have led us to postulate that the circulating factor stimulates TLR3 or TLR4, which are receptors known to induce NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation [16, 17]. Our previous studies have shown that stimulation of TL3 on podocytes is followed by translocation of the p65 subunit from the cytoplasm to the nucleus, documenting the activation of NFκB. Furthermore, we found that the NFκB inhibitor PDTC significantly reduces the expression of CD80 following TL3 stimulation by polyIC, demonstrating that CD80 upregulation in podocytes is dependent on NFκB activation. CD80 activation is followed by re-organization of the actin cytoskeleton with disruption of central stress fibers and increased F-actin at the cortical ring. CD80 silencing by shRNA largely prevents actin re-organization, whereas this does not occur following transfection with a non-relevant shRNA [18]. The reorganization of the podocyte actin cytoskeleton would lead to morphological changes at the slit diaphragm, resulting in increased permeability and proteinuria.
The present study is a corollary to our findings in MCD patients and the aim was to evaluate if serum or lymphocyte cytokines (present in supernatants of PBMC cultures) could induce CD80 expression in cultured podocytes. We found that serum from MCD patients in relapse increased both CD80 mRNA expression and CD80 protein levels in podocyte cultures. In contrast, no such increase was observed when serum from MCD patients in remission was added to podocyte cultures. Consequently, our results demonstrate that MCD patients in relapse do have a circulating factor that induces CD80 expression in podocytes, suggesting that in vivo this circulating factor could lead to the observed proteinuria and increased CD80 podocyte expression in these patients.
Prednisone has been shown to decrease CD80 expression in cultured podocytes. In our study, patients in remission received a significantly increased dose of prednisone compared to those in relapse. However, the lack of increased podocyte CD80 expression when serum from MCD patients in remission was added to the cultures could not be attributed to the effects of prednisone because there was no correlation between CD80 expression and the dose of prednisone in patients in remission. In addition, CD80 expression in podocytes stimulated with serum from MCD patients in remission receiving a low dose of prednisone was not significantly different from that in podocytes stimulated with serum from MCD patients receiving a higher dose of prednisone.
The nature of the circulating factor has yet not been defined, although a role for IL-13 in the pathogenesis of proteinuria has been postulated [19–21]. Overexpression of IL-13 has been reported to cause proteinuria and podoctye CD80 expression in mice [20], but we have not been able to reproduce the results (unpublished data). The results of the present study do not support a role of IL-13 as a circulating factor in MCD, since this cytokine was not detectable in the serum of our patients in relapse and was detectable in only one supernatant from the MCD PBMC cultures from patients in relapse and from one supernatant from cultures of MCD patients in remission.
We have shown that IL-8 could increase the catabolism of heparan sulfate in the GBM and induce proteinuria in rats [22]. Moreover, increased serum IL-8 concentrations have been reported in MCD patients [23]. However, we were unable to observe an effect of IL-8 on CD80 expression in podocytes in this study. This was not due to low levels of IL-8 in the supernatants, since the concentrations in the media culture were equivalent to those observed in MCD serum [23]. However, IL-8 may still play a role in the development of proteinuria by a mechanism other than via CD80, possibly by acting directly on the GBM because we have shown that IL-8 increases the catabolism of heparan sulfate in the GBM in an animal model [22]. The decrease in heparan sulfate in the GBM could contribute to the decreased capillary wall negative charge observed in these patients.
The results of this study suggest the presence of a circulating pathogenetic factor in MCD patients. However, in contrast with Shalhoub’s hypothesis [2], the increased CD80 expression in podocytes observed in our study was not due to a factor released by patients’ lymphocytes because it was not observed when the podocytes were cultured with supernatants from cultured PBMCs sampled from MCD patients in relapse.
It should be pointed out that treatment with steroids could prevent PBMCs from patients in relapse from expressing cytokines, possibly explaining why there was no difference in CD80 expression in podocytes cultured with supernatants from PBMC of either MCD patients in relapse or normal controls. However, PBMC cultures from MCD patients in relapse are supposed to be secreting the factor because patients are in relapse. Finally, there was no correlation between the dose of prednisone and CD80 podocyte expression, and there was no difference in CD80 expression between PBMC supernatants from patients receiving a high dose of prednisone and those from patients on a low dose of steroids.
Although the nature of the serum factor has not been defined in this study, Reiser et al. have suggested that microbial products could trigger podocyte CD80 expression in MCD patients [24]. This hypothesis is supported by the observation that in MCD, relapse is usually triggered by viral upper respiratory infections (URI) [25]. Microbial products in URI are not limited to the respiratory mucosa, but viral fragments have been detected in the circulation of these patients [26]. Consistent with these findings, we have demonstrated that polyIC, which mimics viral RNA, stimulates TLR3 on podocytes, leading to increased expression of CD80 [18]. This hypothesis needs to be confirmed by analysis of serum from MCD patients for the presence of microbial products.
In summary, MCD patients in relapse have a serum factor that increases CD80 expression in podocytes. Because CD80 has been shown to be increased in MCD patients in relapse, this finding can explain, at least in part, the mechanism of proteinuria in these patients. Lymphocyte products do not appear to play a role in the CD80-mediated proteinuria in MCD patients.
References
International Study of Kidney Disease in Children (1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A Report of the International Study of Kidney Disease in Children. Kidney Int 13:159–165
Shalhoub RJ (1974) Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 2:556–560
Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M (1991) A glomerular permeability factor produced by human T-cell hybridomas. Kidney Int 40:453–460
Garin EH, Laflam PF, Muffly K (2006) Proteinuria and fusion of podocyte foot processes in rats after infusion of cytokine from patients with idiopathic minimal lesion nephrotic syndrome. Nephron Exp Nephrol 102:e105–112
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548
Reiser J, von Gersdorff G, Loos M, Oh L, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P (2004) Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113:1390–1397
Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ (2010) Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int 78:296–302
Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ (2009) Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol 20:260–266
Shimada M, Araya C, Rivard C, Ishimoto T, Johnson RJ, Garin EH (2011) Minimal change disease: A “Two hit” podocyte immune disorder? Pediatr Nephrol 26:645–649
International Study of Kidney Disease in Children (1981) Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 20:765–771
Garin EH, Boggs KP (1987) Effect of peripheral blood mononuclear cells from patients with nephrotic syndrome on uptake of 35sulfate by glomerular basement membrane. Int J Pediatr Nephrol 6:189–194
Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13:630–638
Blau EB, Haas JE (1973) Glomerular sialic acid and proteinuria in human renal disease. Lab Invest 28:477–481
Garin EH, Boggs KP (1985) Effect of supernatants from nephrotic peripheral blood mononuclear cells on 35sulfate incorporation in rat glomerular basement membrane. Pediatr Res 19:836–840
Mundel P, Shankland SJ (2002) Podocyte biology and response to injury. J Am Soc Nephrol 13:3005–3015
Chiron D, Pellat-Deceunynck C, Amiot M, Bataille R, Jego G (2009) TLR3 ligand induces NF-{kappa}B activation and various fates of multiple myeloma cells depending on IFN-{alpha} production. J Immunol 182:4471–4478
Yu H, Ha T, Liu L, Wang X, Gao M, Kelley J, Kao R, Williams D, Li C (2012) Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim Biophys Acta 1823:1192–1198
Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Wymer DT, Yamabe H, Mathieson PW, Saleem MA, Garin EH, Johnson RJ (2012) Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-kappaB-dependent pathway. Nephrol Dial Transplant 27:81–89
Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC (1999) Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol 10:529–537
Lai KW, Wei CL, Tan LK, Tan PH, Chiang GS, Lee CG, Jordan SC, Yap HK (2007) Overexpression of interleukin-13 induces minimal change-like nephropathy in rats. J Am Soc Nephrol 18:1476–1485
Cheung W, Wei CL, Seah CC, Jordan SC, Yap HK (2004) Atopy, serum IgE, and interleukin-13 in steroid-responsive nephrotic syndrome. Pediatr Nephrol 19:627–632
Garin EH, West L, Zheng W (2000) Interleukin-8 alters glomerular heparan sulfate glycosaminoglycan chain size and charge in rats. Pediatr Nephrol 14:284–287
Garin EH, Blanchard DK, Matsushima K, Djeu JY (1994) IL-8 production by peripheral blood mononuclear cells in nephrotic patients. Kidney Int 45:1311–1317
Reiser J, Mundel P (2004) Danger signaling by glomerular podocytes defines a novel function of inducible B7-1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol 15:2246–2248
MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E (1986) Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr 108:378–382
Xatzipsalti M, Kyrana S, Tsolia M, Psarras S, Bossios A, Laza-Stanca V, Johnston SL, Papadopoulos NG (2005) Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med 172:1037–1040
Conflict of interest
We, the authors declare that the results presented in this paper have not been published previously in whole or part, except in abstract format. This work was supported by NIH R01DK080764. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishimoto, T., Cara-Fuentes, G., Wang, H. et al. Serum from minimal change patients in relapse increases CD80 expression in cultured podocytes. Pediatr Nephrol 28, 1803–1812 (2013). https://doi.org/10.1007/s00467-013-2498-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2498-4