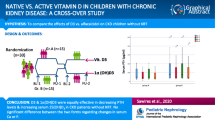
Abstract
Background
We aimed to investigate the effect of single, high-dose intramuscular cholecalciferol on vitamin D3 and intact parathyroid hormone (iPTH) levels in children with chronic kidney disease (CKD).
Methods
Between January 2012 and June 2012, we conducted a prospective, uncontrolled study at the Pediatric Nephrology Unit of King Abdulaziz University Hospital, Jeddah, to investigate the effect of single, high-dose intramuscular vitamin D3 on 25(OH)D3 and iPTH levels in vitamin D insufficient/deficient children with CKD. Serum vitamin D3, iPTH, calcium, phosphate, alkaline phosphatase (ALP), and creatinine levels were measured before intramuscular vitamin D3 (300,000 IU) administration, and these were subsequently repeated at 1 and 3 months after treatment. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA).
Results
Nineteen children fulfilled the criteria. At 3 months after treatment, vitamin D3 levels were significantly higher than at baseline (p < 0.001) but lower than the levels at 1 month. iPTH levels decreased significantly at 3 months (p = 0.01); however, the drop in iPTH levels was not significant at 1 month (p = 0.447). There were no changes in calcium, phosphate, ALP, or creatinine levels after treatment.
Conclusions
Single-dose intramuscular vitamin D3 (300,000 IU) resulted in significant improvement of vitamin D3 and iPTH levels in children with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is associated with disturbance of mineral and bone homeostasis. This condition, termed CKD-mineral bone disorder (CKD-MBD), and possibly its treatment is associated with increased morbidity and mortality [1]. In children with CKD, impaired conversion of 25-hydroxyvitamin D3 (25[OH] D3) to 1, 25-dihydroxyvitamin D3 (1,25 [OH]2D3) and phosphate retention result in low calcium levels. This in turn causes an increase in intact parathyroid hormone (iPTH) levels, leading to defective skeletal mineralization [2]. In contrast, in patients with late CKD-MBD, there are changes in bone turnover, characterized by high levels of PTH and inflammatory markers [3].
In recent studies from different parts of the world, 25 (OH) D3 deficiencies were reported in both adults [4–6] and children [7, 8] with CKD. We reported a high frequency (87.5 %) of vitamin D insufficiency/deficiency that correlated negatively with iPTH levels and CKD stages [9]. The 2009 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline on the management of CKD-MBD recommended that 25 (OH) D3 levels should be monitored and if low, ergo-, or cholecalciferol supplements should be prescribed [10]. Ergocalciferol was reported as safe and effective in delaying the onset of secondary hyperparathyroidism in 25(OH)D3-deficient children with CKD stages 2–3 who were not receiving alfacalcidol [11]. In our previous report, the administration of oral vitamin D3 2000 IU/day resulted in significant improvement of vitamin D levels in children with CKD, but only 11 % of the patients achieved normal levels after 3 months of treatment; however, oral vitamin D supplementation had no effect on the iPTH levels of the children [12].
In this study, we investigate the effect of single, high-dose intramuscular vitamin D3 on the levels of 25(OH) D3 and iPTH in vitamin D-insufficient/-deficient children with CKD who failed to respond to low-dose maintenance therapy with oral vitamin D3. We are testing the hypothesis that in children with high iPTH who had received alfacalcidol, CKD-MBD can be improved by normalizing vitamin D3 levels.
Patients and methods
This prospective, uncontrolled study was conducted to investigate the effect of single, high-dose intramuscular vitamin D3 on the levels of 25(OH) D3 and iPTH in vitamin D insufficient/deficient children with CKD. All the children were followed up at the Pediatric Nephrology Unit of King Abdulaziz University Hospital, Jeddah, between January 2012 and June 2012. Ethical approval for the study was obtained from the Biomedical Ethics Research Committee of King Abdulaziz University. Written informed consent was obtained from all the parents.
Children with CKD stages 2–5 were included in the study provided they met the following criteria: low vitamin D3 despite supplementation with oral cholecalciferol 2000 IU/day for 6 months, high iPTH, and normal calcium. We excluded all children who did not complete 6 months of treatment with oral cholecalciferol. As reported previously, all the children had received alfacalcidol (15–30 ng/kg/day) and calcium carbonate (300 mg–1.25 g three to four times daily) for at least 3 months prior to the administration of oral cholecalciferol 2,000 IU/day (for 6 months) [12]. The doses were maintained throughout the study period.
For all children included in the study, laboratory investigations, including serum vitamin D3, iPTH, calcium, phosphate, alkaline phosphatase (ALP), and creatinine levels were performed before the administration of intramuscular vitamin D3 300,000 IU (1 ml of cholecalciferol; Streuli, Switzerland). The investigations were subsequently repeated at 1 and 3 months after treatment. Calcidiol levels were measured by using radioimmunoassay (Diasorin, Stillwater, MN, USA). Serum iPTH was measured by Elecsys 2010 autoanalyzer system (Roche Diagnostics, Basel, Switzerland).
The stages of CKD were classified according to the estimated glomerular filtration rate, which was calculated using the Schwartz formula [13]. Based on the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines [10], vitamin D sufficiency was defined as 25(OH)D level ≥30 ng/ml; insufficiency, 16–29 ng/ml; deficiency, 5–15 ng/ml; and severe deficiency if <5 ng/ml.
Statistical method
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS). Kolmogorov–Smirnov test of normality was conducted to test numerical variables for normality. A p value > 0.05 implied normal distribution of the data.
Descriptive statistics was conducted for all variables that were normally distributed by using mean and standard deviation. Paired t test was used to compare the change in vitamin D3 levels from baseline to the first and third months after intramuscular vitamin D3 administration. Significance was set at p < 0.05, with 95 % confidence interval that did not include 0.
Results
Nineteen children fulfilled the inclusion criteria. The amount of elapsed time between completion of the oral trial and entry into the intramuscular trial ranged from 2 to 6 months (median, 3 months). The mean (SD) age of the children was 11.8 (4.6) years (median, 12 years; range, 2–16 years). The study population comprised 11 boys and eight girls (Table 1). Twelve children were Saudis and seven were of other nationalities. Two children had stage 2 CKD, one had stage 3 CKD, three had stage 4 CKD, and 13 had stage 5 CKD.
There was significant improvement in vitamin D3 levels in 15 children (79 %) (p < 0.001) who achieved normal vitamin D3 levels (≥30 ng/ml), 1 month following the administration of intramuscular cholecalciferol. Among the 15 children who achieved normal vitamin D3 levels, 11 had stage 5 CKD and four had stage 2–4 CKD; four children (two with stage 5 CKD and two with stage 2–4) failed to achieve normal vitamin D3 levels. Four children had vitamin D3 levels ≥30 ng/ml (range, 53.2–61.2 ng/ml), but none of them had hypercalcemia.
Three months after intramuscular injection of cholecalciferol, the vitamin D3 levels were significantly higher than at baseline (p < 0.001) but lower than the levels after 1 month. There was no case of high vitamin D3 levels 3 months after treatment, and only nine children (47.4 %) had normal vitamin D3 levels. Of these, seven had stage 5 CKD, one had stage 2 CKD, and one had stage 4 CKD. Their median age was 13 years (range, 3–16 years).
Similarly, there was a significant improvement in iPTH levels, from mean (SD) 107.7 (78.0) pmol/l at baseline to 90.6 (79.4) pmol/l, 3 months after intramuscular administration of cholecalciferol (p = 0.01); however, the drop in iPTH levels was not significant 1 month after vitamin D3 injection (p = 0.447). There were no changes in the levels of calcium, phosphate, ALP, or creatinine after intramuscular administration of vitamin D3 (Table 2).
Table 3 shows the results of laboratory investigations of the 19 children before and after administration of oral cholecalciferol 2,000 IU/day prior to the administration of single-dose intramuscular cholecalciferol. When compared with the results observed after intramuscular administration of high-dose intramuscular cholecalciferol, there was no significant improvement of the vitamin D3 and iPTH levels.
Discussion
Vitamin D insufficiency/deficiency is a common health problem in Saudi Arabia, a country with abundant sunshine throughout the year, due to lack of exposure to sunlight, poor supplementation, consumption of cola soft drinks, and prolonged breastfeeding [14–16], which are common practices among the local population. In our previous study investigating the effect of daily oral vitamin D3 on the levels of 25(OH)D3 and iPTH in vitamin D-insufficient/-deficient children with CKD [12], maintenance therapy with oral vitamin D3 (2000 IU/day for 6 months) did not result in improved iPTH levels, and only 11 % of the children achieved normal 25 (OH) D3 levels (≥30 ng/ml). Furthermore, all the children had received alfacalcidol, the active form of vitamin D, which is recommended for use in children with CKD and high iPTH, with the aim of normalizing iPTH levels in these children. We believe that non-adherence to treatment may explain the poor response to vitamin D supplementation in our study, as there is evidence that the administration of oral vitamin D3 resulted in improved vitamin D3 levels and a reduction in iPTH [8, 17, 18]. The poor response in our study may also be attributed to the low dose of oral vitamin D that we administered to the children since the current recommendation is to use doses of 1,000 IU/day for infants <1 month old, 1,000 to 5,000 IU/day for infants 1 to 12 months old, and >5,000 IU/day for children >12 months old [19].
According to our findings, vitamin D3 is useful in children with renal bone disease, who have low vitamin D3. We found that 3 months after intramuscular therapy, vitamin D3 levels were sustained in nine children, who were older in age. While it is known that children with CKD stages 2–4 show a better response to vitamin D therapy [11], only two of the children with CKD stages 2–4 and seven with stage 5 CKD sustained normal vitamin D3 levels 3 months after therapy. However, this finding can be explained by the small size of our cohort. We also did not observe any adverse effect, such as hypercalcemia as confirmed in previous studies on normal children [20] or those with CKD [17, 18]. Despite the improvement of vitamin D3 level after the intramuscular dose, however, iPTH was still higher than its level at the end of the oral trial. This could be explained by the time delay between the end of the oral trial and the administrations of the intramuscular high dose as the same method of essays were used in both occasions.
As mentioned earlier, high doses of oral vitamin D3 were associated with increased vitamin D3 and decreased iPTH levels. Hari et al. from India reported using oral vitamin D3 600,000 IU daily for 3 days in children aged 2–15 years, and they observed a significant improvement in vitamin D levels as well as a significant reduction in iPTH, 6 weeks after treatment [17]. Belostotsky et al. from Manchester used single dose oral vitamin D3 (100,000 IU for those 5–10 years of age and 150,000 IU for those over 10 years old), with the nephrologist or clinic nurse witnessing ingestion of the medication at the clinic [18]. They observed a significant increase in vitamin D levels, but they did not measure iPTH levels in their patients. In the current study, to curb non-adherence to treatment and because of the unavailability of oral high-dose formulations locally, we administered a single, high dose of vitamin D3 (300,000 IU) intramuscularly to the children who failed to respond to low-dose maintenance therapy with oral vitamin D3. This is an unconventional mode of drug delivery, which could be avoided by using large oral doses of vitamin D3.
However, we observed that children with vitamin D3 deficiency benefited from single, high-dose cholecalciferol injections as reflected by the significant improvement with a rise in the levels of vitamin D3 at 1 and 3 months after treatment. Similar improvements with a reduction in the serum levels of iPTH were also observed after 3 months of treatment which is of clinical relevance as it could improve CKD-MBD. Nevertheless, within a few months, the levels of vitamin D3 should be expected to drop as observed in our cases. Thus, monitoring 25(OH) D3 levels and repeating the dose of vitamin D3 every 3 months, as suggested by Belostotsky et al. [18], seems reasonable.
The Cochrane reviewers found 15 randomized controlled trials comparing different interventions used to prevent or treat bone disease in children with CKD stages 2–5. They concluded that bone disease, assessed by changes in PTH levels, is improved by all vitamin D preparations. However, no consistent differences between routes of administration (oral or intraperitoneal), frequencies of dosing, or vitamin D preparations could be recommended [21].
Shroff et al. used oral vitamin D3 in vitamin D-deficient or severely deficient children with CKD who had normal iPTH levels [11]. They used intensive replacement treatment with ergocalciferol as per the Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines for nutrition in CKD [22], and they found that after 3 months of therapy, there was a significant improvement in 25(OH)D levels in the treated group as compared with the placebo group. There was also an improvement in the levels of 1,25(OH)2D in the treated group compared with the placebo group [11]. They concluded that ergocalciferol was effective in delaying the development of secondary hyperparathyroidism in children with CKD 2–3 who had normal iPTH.
This study had some limitations in that it was uncontrolled and hence had the fundamental defect of lacking a contemporaneous comparison group. More so, because it was single-arm comparing to a historic control of oral vitamin D treated patients, the time-to-event endpoints in the single-arm study relied on historical controls. This study was also limited in that the study sample was small, especially the number of patients with CKD stages 2–4. On the one hand, because all the patients initially received low doses of oral vitamin D (2,000 IU/day), the poor response was expected in the historical group since the current recommendation is to use doses of >5,000 IU/day for children >12 months old [19]. On the other hand, we administered high doses of intramuscular vitamin, which have been reported to cause a biochemical response within 1–2 weeks. Because of the possible toxicity related to the administration of high doses of vitamin D3, it is important to obtain at this period serum calcium, phosphorus, magnesium, ALP, 25 (OH) D, PTH levels, and a urine sample to determine the calcium/creatinine ratio, the first sign of which is an increase in phosphate [23]. Unfortunately, we did not perform these tests 1–2 weeks after intramuscular injection of vitamin D3.
Conclusions
High-dose intramuscular vitamin D3 is effective and safe in children with CKD, and it improves hyperparathyroidism. Vitamin D3 levels should be measured even in children who had received 1,25(OH)2 vitamin D3.
References
Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K (2012) The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrol Dial Transplant 27(8):3063–3071
Sanchez CP (2008) Mineral metabolism and bone abnormalities in children with chronic renal failure. Rev Endocr Metab Disord 9(2):131–137
Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR et al (2000) Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58(1):396–399
Rucker D, Tonelli M, Coles MG, Yoo S, Young K, McMahon AW (2009) Vitamin D insufficiency and treatment with oral vitamin D3 in northern-dwelling patients with chronic kidney disease. J Nephrol 22(1):75–82
Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL (2010) Vitamins K and D status in stages 3–5 chronic kidney disease. Clin J Am Soc Nephrol 5(4):590–597
Jabbar Z, Aggarwal PK, Chandel N, Kohli HS, Gupta KL, Sakhuja V, Jha V (2009) High prevalence of vitamin D deficiency in north Indian adults is exacerbated in those with chronic kidney disease. Nephrology (Carlton) 14(3):345–349
Belostotsky V, Mughal MZ, Berry JL, Webb NJ (2008) Vitamin D deficiency in children with renal disease. Arch Dis Child 93(11):959–962
Menon S, Valentini RP, Hidalgo G, Peschansky L, Mattoo TK (2008) Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney disease. Pediatr Nephrol 23(10):1831–1836
Kari JA, El-Desouky SM, EL-Morshedy SM, Habib HS (2012) Vitamin D insufficiency and deficiency in children with chronic kidney disease. Annals Saudi Med 32(5):473–478
Moe SM, Drüeke TB, Block GA, Cannata-Andía JB, Elder GJ, Fukagawa M, Jorgetti V, Ketteler M, Langman CB, Levin A, MacLeod AM, McCann L, McCullough PA, Ott SM, Wang AY, Weisinger JR, Wheeler DC, Persson R, Earley A, Moorthi R, Uhlig K (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (113):S1–130
Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, Wells D, Aitkenhead H, Manickavasagar B, van’t Hoff W, Rees L (2012) Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol 7(2):216–223
Kari JA, Eldesoky SM, Bagdadi OT (2012) Vitamin D insufficiency and treatment with oral vitamin D3 in children with chronic kidney disease. Saudi Med J 33(7):740–744
Kemperman FA, Krediet RT, Arisz L (2002) Formula-derived prediction of the glomerular filtration rate from plasma creatinine concentration. Nephron 91(4):547–558
Al-Atawi MS, Al-Alwan IA, Al-Mutair AN, Tamim HM, Al-Jurayyan NA (2009) Epidemiology of nutritional rickets in children. Saudi J Kidney Dis Transpl 20:260–265
Al-Jurayyan NA, El-Desouki ME, Al-Herbish AS, Al-Mazyad AS, Al-Qhtani MM (2002) Nutritional rickets and osteomalacia in school children and adolescents. Saudi Med J 23:182–185
Libuda L, Alexy U, Remer T, Stehle P, Schoenau E, Kersting M (2008) Association between long-term consumption of soft drinks and variables of bone modeling and remodeling in a sample of healthy German children and adolescents. Am J Clin Nutr 88:1670–1677
Hari P, Gupta N, Hari S, Gulati A, Mahajan P, Bagga A (2010) Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol 25(12):2483–2488
Belostotsky V, Mughal Z, Webb NJ (2009) A single high dose of ergocalciferol can be used to boost 25-hydroxyvitamin D levels in children with kidney disease. Pediatr Nephrol 24(3):625–626
Munns C, Zacharin MR, Rodda CP, Batch JA, Morley R, Cranswick NE, Craig ME, Cutfield WS, Hofman PL, Taylor BJ, Grover SR, Pasco JA, Burgner D, Cowell CT, Paediatric Endocrine Group, Paediatric Bone Australasia (2006) Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 185(5):268–272
Carnes J, Quinn S, Nelson M, Jones G, Winzenberg T (2011) Intermittent high-dose vitamin D corrects vitamin D deficiency in adolescents: a pilot study. Eur J Clin Nutr 66(4):530–532
Geary DF, Hodson EM, Craig JC (2010) Interventions for bone disease in children with chronic kidney disease. Cochrane Database Syst Rev (1):CD008327. doi:10.1002/14651858.CD008327
KDOQI Work Group (2009) KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53(3 Suppl 2):S11–104
Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M (2008) Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122(2):398–417
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kari, J.A., Baghdadi, O.T. & El-Desoky, S. Is high-dose cholecalciferol justified in children with chronic kidney disease who failed low-dose maintenance therapy?. Pediatr Nephrol 28, 933–937 (2013). https://doi.org/10.1007/s00467-012-2407-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2407-2