Abstract
Background
Growth hormone had been applied to treat pediatric renal allograft recipients with growth retardation. In this systemic review and meta-analysis, we assess the efficiency and safety of growth hormone use in post-renal transplant children.
Methods
A literature search revealed five prospective randomized controlled trials assessing this therapy, with a total of 401 patients. The outcomes, including the baseline height standard deviation score (HSDS), HSDS after a 1-year therapy, delta height standard deviation score (△HSDS), allograft rejection rates and changes in the glomerular filtration rates (GFR) were analyzed.
Results
Pooled data of the five studies showed that 1 year after the randomized controlled trials, the experimental group receiving growth hormone had a significantly higher growth velocity than the control group, with a mean HSDS difference of 0.68 [95 % confidence interval (CI) 0.25–1.11, P = 0.002] between the two groups. The mean difference in the △HSDS between the treated and control group was 0.52 (95 % CI 0.37–0.68, P < 0.00001). The rejection episode rates were 35/205 and 19/185, respectively (number of patients with rejection/ total number of patients) (risk ratio 1.56, 95 % CI 0.97–2.53, P = 0.07), and the mean difference in the △GFR was 3.27 ml/min per 1.73 m2 (95 % CI −3.54–10.09, P = 0.35), which was not statistically significant.
Conclusions
Based on these studies, we suggest that the application of growth hormone is an effective treatment to promote the growth velocity of children after kidney transplantation. However, the safety of this treatment needs further evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
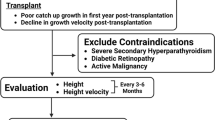
Renal transplantation is considered to be an optimal therapy for children with end stage renal disease (ESRD) [1–4]. Growth retardation is a common phenomenon in pediatric renal allograft recipients [5, 6], and various mechanisms may account for the failure of these children to achieve normal or accelerated catch-up growth, including post-transplant glucocorticoid use, disturbed growth hormone/insulin-like growth factor-I (IGF-I) axis, abnormal allograft function and secondary hyperparathyroidism [7–10].
Growth hormone therapy is widely used in children showing growth retardation due to various causes. In a number of preliminary studies, the administration of growth hormone to pediatric patients after renal transplantation has been demonstrated to improve growth velocity. The safety of growth hormone on renal function and rejection rates has also been evaluated in these trials, but with contradictory results. Thus, the efficiency and safety of growth hormone therapy for children after renal transplantation remains a concern for clinical practitioners.
The aim of our systemic review and meta-analysis was to identify and analyze high-quality clinical trials of growth hormone use in pediatric renal allograft recipients. Specifically, we focused on data on the delta standard height deviation score (△HSDS), change in the glomerular filtration rate (△GFR) as an indication of renal function and rejection episodes of the patients to evaluate the efficiency and safety of this therapy.
Methods
Inclusion criteria of trials
We included trials that meet all the following criteria for analysis: (1) the study is a prospective randomized controlled test (RCT); (2) all participants involved are pediatric renal allograft recipients (range 0–18 years of age); (3) the study was designed to compare children receiving or not receiving recombinant human growth hormone after transplantation; (4) the following outcome measurements were all or partially reported: the delta height SDS (standard height), change in the GFR and rejection episodes of each group during the trial.
Search strategy
We searched MEDLINE (1966 to December 2011), EMBASE (1980 to December 2011) and the Cochrane Controlled Trials Register using the following subject heading or keywords: “pediatric”, “children”, “renal/kidney transplantation”, “growth”, “height”, “rejection” and “growth hormone.” The search was restricted to English-language articles. Two reviewers (Y Wu and J Pei) independently carried out the search and analyzed the quality of the studies that met the inclusion criteria. The reference lists of relevant textbooks, review articles and abstracts of scientific meetings were also searched.
Data extraction
Each eligible paper was assessed by two reviewers (Y Wu and J Pei) independently, and the following data were extracted from each of the RCTs: general information (year, author, study type and design), baseline information of patients (age, gender, height SDS before transplant, GFR, follow-up time, among others), change in height SDS and SDS at the end of the trial year, allograft rejection episodes and change in the GFR during the trial.
Statistical analysis
Review Manager 5.0, which was created by the Cochrane Collaboration for meta-analysis (http://www.cochrane.org), was used to analyze the statistics. Heterogeneity between studies was assessed using Cochran’s Q statistic to determine whether a fixed (P > 0.1) or random (P < 0.1) effect model should be used. Continuous (baseline SDS, delta height SDS, SDS at the end of year and change in the GFR) and dichotomous (acute rejection episodes) outcomes were expressed as mean differences and pooled risk ratio (RR), respectively, with their 95 % confidence intervals (CI). Statistical significance was assessed by the Z test, and the pooled data were considered to be statistically significant at p < 0.05.
Results
Identification of eligible trials
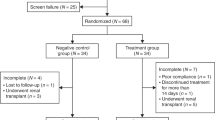
The initial search yielded a total of 121 potentially relevant studies. We screened the abstracts of these preliminary results and excluded 105 of them that were not related with growth hormone use after pediatric kidney transplantation. After reading through the full texts of the remaining articles, we then excluded a further 11 as they were narrative reviews that provided insufficient numerical results or they were not prospective RCTs.
Trial characteristics
Five randomized controlled trials were ultimately included in our review, with a total of 401 pediatric renal allograft recipients [11–15]. The characteristics and baseline information of the five trials are listed in Table 1. Four of the five trials analyzed the height SDS at the end of the trial year and another three evaluated the change in the standard height (△HSDS). In all five RCTs, the rejection episode rates were compared between two groups of patients during the first post-transplant year: those receiving growth hormone therapy and those who did not. Four studies measured the change in renal function (△GFR; ml/min per 1.73 m2) at the start and end of the trial year. Placebo was not been used for ethical reasons.
Effect of growth hormone on growth velocity
The meta-analysis of the pooled data showed that growth hormone significantly improved the height SDS 1 year after initiation of the trials. The mean difference of HSDS was 0.68 (95 % CI 0.25–1.11, P = 0.002) at the end of the trial year (Fig. 1), while no statistical difference was noted at the baseline between the two groups at the start of the year. The mean difference in the △HSDS was 0.52 (95 % CI 0.37–0.68, P < 0.00001).
Rejection episodes
Rejection episode rates were not statistically different during the trials between patients receiving growth hormone and those you received no such treatment: 35/205 vs. 19/185 (number of patients with rejection/total number of patients). The risk ratio was 1.56 (95 % CI 0.97–2.53, P = 0.07) using the fixed effect model with good homogeneity among the five studies (Fig. 2).
Renal function
Renal function was measured by determining the GFR. The GFR showed a better improvement in the growth hormone group versus the no-treatment group, although the difference was not statistically significant. The mean difference in the △GFR was 3.27 (95 % CI: −3.54–10.09, P = 0.35) between the two groups (Fig. 3).
Discussion
Growth retardation in children following kidney transplantation had led to many clinical trials on the efficacy of growth hormone use to attain a better or even normal growth velocity in these patients. Among those studies evaluating the effect of growth hormone, we only included the prospective RCTs in our systemic review and meta-analysis. The results of our analysis of these five RCTs provide pooled data on the effect and safety of growth hormone therapy for children receiving a kidney transplant.
The height SDS is used to measure the degree of a child’s height deviated from the standard height according to a specific age group. As such, SDS can be applied to compare growth states among children of different ages and races. Before the intervention, all children of both groups (treatment and no-treatment groups) exhibited a negative height SDS, suggesting a retarded growth state relative to the average normal height. Our meta-analysis revealed that growth hormone therapy is beneficial in terms of improving the growth velocity. Four of the five studies provided the control group with growth hormone after the trial year [11–14]. Growth data on these children revealed a significantly increased growth velocity compared with the previous year (no treatment), although it remained inferior to that of the treatment group which had been started on growth hormone therapy 1 year earlier. This catch-up growth phenomenon shown by the delta height SDS of two groups definitively demonstrates the benefits of growth hormone on height increase of these children [11–15]. Meta-regression analysis did not reveal a dose–growth response relationship, and one possible explanation for this may be the limited number of trials.
The safety of growth hormone therapy was mainly measured by the rejection rate and renal function change. The five studies provided conflicting answers to this question. Experimental findings indicated that growth hormone may interact with the immune system and thus influence the immunological reaction after transplant [16]. Growth hormone is suspected to augment the proliferative and cytotoxic responses to promote the alloantigen reaction during a mixed leukocyte culture. Several clinical trials [1, 17], including one of the RCTs included in our meta-analysis, have also shown that growth hormone might increase the risk of transplant rejection and accelerate the decline of the allograft function. In our meta-analysis of all five RCTs, the rejection rate based on the pooled data was higher in the treatment group (35/205) group than in the control group (19/185). Although this difference is not statistically significant (RR 1.56, 95 % CI: 0.97–2.53), the p value of 0.07, which is close to the limit of significance, indicates that any conclusion should be drawn with caution. The change in the GFR of the patients receiving growth hormone was not inferior to that of the control group during the trial (P = 0.35).
Two of the included trials found that having had more than one rejection episode prior to the growth hormone therapy was a risk factor for allograft rejection after the initiation of this therapy [12, 14]. Different previous rejection rates among patients might be a potential confounding factor in any evaluation of the effects of growth hormone on immune rejection. It is also possible that inappropriate immunosuppressant regimen or poorer HLA match or other factors might explain the higher rates of rejection in these patients—rather than growth hormone itself. Diagnoses of allograft rejection were confirmed by renal biopsy in three of the trials [12, 14, 15] and partially by biopsy in one [11]. One trial did not provide the diagnostic method [13]. In the trial conducted by Fine RN et al. [14] patients underwent systematic renal biopsy; a lower rejection rate was reported. This evidence suggests that different diagnostic methods of rejection might influence the outcome of any evaluation of the effects of GH on renal rejection. Consequently, we suggest that it will be necessary to further evaluate the effects of GH on rejection, especially in the long term.
The pooled data of our analysis seem to support the notion that growth hormone use in post-renal transplant children is safe and does not increase the risk of decline or loss of function of the allografts.
There are a number of limitations to this systemic review. Studies such as unpublished trials, abstracts of conferences or any other works may have been overlooked in our search. The omission of these potentially related studies may have influenced our conclusion based on our meta-analysis. In addition, none of the included RCTs was double-blinded. The dosage of the growth hormone did not differ greatly among trials, yet whether a lower dose of drug can work as effectively and safely as a higher one needs to be explored. All five studies only compared the growth velocity of two groups for 1 year. Consequently, the long-term efficiency and safety of growth hormone use on pediatric renal transplant recipients remain to be evaluated in future high-quality clinical trials. The effect of growth hormone on the final height of children also needs to be determined.
In conclusion, the administration of growth hormone in the post-renal transplant children improves growth. Future research should focus on its safety on allograft rejection and renal function in the long term.
References
McEnery PT, Alexander SR, Sullivan K, Tejani A (1993) Renal transplantation in children and adolescents: the 1992 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 7:711–720
McEnery PT, Stablein DM, Arbus G, Tejani A (1992) Renal transplantation in children. A report of the North American Pediatric Renal Transplant Cooperative Study. N Engl J Med 326:1727–1732
Feld LG, Stablein D, Fivush B, Harmon W, Tejani A (1997) Renal transplantation in children from 1987–1996: the 1996 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 1:146–162
Gulati A, Sarwal MM (2010) Pediatric renal transplantation: an overview and update. Curr Opin Pediatr 22:189–196
Hokken-Koelega AC, van Zaal MA, van Bergen W, de Ridder MA, Stijnen T, Wolff ED, de Jong RC, Donckerwolcke RA, de Muinck Keizer-Schrama SM, Drop SL (1994) Final height and its predictive factors after renal transplantation in childhood. Pediatr Res 36:323–328
Hokken-Koelega AC, Van Zaal MA, de Ridder MA, Wolff ED, De Jong MC, Donckerwolcke RA, De Muinck Keizer-Schrama SM, Drop S (1994) Growth after renal transplantation in prepubertal children: impact of various treatment modalities. Pediatr Res 35:367–371
Messa P, Sindici C, Cannella G, Miotti V, Risaliti A, Gropuzzo M, Di Loreto PL, Bresadola F, Mioni G (1998) Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int 54:1704–1713
Kiepe D, Rüth EM, Blum WF, Mohan S, Weber LT, Tonshoff B (2010) The IGF/IGFBP system in relation to macroscopic bone architecture in pediatric renal transplant patients. Pediatr Nephrol 25:659–667
Ingulli E, Singh A, Moazami S, Tejiani A (1993) Prednisone inhibits the efficacy of recombinant human growth hormone in pediatric renal transplant recipients. Kidney Int 44:65–70
Castañeda DA, López LF, Ovalle DF, Buitrago J, Rodríguez D, Lozano E (2011) Growth, chronic kidney disease and pediatric kidney transplantation: is it useful to use recombinant growth hormone in Colombian children with renal transplant. Transplant Proc 43:3344–3349
Broyer M (1996) Results and side-effects of treating children with growth hormone after kidney transplantation—a preliminary report. Acta Pediatr Suppl 417:76–79
Guest G, Bérard E, Crosnier H, Chevallier T, Rappaport R, Broyer M (1998) Effects of growth hormone in short children after renal transplantation. Pediatr Nephrol 12:437–446
Maxwell H, Rees L (1998) Randomised controlled trial of recombinant human growth hormone in prepubertal and pubertal renal transplant recipients. Arch Dis Child 79:481–487
Fine RN, Stablein D, Cohen AH, Tejani A, Kohaut E (2002) Recombinant human growth hormone post-renal transplantation in children: a randomized controlled study of the NAPRTCS. Kidney Int 62:688–696
Sanchez CP, Kuizon BD, Goodman WG, Gales B, Ettenger RB, Inez Boechat M, Wang Y, Elashoff R, Salusky IB (2002) Growth hormone and the skeleton in pediatric renal allograft recipients. Pediatr Nephrol 17:322–328
Kelley KW (1989) Growth hormone, lymphocytes and macrophages. Biochem Pharmacol 38:705–713
Tyden G, Berg U, Reinholt F (1990) Acute renal graft rejection after treatment with human growth hormone. Lancet 336:1455–1456
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Cheng, W., Yang, Xd. et al. Growth hormone improves growth in pediatric renal transplant recipients—a systemic review and meta-analysis of randomized controlled trials. Pediatr Nephrol 28, 129–133 (2013). https://doi.org/10.1007/s00467-012-2208-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2208-7