Abstract
Advances in genetic mapping and sequencing techniques have led to substantial progress in the study of rare monogenic (Mendelian) forms of abnormal blood pressure. Many disease-defining pathways for hypertension have been identified in the past two decades. Perturbations in renal salt handling appear to be a common mechanism underlying these rare syndromes of hypertension. Excess activation at various points in the mineralocorticoid signaling pathway and malfunctioning of the autonomic (specifically sympathetic) nervous system have both been implicated in inducing hypertension, while complementary studies examining low blood pressure phenotypes have identified novel pathways exclusively linked to renal salt wasting in either the thick ascending limb or the distal nephron. The genetic defects and the physiological and cellular pathways affected in these various disorders are reviewed here. Importantly, studies have suggested that genetic variation affecting these same genes and pathways may play an important role in explaining the variation of blood pressure levels in the general population. The investigation of rare syndromes of human blood pressure variation has important implications for improving the diagnosis and treatment of hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is a substantial public health problem affecting approximately 25 % of the adult population in industrialized societies and over 1 billion people worldwide [1]. It is a major risk factor for many causes of morbidity and mortality in the general population, including stroke, myocardial infarction, congestive heart failure, and end-stage renal disease [2]. Despite the important role of hypertension as a cause of disease, its pathogenesis remains largely unknown. Extensive investigations over the last several decades have revealed that hypertension has a multifactorial etiology, including demographic, dietary, and genetic factors. Known demographic components are numerous and include age, gender, and body mass [3]. Dietary factors include salt, potassium, and calcium intake [4]. The influence of genetic factors on blood pressure (BP) variation is known from twin studies and studies of biological versus adopted siblings. Monozygotic twins show greater concordance of BP variation than dizygotic twins, and biological siblings have similar BP values when compared with adopted siblings [5, 6]. The identification of genes underlying BP variation has the capacity not only to define primary physiologic mechanisms, but also to reveal disease mechanisms and thereby develop novel therapies. Different approaches have been made to study the molecular basis of BP variation, including in vitro and in vivo studies, animal models, and population genetics. The most successful approach has been the investigation of Mendelian (monogenic) forms of arterial hypertension and hypotension in men, where single genes have a large effect on BP [7]. In the last two decades, a number of disease-causing mutations have been identified in various genes, and several disease mechanisms have been described [8]. In the 1990s, the identification of a disease gene for BP variation was mainly achieved by studying large pedigrees of individuals with BP variation in conjunction with advances in genotyping technology and computational (linkage) analysis. Advances in analyzing polymorphic microsatellite markers on a genome-wide level were followed by single nucleotide polymorphism technology in the 2000s. In addition, the candidate gene approach has been successful in conditions that had been previously studied in detail by physiologists. In the last few years, new advances in sequencing (“next generation sequencing”) and computational technology have made it possible to identify additional disease genes in small pedigrees and single individuals with extreme phenotypes [9, 10]. Importantly, genetic studies in the general population have shown that rare allelic variations of Mendelian disease genes affecting BP have implications for the genetic structure of BP variation in the general population. Participants of the Framingham Heart Study (FHS) were screened for allelic variations in rare disease genes affecting renal salt handling. Several allelic gene variants were identified and proven or inferred to be functional in lowering BP [11]. In the following report, several rare conditions eliciting high and low BP will be reviewed and compared.
Genes that increase BP
Pathways of enhanced salt reabsorption in the collecting duct
Glucocorticoid-remediable aldosteronism (familial hyperaldosteronism type I)
The BP in patients with glucocorticoid-remediable aldosteronism (GRA) is salt sensitive and increases with salt intake. Laboratory tests show that these patients have mild hypokalemia and metabolic alkalosis. Renin levels are typically low, while aldosterone values can be elevated (Table 1). Patients with this condition are often suspected of having primary hyperaldosteronism. However, computed tomography (CT) scanning of the adrenal glands is negative for (unilateral) adrenal adenomas. A family history of hypertension is often positive, suggesting autosomal dominant inheritance. The BP in GRA improves with all diuretics, including blockers of the epithelial sodium channel (ENaC) in the distal nephron. A distinguishing biochemical feature of GRA is the presence of steroid metabolites not normally found in urine. Urine testing in affected individuals is positive for 18-hydroxycortisol and 18-oxocortisol. Recognition of these abnormal products in the urine helped solve the etiology of this condition. Linkage analysis of a large kindred with GRA localized the responsible gene(s) to chromosome 8q21. The enzyme 11-β-hydroxylase (CYP11B1), expressed in the zona fasciculata of the adrenal gland, resides within this locus. CYP11B1 is responsive to adrenocorticotropic hormone and involved in the terminal step of glucocorticoid biosynthesis. The gene for aldosterone synthase (CYPB11B2) resides at the same locus on chromosome 8q21, and this gene has a highly similar DNA sequence (approx. 95 %) to CYB11B1 and encodes an enzyme involved in mineralocorticoid synthesis. In affected individuals, a chimeric gene is formed by an unequal crossing-over at chromosomal location 8q21, consisting of the promoter (regulatory) region of the 11-β-hydroxylase gene and the structural portion of the aldosterone synthase gene. The protein of this chimeric gene performs all of the same actions as aldosterone; however, protein expression is regulated by the adrenocorticotropic hormone (ACTH) and not by angiotensin 2 (Fig. 1). Metabolism of this chimeric gene product results in the unusual urine metabolites mentioned above. Steroid treatment with prednisone ameliorates hypertension in GRA by suppressing the adrenal zona fasciculata, giving this condition its name [12, 13].
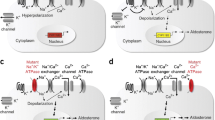
Mutations in genes expressed in distal nephron epithelia cause salt retention and blood pressure (BP) elevation. Schematic illustration of ten molecular mechanisms (red) leading to increased salt reabsorption in distal nephron epithelia. Red double lines indicate loss-of-function effect on downstream target(s). WNK1 gain-of-function mutations lead to increased suppression of WNK4, which has dual function at baseline (NCCT activation and ROMK suppression). Aldo Aldosterone, cAldo chimeric aldosterone, MR mineralocorticoid receptor, APA aldosterone-producing adrenal adenoma, CAH congenital adrenal hyperplasia, ACTH adrenocorticotropic hormone, DOC deoxycorticosterone, Cul3 Cullin 3, KLHL3 Kelch-like 3, 11-β-HSD 11-β hydroxysteroid dehydrogenase, NCCT Na+–Cl− cotransporter, ROMK renal outer medullary K+ channel
Apparent mineralocorticoid excess
The biochemical presentation of apparent mineralocorticoid excess (AME) is similar to that of GRA (Table 1). In contrast to GRA, urine analysis is negative for abnormal steroid metabolites, but the urinary free cortisol to cortisone ratio is increased (ratio > 0.5) despite normal cortisol levels. This finding helped to identify the disease gene for AME, which is inherited in an autosomal recessive fashion. Glycyrrhetinic acid was found to inhibit the enzyme 11-β-hydroxysteroid-dehydrogenase (11-β-HSD), which converts cortisol to cortisone in renal tubular epithelial cells. In the distal renal tubule, the kidney-specific isoform of 11-β-HSD “protects” the mineralocorticoid receptor (MR) from cortisol, which has the same affinity for the MR as aldosterone. Candidate gene analysis showed that individuals with AME have loss-of-function mutations in a renal isoform of 11-β-HSD (11-β-HSD2), rendering the product incapable of converting cortisol to cortisone (Fig. 1). The hypertension in this condition responds to both spironolactone and ENaC blockers. Individuals ingesting large amounts of licorice, which contains glycyrrhetinic acid, and individuals treated with carbenoxolone, which also contains glycyrrhetinic acid and is licensed in the UK for treatment of mucosal ulcerations, develop features of AME [14, 15].
Liddle’s syndrome
In 1963, Grant W. Liddle described patients with an autosomal dominant form of hypertension associated with hypokalemia, metabolic alkalosis, and low aldosterone levels [16] (Table 1). Liddle speculated that his patients had a distal tubular defect of enhanced sodium reabsorption. Renal transplantation in Liddle’s patients who developed renal failure “cured” their hypertension, suggesting that the etiology of this condition resided within the kidney. The hypertension in affected individuals did not respond to spironolactone treatment. However, affected individuals showed significant improvement with blockers of ENaC [17]. Candidate gene analysis of ENaC identified gain-of-function mutations in two out of three subunits as the responsible cause for Liddle’s syndrome. Missense mutations and deletions in the cytoplasmic tails of the β- or γ-subunits of ENaC, which share approximately 35 % identity in their amino acid sequences, lead to impaired deactivation of the channel in the distal nephron [18, 19]. Disease-causing mutations typically occur in the proline-rich PY motif (PPPXY) of the cytoplasmic tails of these subunits. This domain interacts with WW-domains of proteins that are known for their ubiquitination and degradation of cell surface proteins. The PY motif of the cytoplasmic tails is also important for endocytosis via clathrin-coated pits [20]. As a consequence, internalization of the ENaC channels is impaired, and the ENaC channels remain active on the apical cell surface (Fig. 1). This mechanism explains the superb efficacy of ENaC blockers, such as amiloride and triameterene, in the treatment of this disease.
Syndrome of hypertension exacerbated in pregnancy
Candidate gene screening of the MR gene (NR3C2) in patients with features resembling Liddle’s syndrome, who tested negative for ENaC gene mutations, led to the identification of one family with activating mutations in NR3C2 [21]. One index case was found to have a heterozygous mutation at codon 810 of the MR that resulted in a leucine (L) substitution for serine (S). All four affected family members carried the same MR-S810L mutation, whereas unaffected members were negative. The mode of transmission was autosomal dominant (Table 1). Interestingly, affected women in this family exhibited a worsening of hypertension in pregnancy (HiP), suggesting that progesterone acted as an agonist of the mutated MR-S810L. Structural protein analysis revealed that the mutated MR gene allowed for MR activation by steroids lacking the 21-hydroxyl group, which under normal conditions is not possible. The leucine residue at position 810 lies within helix 5 of the ligand-binding domain of the MR and creates a novel interaction with alanine at a position of MR helix 3. This modification explains why, in in vitro studies, compounds that are normally antagonists, such as spironolactone, act as agonists in MR-S810L [21].
Aldosterone-producing adrenal adenomas (familial hyperaldosteronism type III)
At least 5 % of patients referred for evaluation of primary hypertension have aldosterone-producing adrenal adenomas (APA, or unilateral adrenal hyperplasia) [22]. As in GRA and AME, patients with APA show findings consistent with excessive aldosterone secretion (Table 1). Family history is often negative. Patients are identified due to hypokalemia and frequently feature a characteristic adrenal mass on CT scans. Adrenal vein sampling demonstrates predominant aldosterone secretion from the gland harboring the tumor, which is crucial for the correct diagnosis as it allows APA to be distinguished from idiopathic hyperaldosteronism (familial hyperaldosteronism type II). Surgical removal of the affected adrenal gland ameliorates hypertension in most patients [22]. Lifton and colleagues performed exome sequencing of 22 adrenal adenoma tissues and identified that approximately one-third of all adenomas harbored novel somatic mutations at highly conserved residues (G151R and L168R) of the inwardly rectifying potassium channel KCNJ5 (Kir3.4) [23]. KCNJ5 was further implicated as a cause of a Mendelian form of primary aldosteronism through the identification of a family (father and both daughters) with familial adrenal adenomas and severe hypertension [24]. Mutational analysis in this family revealed a unique germline mutation within a highly conserved residue of KCNJ5 (T158A). Structural proteomics and in vitro experiments suggest that these rare KCNJ5 mutations alter Kir3.4 channel function and lead to chronic depolarization of adrenal zona glomerulosa cells, thereby causing constitutive aldosterone production as well as adrenal cell proliferation [25] (Fig. 1).
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) refers to several autosomal recessive diseases resulting from mutations in genes encoding enzymes mediating biochemical steroidogenesis in the adrenal gland. In CAH, the adrenal glands secrete excessive or deficient amounts of sex hormones and mineralocorticoids during prenatal development [26]. Poor cortisol production is a hallmark of these conditions. CAH is often classified into the common classical (“salt-wasting” and “simple virilizing” mostly due to 21-α-hydroxylase deficiency) and the rare non-classical forms (<5–10 %), which can be associated with hypertension due to elevated adrenocorticotropic hormone (ACTH) levels. Loss-of-function mutations in CYP11B1 (11-β-hydroxylase) and CYP17A1 (cytochrome P450 17A1, also known as 17α-hydroxylase) are both causes of rare forms of CAH [26]. Both cortisol and sex steroids are decreased in CAH, leading to increased mineralocorticoid precursor production. The aldosterone precursors 11-deoxy corticosterone (DOC) and corticosterone are elevated, activating the MR (Fig. 1). Patients typically develop hypertension in childhood due to volume expansion and feature hypokalemia and metabolic alkalosis (Table 1). Treatment with glucocorticoids suppresses ACTH, thereby returning mineralocorticoid precursor production back toward normal and lowering the BP [27]. Female virilization (11-β-hydroxylase deficiency) and ambiguous genitalia in genetic males or failure of the ovaries to function at puberty in genetic females (17-α-hydroxylase deficiency) are other features of CAH.
Pathway of enhanced salt reabsorption in the distal convoluted tubule associated with impaired potassium secretion
Pseudohypoaldosteronism type 2 (PHA II, also known as Gordon’s syndrome or familial hyperkalemia and hypertension) is a unique form of hypertension associated with hyperkalemia and metabolic acidosis transmitted in an autosomal dominant fashion [28] (Table 1). Hypercalciuria has been reported in some cases, making this syndrome a near mirror image of Gitelman’s syndrome, which is described further below [29]. Renin activity in PHA II is typically suppressed, and aldosterone levels can be normal or slightly elevated. Thiazide diuretics represent a highly effective treatment for this syndrome and also commensurate with salt-sensitivity. The hypertension is chloride dependent because the exchange of sodium bicarbonate or citrate infusions for sodium chloride infusion was found to ameliorate BP elevation [30]. In recent years, several genes have been identified for the etiology of PHA II. Intronic deletions in the kinase WNK1 and missense mutations in the kinase WNK4 have been identified in large pedigrees of this condition by linkage analysis [31]. Both kinases belong to a novel kinase family that is lacking lysine (K) at a typical location, giving them their name (“With No K”). Both of these kinases are expressed in the distal nephron and have been implicated in the regulation of several transporters and channels since their discovery. Dominant gain-of-function mutations in WNK1 and loss-of-function mutations in WNK4 lead to increased salt reabsorption in the distal nephron by activating the Na+–Cl− cotransporter (NCCT) regardless of volume status, resulting in salt-sensitive hypertension and inhibition of K+ excretion despite marked hyperkalemia. The role of NCCT activation in the pathophysiology of PHA II explains why this condition is so susceptible to treatment with thiazide diuretics. At baseline, WNK1 functions as a suppressor of WNK4 by associating with WNK4 in a protein complex involving the kinase domains. WNK4 has also been found to regulate the renal outer medullary K+ channel (ROMK) in the distal convoluted tubule (DCT) [32]. Recently, two more gene defects for PHA II have been identified by exome sequencing [33]. The majority of patients with PHA II in this study (87 %) were negative for WNK mutations and 52 such kindreds were included. Many of these did not display the usual autosomal-dominant inheritance; rather, a recessive model was suggested. Novel, protein-altering allelic variants were identified primarily in two genes. Twenty-four PHA II index cases revealed novel mutations in the gene KLHL3 (Kelch-like 3) that were predominantly at positions conserved among orthologs. Among the remaining index cases of PHA II without mutations in WNK1, WNK4, or KLHL3, 17 were identified with novel allelic variants in the gene CUL3 (Cullin 3). Eight of these mutations were de novo and not present in parents. The molecular mechanism of KLHL3 and CUL3 in causing PHA II remains unclear; however, both proteins are expressed in the DCT and co-localize with WNK1, WNK4, and NCCT. Impaired ubiquitination of NCCT from the luminal cell surface in the DCT has been speculated as a mechanism for PHA II development in patients with KLHL3 and CUL3 mutations [33] (Fig. 1). The phenotype of patients with different gene defects in PHA II differs. Patients with CUL3 appear more severely affected as they develop PHA II at younger age and present with more severe hyperkalemia and also a failure to thrive. However, thiazide diuretics are the treatment of choice in all forms of PHA II due to their inhibition of NCCT [29] (Table 1).
Pathways affecting the autonomic (sympathetic) regulation of BP
Hypertension with brachydactyly (Bilginturan’s syndrome)
Autosomal dominant hypertension with brachydactyly (HBS) was first described in 1973 [34]. The affected family members were short in stature, developed hypertension in childhood, and died typically of stroke before the age of 50. The clinical findings of short metacarpal bones (= brachydactyly type E), cone-shaped epiphysis, and short end-phalanx of the thumb (= brachydactyly type B) are 100 % concordant with the elevated BP values in identified families [35]. Contrary to the previously described syndromes, HBS does not feature any associated biochemical abnormalities, and BP levels do not appear to be salt-sensitive (Table 1). Evaluation of the renin–angiotensin–aldosterone axis, as well as catecholamines, has revealed no abnormalities [36]. Diuretics do not play a significant role in the treatment of this condition, and patients typically require multiple anti-hypertensive drugs [37]. Autonomic nervous system testing has revealed an abnormal baroreceptor reflex response, resulting in an excessive increase of BP with sympathetic stimuli [38]. The BP of affected individuals at baseline is much more sensitive to the alpha-agonist phenylephrine than that of controls. This difference is diminished when the baroreceptor reflex mechanism is blocked with the ganglion blocker trimethaphan. All tested affected patients (n = 15) in the originally described family feature neurovascular anomalies in the area of the left ventrolateral medulla oblongata in MRI studies, whereas these anomalies are absent in unaffected family members (n = 12) [39]. It is unknown if neurovascular arterial compression of the brainstem in this location is responsible for the abnormal baroreceptor function and hypertension in this syndrome. The gene(s) for this condition was (were) located on chromosome 12p [35]. A complex rearrangement of the HBS locus has been identified, and several promising candidate genes have been screened, but no definitive underlying cause has been as yet identified. It has been speculated that the rearrangement at this locus could affect microRNA expression and cause translational repression of gene transcripts or gene silencing [40].
Hereditary familial pheochromocytoma
Pheochromocytoma is caused by catecholamine-producing adrenal tumors and is associated with various symptoms depending on the type and secretory pattern of the produced catecholamine(s). Hypertension can present as paroxysmal, labile hypertension, complicated by orthostatic hypotension, as well as persistent hypertension. Hypokalemia can often be found, and renin and aldosterone levels can be elevated due to decreased intravascular volume [41]. The frequency of hereditary familial forms of pheochromocytoma has been reported to be approximately 25 %. The majority of these are associated with the type II multiple endocrine neoplasia syndrome (MEN II) and caused by gain-of-function mutations in the RET proto-oncogene [42]. In addition to pheochromocytoma, MEN II features medullary thyroid cancer (types IIA and IIB), hyperparathyroidism (type IIA), and mucosal neuromas (type IIB). Including RET, more than ten gene defects have been associated with pheochromocytoma. Other examples are neurofibromatosis type 1 (NF1), Von Hippel–Lindau disease (VHL), and familial extra-adrenal paragangliomas (SDHB, SDHC, SDHD) [42]. The genes encoding for the succinate dehydrogenase subunits B (SDHB), C (SDHB), and D (SDHD) are three of four proteins forming the succinate dehydrogenase protein complex, which participates in the Krebs cycle and in mitochondrial electron chain transport. The treatment of choice is surgical resection of the affected adrenal gland(s) or paraganglioma, respectively. Treatment with irreversible alpha-blockade prior to surgery is mandatory to prevent hypertensive complications [41].
Pathway with unknown mechanism: mitochondrial gene mutation in tRNA-Ile resembling metabolic syndrome
Richard Lifton and colleagues described a familial form of hypertension, hypomagnesemia, and hyperlipidemia along the maternal lineage of a large family, indicating mitochondrial inheritance of this syndrome [43]. Sequencing of the mitochondrial genome of the maternal lineage identified a homoplasmic mutation substituting cytidine for uridine immediately 5′ to the mitochondrial tRNA anti-codon for isoleucin (Ile). In silico analysis showed that uridine at this position is almost invariant among tRNAs, stabilizing the tRNA anticodon loop. Hypertension, hypomagnesemia, and hypercholesterolemia each showed 50 % penetrance among adults on the maternal lineage. The prevalence of hypertension on the maternal lineage showed marked age dependence, increasing from 5 % in subjects under 30 years of age to 95 % in those over 50 years of age . The mechanism of BP elevation in this syndrome is as yet unexplained. In vivo nuclear magnetic resonance spectroscopy of skeletal muscle in one affected patient showed decreased ATP production (in the setting of normal Krebs cycle function) [43]. Given the known loss of mitochondrial function with aging due to increased mitochondrial mutations, increasing BP could be secondary to the loss of ATP production, which has been associated with hypertension in the animal model [43]. Another possibility is the increased presence of reactive oxygen species secondary to mitochondrial dysfunction that has been associated with hypertension as well [44]. Epidemiological studies have shown that children of hypertensive mothers are more likely to develop hypertension, also suggesting that the mitochondrial genome could be associated with inheriting hypertension [45, 46].
Genes that decrease BP
Pathway of renal salt wasting in the thick ascending limb: Bartter’s syndrome
Barrter’s syndrome is a rare defect of the thick ascending limb (TAL) of the loop of Henle [47]. All patients with this condition feature varying degrees of hypokalemic metabolic alkalosis and low-to-normal BP with elevated renin levels (Table 2). Some patients also have hypercalciuria (Bartter’s types 1, 2 and 5). Most of these findings are identical to those of patients who are on loop diuretics. To date, five different disease genes have been identified for this syndrome, all encoding for proteins facilitating salt reabsorption in the TAL (Fig. 2). Bartter’s syndrome is classified into five different genetic subtypes, which differ in disease severity. The transmission of Bartter’s syndrome is typically autosomal recessive except for type 5, which is transmitted in an autosomal-dominant fashion. Neonatal Bartter’s syndrome is the most common form (approx. 90 % of all patients) and is typically noticed during pregnancy due to polyhydramnios (excess amniotic fluid). Neonatal infants feature severe polyuria and polydipsia. Life-threatening volume contraction may result if the infant does not receive adequate fluids. The majority of infants are hypercalciuric and will develop nephrocalcinosis, which can progress to renal failure. Failure to thrive is a typical occurrence in children with neonatal Bartter’s syndrome, which is caused by loss-of-function mutations in the Na+–K+–2Cl− cotransporter (NKCC2, Bartter’s type 1) and the renal outer medullary K+ channel (ROMK, Bartter’s type 2), both of which are expressed at the apical membrane of TAL epithelia [48, 49]. In comparison, the classic Bartter’s syndrome (type 3) is caused by loss-of-function mutations in the basolateral Cl− channel Kb (CLCNKB) and is usually diagnosed at school age or later, although symptoms of renal salt wasting may occur earlier in life [50]. In classic Bartter’s syndrome, increased urinary calcium excretion is significantly milder, and kidney stones can develop later in life, if at all. Renal function is typically normal; however, progression to end-stage renal disease has been described. Since CLCNKB is also expressed in the DCT, type III Bartter’s syndrome is classified by some authors as a mixed disorder of the TAL and DCT or as a disorder of the thiazide–furosemide pharmacotype [51]. Mild hypomagnesemia can be present in classic Bartter’s syndrome. Type 4 Bartter’s syndrome is caused by mutations in Barttin (BSND), which is an accessory β-subunit of the CLCNKB [52]. Since Barttin is also expressed in the inner ear (where it interacts with the Cl− channel CLCNKA), patients with Bartter’s type 4 also suffer from sensorineural deafness. Gain-of-function mutations in the calcium-sensing receptor gene (CASR) feature renal salt wasting and hypercalciuria [53]. Although parathyroid hormone (PTH) levels are severely suppressed in this syndrome, which is also known as autosomal-dominant hypocalcemia (ADH), this condition is classified by some as Bartter’s type 5 due to the expression of the CASR on the basolateral membrane of TAL epithelia. However, rather than being considered a variant of Bartter’s syndrome with specific dysfunction of TAL epithelia, this condition could also be interpreted as a phenocopy of Bartter’s syndrome.
Mutations in genes expressed in thick ascending limb (TAL) epithelia cause Bartter’s syndrome. Schematic illustration of five molecular mechanisms (blue) leading to renal salt wasting in epithelia of the TAL. CASR Calcium-sensing receptor, NKCC2 Na+–K+-2Cl− cotransporter, CLCNKB Cl− channel Kb. For other abbreviations, see caption to Fig. 1
Pathways of renal salt wasting in the distal nephron
Gitelman’s syndrome
Patients with Gitelman’s syndrome present with symptoms identical to those who are on thiazide diuretics. Lifton and colleagues performed linkage analysis in several unrelated families with Gitelman's syndrome and identified the locus for the thiazide-sensitive NCCT gene (SLC12A3). Several homozygous or compound heterozygous loss-of-function mutations in SLC12A3 were identified in their study [54], which inactivate NCCT expressed in the apical membrane of DCT epithelia (Fig. 3). The clinical symptoms are a mirror image of those of PHA II, with the exception of hypomagnesemia, and include hypochloremic metabolic alkalosis, hypokalemia, and hypocalciuria (Table 2). Affected individuals are typically asymptomatic; however, muscular cramps, weakness/fatigue, and irritability have been described. More severe symptoms, such as tetany and paralysis, are rare. Individuals with heterozygous loss-of-function mutations in NCCT may have a survival benefit due to a lower BP and increased bone mineral density [11, 55].
Mutations in genes expressed in distal nephron epithelia cause salt wasting, electrolyte abnormalities, and low blood pressure. Schematic illustration of four molecular mechanisms (blue) leading to renal salt wasting in distal nephron epithelia. ENaC Epithelial Na+ channel, Kir4.1 K+ channel, inwardly rectifying, subfamily J, member 10. For other abbreviations, see captions to Figs. 1 and 2
Pseudohypoaldosteronism type 1
Pseudohypoaldosteronism type 1 (PHA I) is characterized by salt wasting resulting from renal unresponsiveness to mineralocorticoids [56, 57]. Patients may present with neonatal renal salt wasting with hyperkalemic acidosis despite high aldosterone levels (Table 2). Two genetic subtypes can be distinguished; type I A, which is inherited in an autosomal dominant fashion, and type I B, which is transmitted in an autosomal recessive pattern. PHA I A is caused by loss-of-function mutations in the MR gene and is typically milder than PHA I B [56]. It could be considered as a mirror image of the syndrome of hypertension in pregnancy. Patients improve with age and usually become asymptomatic without treatment when they reach adulthood. Some adult patients are found to have elevated aldosterone levels, however, they lack a history of the disease. This observation suggested that only those infants whose salt homeostasis is “stressed” by intercurrent illness and volume depletion develop clinically recognized PHA I. The recessive form, PHA 1B, is caused by loss-of-function mutations in any one of the three genes encoding the α-, β- or γ-subunits of ENaC, leading to decreased channel activity and renal salt wasting (Fig. 3) [57]. PHA 1B is a mirror image of Liddle’s syndrome. Patients with this form can feature a severe systemic disorder starting in infancy and persisting into adulthood.
SeSAME/EAST syndrome
The SeSAME (seizures, sensorineural hearing loss, ataxia, mental retardation, and electrolyte imbalance) or EAST (epilepsy, ataxia, sensorineural deafness, and tubulopathy) syndrome features renal salt wasting and electrolyte imbalance, and its study has added considerable new insight into renal electrolyte homeostasis in the distal nephron. This syndrome is accompanied by several additional findings giving this condition its names. Lifton and colleagues named it the SeSAME syndrome in order to describe the presence of seizures, sensorineural hearing loss, ataxia, mental retardation and electrolyte imbalance [58]. Bockenhauer and colleagues named it the EAST syndrome for its association with apparent epilepsy, ataxia, sensorineural deafness, and renal tubulopathy [59]. The mode of inheritance is autosomal recessive, and consanguinity has been described in some families. The responsible gene, KCNJ10, was identified by linkage analysis and encodes for the K+-channel Kir4.1, which is expressed in the basolateral membranes of the DCT, connecting tubule (CNT), and CD epithelia. The identified electrolyte and acid–base abnormalities are similar to those seen in Gitelman’s syndrome and include hypokalemia, hypomagnesemia, and metabolic alkalosis (Table 2). Renin and aldosterone levels are elevated. Patients typically have normal BP values but still crave salt, suggesting that they compensate for renal salt losses with an increased consumption of salt to maintain normal BP values [59]. In vitro studies suggest that loss-of-function mutations in KCNJ10 impair the activity of the Na+–K+ ATPase, which is also located at the basolateral membrane of epithelia of the same nephron segments. Loss of Kir4.1 function probably impairs K+ cycling at the basolateral membrane and thereby inhibits the Na+–K+ ATPase function and Na+ reabsorption [58]. The additional features seen in this syndrome are due to the expression of Kir4.1 in neuronal tissue and in cells of the inner ear. KCNJ10-deficient mice exhibit a striking pathology of the entire central nervous system and display renal salt wasting and volume contraction [60].
Severe hypotension due to renal tubular dysgenesis
Autosomal recessive renal tubular dysgenesis (RTD) is a severe developmental disorder of abnormal renal tubular formation characterized by persistent fetal oligoanuria frequently associated with in utero or perinatal death [61]. Parental consanguinity is present in approximately one-third of all reported families [62]. Surviving newborn infants display severe and refractory hypotension that requires vasopressor treatment, respiratory assistance, and dialysis after birth. Death often occurs due to pulmonary hypoplasia and respiratory failure from early-onset oligohydramnios (Potter sequence). Gubler et al. reported that to date only four patients have survived after days or weeks of intensive care [62]. The absence or paucity of differentiated proximal tubules is the histopathologic hallmark of this disorder, which is often associated with postnatal skull ossification defects (hypocalvaria). In RTD, all tubules appear to be abnormally developed, primitive, and reminiscent of collecting tubules. RTD can also be found in children of women using angiotensin-converting-enzyme inhibitors (ACEi) during pregnancy [63]. Hypocalvaria is also present in this acquired (secondary) form of RTD, also known as ACEi fetopathy. The genetic forms of RTD are caused by loss-of-function mutations in four genes encoding for proteins of the renin–angiotensin system. These genes are shown in Table 2 and include REN (renin), AGT (angiotensinogen), ACE, and AGT1R (angiotensin II receptor type 1). In one study involving 160 cases, no correlation could be established between the clinical course of the disease and the type of mutation[62].
Molecular basis of essential hypertension
The genetic causes of (essential) hypertension in the general population remain unknown, probably due to the polygenic nature of BP homeostasis involving many different systems, including vasculature, the central and autonomic nervous system, the kidney, and various different hormonal pathways [64]. It is probable that multiple genes with small effects determine overall BP levels by either lowering or increasing BP. In the last two decades, several DNA sequence-based strategies of gene identification were applied to identify genes for essential hypertension, including hypothesis-based (candidate gene analysis, candidate gene association studies) and hypothesis-free [linkage analysis, genome-wide association studies (GWAS)] strategies. Most gene loci for essential hypertension have been identified by GWAS, which require a large sample size and population stratification to succeed. Additional limitations of GWAS are the frequent inability to replicate findings in independent data sets and the lack of causal (functional) allelic gene variations at the identified loci.
Four large GWAS for essential hypertension with 29,000–200,000 participants identified 29 gene loci determining systolic and diastolic BP as quantitative traits [65–68] (Table 3). Most of the identified loci have no obvious connections to known pathways affecting BP and contribute only minimally to phenotypic variation, explaining <1 % of systolic and diastolic BP variance in these cohorts, despite an estimated BP heritability of 30–60 % [5, 6, 64]. Two of the BP loci, 1p36 and 12q24, were identified in all four GWAS despite the varying ancestry of the study populations. Interestingly, genes located at 1p36 include MTHFR (methylene-tetrahydrofolate reductase) and NPPB (brain natriuretic peptide). MTHFR has been associated with pre-ecclampsia [69], and deletions in NPPB have been associated with salt-sensitive hypertension in the mouse [70]. Another interesting candidate gene is SLC4A7 (Na+–HCO3− cotransporter 3) on 3p23. SLC4A7-deficient mice develop arterial hypertension, probably by inhibition of NO-mediated vasorelaxation [71]. CYP17A1 on 10q24 is the only locus previously identified in a rare syndrome of BP variation (loss-of-function mutations cause CAH). Similar to the genes responsible for rare forms of BP variation, most of the genes found at the “GWAS loci” for essential hypertension are expressed in the kidney, underscoring the importance of the kidney in BP regulation (Table 3).
One (candidate gene-based) study showing that rare functional variants do determine BP in the general population was conducted in approximately 5,000 participants of the FHS population [11]. Several rare, heterozygous mutations in SLC12A3 (NCCT), SLC12A1 (NKCC2), and KCNJ1 (ROMK) were identified, and a causal reduction of BP was postulated based on data obtained from comparative genomics, genetics, and biochemistry. In the heterozygous state, 30 mutations in these three genes were associated with protection from BP changes. This study underlined the contribution of genes altering renal salt handling on BP homeostasis and showed the importance of studying rare forms of hypertension with extreme phenotypes to unravel the genetics of hypertension. The identification of the ROMK pathway as a potentially important player in BP regulation in the general population was particularly interesting since it could motivate the investigation of a new antihypertensive diuretic agent that might not produce the hypokalemia seen with the known loop and thiazide diuretics.
Conclusion
Guyton and colleagues postulated over 3 decades ago that the kidney plays a central role in BP regulation by managing urinary sodium excretion (“pressure natriuresis”) [72]. Although this hypothesis has been debated over several decades, there is overwhelming clinical evidence that sodium intake is associated with hypertension and that diuretic therapy is beneficial. The vast majority of genes identified in rare forms of hypertension are expressed in the kidney, which supports Guyton et al.’s hypothesis that renal salt handling is the final determinant of (abnormal) BP homeostasis [7, 8]. These syndromes have led us to understand the primary physiology of BP regulation and taught us about disease mechanisms, which can lead to arterial hypertension, hypotension, and abnormal electrolyte and acid–base homeostasis. Some of the genes discussed in this review have been tested in the FHS population and have been shown to have an effect on BP variation in the general population [11]. Based on the FHS data, it is probable that the combined effects of rare independent mutations in genes described in this review may account for a substantial fraction of BP variation in the general population. Therefore, continuing to study these rare conditions is of great importance, and further knowledge will enable us to understand, prevent, and treat hypertension better than we do at present and lay the foundation for future individualized medical care based on genetic predisposition.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365:217–223
Mosterd A, D'Agostino RB, Silbershatz H, Sytkowski PA, Kannel WB, Grobbee DE, Levy D (1999) Trends in the prevalence of hypertension, antihypertensive therapy, and left ventricular hypertrophy from 1950 to 1989. N Engl J Med 340:1221–1227
Stanton JL, Braitman LE, Riley AM Jr, Khoo CS, Smith JL (1982) Demographic, dietary, life style, and anthropometric correlates of blood pressure. Hypertension 4(III):135–142
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N (1997) A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336:1117–1124
Feinleib M, Garrison RJ, Fabsitz R, Christian JC, Hrubec Z, Borhani NO, Kannel WB, Rosenman R, Schwartz JT, Wagner JO (1977) The NHLBI twin study of cardiovascular disease risk factors: methodology and summary of results. Am J Epidemiol 106:284–285
Rice T, Vogler GP, Perusse L, Bouchard C, Rao DC (1989) Cardiovascular risk factors in a French Canadian population: resolution of genetic and familial environmental effects on blood pressure using twins, adoptees, and extensive information on environmental correlates. Genet Epidemiol 6:571–588
Lifton RP, Gharavi AG, Geller DS (2001) Molecular mechanisms of human hypertension. Cell 104:545–556
Toka HR, Luft FC (2002) Monogenic forms of human hypertension. Semin Nephrol 22:81–88
Bockenhauer D, Medlar AJ, Ashton E, Kleta R, Lench N (2012) Genetic testing in renal disease. Pediatr Nephrol 27:873–883
Bailey-Wilson JE, Wilson AF (2011) Linkage analysis in the next-generation sequencing era. Hum Hered 72:228–236
Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP (2008) Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40:592–599
Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM (1992) A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355:262–265
Lifton RP, Dluhy RG, Powers M, Rich GM, Gutkin M, Fallo F, Gill JR Jr, Feld L, Ganguly A, Laidlaw JC, Murnaghan DJ, Kaufman C, Stockigt JR, Ulick S, Lalouel JM (1992) Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet 2:66–74
Mune T, Rogerson FM, Nikkila H, Agarwal AK, White PC (1995) Human hypertension caused by mutations in the kidney isozyme of 11 beta-hydroxysteroid dehydrogenase. Nat Genet 10:394–399
White PC, Mune T, Agarwal AK (1997) 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 18:135–156
Liddle GW, Bledsoe T, Coppage WS Jr (1963) A familial renal disorder stimulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians 199–213
Botero-Velez M, Curtis JJ, Warnock DG (1994) Brief report: Liddle's syndrome revisited–a disorder of sodium reabsorption in the distal tubule. N Engl J Med 330:178–181
Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP (1994) Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79:407–414
Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP (1995) Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 11:76–82
Rotin D, Kanelis V, Schild L (2001) Trafficking and cell surface stability of ENaC. Am J Physiol Renal Physiol 281:F391–F399
Geller DS, Farhi A, Pinkerton N, Fradley M, Moritz M, Spitzer A, Meinke G, Tsai FT, Sigler PB, Lifton RP (2000) Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science 289:119–123
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F, PAPY Study Investigators (2006) A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 48:2293–2300
Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP (2011) K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331:768–772
Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP (2008) A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 93:3117–3123
Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, Wang WH, Lifton RP (2012) Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA 109:2533–2538
Speiser PW, White PC (2003) Congenital adrenal hyperplasia. N Engl J Med 349:776–788
Speiser PW (2011) Medical treatment of classic and nonclassic congenital adrenal hyperplasia. Adv Exp Med Biol 707:41–45
Gordon RD, Geddes RA, Pawsey CG, O'Halloran MW (1970) Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Austr Ann Med 19:287–294
Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z (2002) Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87:3248–3254
Schambelan M, Sebastian A, Rector FC Jr (1981) Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int 19:716–727
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293:1107–1112
Kahle KT, Wilson FH, Lalioti M, Toka H, Qin H, Lifton RP (2004) WNK kinases: molecular regulators of integrated epithelial ion transport. Curr Opin Nephrol Hypertens 13:557–562
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Välimäki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102
Bilginturan N, Zileli S, Karacadag S, Pirnar T (1973) Hereditary brachydactyly associated with hypertension. J Med Genet 10:253–259
Toka HR, Bahring S, Chitayat D, Melby JC, Whitehead R, Jeschke E, Wienker TF, Toka O, Schuster H, Luft FC (1998) Families with autosomal dominant brachydactyly type E, short stature, and severe hypertension. Ann Intern Med 129:204–208
Schuster H, Wienker TF, Toka HR, Bähring S, Jeschke E, Toka O, Busjahn A, Hempel A, Tahlhammer C, Oelkers W, Kunze J, Bilginturan N, Haller H, Luft FC (1996) Autosomal dominant hypertension and brachydactyly in a Turkish kindred resembles essential hypertension. Hypertension 28:1085–1092
Schuster H, Toka O, Toka HR, Busjahn A, Oztekin O, Wienker TF, Bilginturan N, Bähring S, Skrabal F, Haller H, Luft FC (1998) A cross-over medication trial for patients with autosomal-dominant hypertension with brachydactyly. Kidney Int 53:167–172
Jordan J, Toka HR, Heusser K, Toka O, Shannon JR, Tank J, Diedrich A, Stabroth C, Stoffels M, Naraghi R, Oelkers W, Schuster H, Schobel HP, Haller H, Luft FC (2000) Severely impaired baroreflex-buffering in patients with monogenic hypertension and neurovascular contact. Circulation 102:2611–2618
Naraghi R, Schuster H, Toka HR, Bähring S, Toka O, Oztekin O, Bilginturan N, Knoblauch H, Wienker TF, Busjahn A, Haller H, Fahlbusch R, Luft FC (1997) Neurovascular compression at the ventrolateral medulla in autosomal dominant hypertension and brachydactyly. Stroke 28:1749–1754
Bahring S, Kann M, Neuenfeld Y, Gong M, Chitayat D, Toka HR, Toka O, Plessis G, Maass P, Rauch A, Aydin A, Luft FC (2008) Inversion region for hypertension and brachydactyly on chromosome 12p features multiple splicing and noncoding RNA. Hypertension 51:426–431
Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS (2001) Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med 134:315–329
Tischler AS (2006) Molecular and cellular biology of pheochromocytomas and extra-adrenal paragangliomas. Endocr Pathol 17:321–328
Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, Scheinman SJ, Lifton RP (2004) A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 306:1190–1194
Mori T, Ogawa S, Cowely AW Jr, Ito S (2012) Role of renal medullary oxidative and/or carbonyl stress in salt-sensitive hypertension and diabetes. Clin Exp Pharmacol Physiol 39:125–131
Yang Q, Kim SK, Sun F, Cui J, Larson MG, Vasan RS, Levy D, Schwartz F (2007) Maternal influence on blood pressure suggests involvement of mitochondrial DNA in the pathogenesis of hypertension: the Framingham Heart Study. J Hypertens 25:2067–2073
DeStefano AL, Gavras H, Heard-Costa N, Bursztyn M, Manolis A, Farrer LA, Baldwin CT, Gavras I, Schwartz F (2001) Maternal component in the familial aggregation of hypertension. Clin Genet 60:13–21
Hebert SC (2003) Bartter syndrome. Curr Opin Nephrol Hypertens 12:527–532
Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP (1996) Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13:183–188
Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP (1996) Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14:152–156
Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP (1997) Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17:171–178
Seyberth HW, Schlingmann KP (2011) Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol 26:1789–1802
Estevez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl- channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414:558–561
Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C (2002) Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13:2259–2266
Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP (1996) Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12:24–30
Nicolet-Barousse L, Blanchard A, Roux C, Pietri L, Bloch-Faure M, Kolta S, Chappard C, Geoffroy V, Morieux C, Jeunemaitre X, Shull GE, Meneton P, Paillard M, Houillier P, De Vernejoul MC (2005) Inactivation of the Na-Cl co-transporter (NCC) gene is associated with high BMD through both renal and bone mechanisms: analysis of patients with Gitelman syndrome and Ncc null mice. J Bone Miner Res 20:799–808
Geller DS, Rodriguez-Soriano J, Vallo Boado A, Schifter S, Bayer M, Chang SS, Lifton RP (1998) Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism type I. Nat Genet 19:279–281
Chang SS, Grunder S, Hanukoglu A, Rösler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP (1996) Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12:248–253
Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP (2009) Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106:5842–5847
Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben-Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R (2010) KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA 107:14490–14495
Rozengurt N, Lopez I, Chiu CS, Kofuji P, Lester HA, Neusch C (2003) Time course of inner ear degeneration and deafness in mice lacking the Kir4.1 potassium channel subunit. Hear Res 177:71–80
Schreiber R, Gubler MC, Gribouval O, Shalev H, Landau D (2010) Inherited renal tubular dysgenesis may not be universally fatal. Pediatr Nephrol 25:2531–2534
Gubler MC, Antignac C (2009) Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int 77:400–406
Sedman AB, Kershaw DB, Bunchman TE (1995) Recognition and management of angiotensin converting enzyme inhibitor fetopathy. Pediatr Nephrol 9:382–385
Coffman TM (2011) Under pressure: the search for the essential mechanisms of hypertension. Nat Med 17:1402–1409
Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM (2009) Genome-wide association study of blood pressure and hypertension. Nat Genet 41:677–687
Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Wellcome Trust Case Control Consortium, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB (2009) Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41:666–676
Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J (2011) Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 43:531–538
Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Artigas MS, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O'Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T, International Consortium for Blood Pressure Genome-Wide Association Studies; CARDIoGRAM consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen consortium; CHARGE-HF consortium (2011) Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478:103–109
Nagy B, Hupuczi P, Papp Z (2007) High frequency of methylenetetrahydrofolate reductase 677TT genotype in Hungarian HELLP syndrome patients determined by quantitative real-time PCR. J Hum Hypertens 21:154–158
Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ (2009) Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 41:348–353
Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Füchtbauer AC, Simonsen U, Füchtbauer EM, Aalkjaer C (2011) Disruption of Na+, HCO cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca(2) sensitivity, and hypertension development in mice. Circulation 124:1819–1829
Guyton AC (1991) Blood pressure control—special role of the kidneys and body fluids. Science 252:1813–1816
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toka, H.R., Koshy, J.M. & Hariri, A. The molecular basis of blood pressure variation. Pediatr Nephrol 28, 387–399 (2013). https://doi.org/10.1007/s00467-012-2206-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2206-9