Abstract
Background
Three patients with Dent’s disease presented with complaints of impaired night vision or xerophthalmia and were found to have severely decreased serum retinol concentrations. Retinol, bound to its carrier retinol-binding protein (RBP), is filtered at the glomerulus and reabsorbed at the proximal tubule. We hypothesized that urinary loss of retinol-RBP complex is responsible for decreased serum retinol.
Objective and methods
The study aim was to investigate vitamin A status and RBP in serum and urine of patients with genetically confirmed Dent’s disease.
Results
Eight patients were studied, three boys had clinical vitamin A deficiency, three had asymptomatic deficiency, and two young men with Dent’s disease and impaired renal function had normal retinol values. Serum RBP concentrations were low in patients with vitamin A deficiency and were correlated with vitamin A levels. Urinary RBP concentrations were increased in all patients (2,000-fold), regardless of vitamin A status. This was in contrast to patients with glomerular proteinuria who had only mildly increased urinary RBP with normal serum RBP and vitamin A, and patients with cystinosis with impaired renal function who had massive urinary RBP losses but without a decrease in serum RBP or vitamin A levels. Treatment with vitamin A supplements in patients with retinol deficiency resulted in rapid resolution of ocular symptoms and an increase in serum retinol concentrations.
Conclusions
Vitamin A deficiency is common in patients with Dent's disease and preserved renal function. We therefore recommend screening these patients for retinol deficiency and treating them before visual symptoms develop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dent’s disease is an X-linked renal tubular disorder manifesting as low molecular weight (LMW) proteinuria as well as hypercalciuria, nephrocalcinosis, and progressive renal impairment. Other features of proximal tubulopathy may be present in Dent’s disease, such as phosphaturia leading to rickets, glycosuria, aminoaciduria, hypokalemia, and salt wasting [1, 2]. LMW proteins are readily filtered in the glomerulus and are reabsorbed in the proximal tubule by the endocytic machinery, which involves the multiligand luminal receptors megalin and cubilin. Among the LMW proteins are a number of hormones and their binding proteins, for instance, parathyroid hormone (PTH) and vitamin-D-binding protein, respectively. Increased excretion of LMW proteins is clinically estimated by measuring the urinary concentration of beta-2 microglobulin or retinol-binding protein (RBP). Mutations in the CLCN5 gene encoding the ClC5 renal chloride/proton exchanger are responsible for the disease in most patients. Some patients are found to have mutations in the OCRL1 gene, which was previously shown to be responsible for Lowe’s syndrome [3]. Other genes may be involved in the pathogenesis of Dent’s disease in patients who do not demonstrate mutations in either of these genes. Three patients with Dent’s disease who were followed at our clinic presented with complaints of night blindness and xerophthalmia. Laboratory evaluation showed severely decreased serum retinol concentrations. RBP is a transport protein for retinol, delivering it from the liver to target organs. The retinol-RBP complex is filtered at the glomerulus and normally undergoes reabsorption at the proximal tubule. We hypothesized that urinary loss of retinol-RBP complex may be responsible for symptomatic vitamin A deficiency in these patients. Therefore, we systematically investigated patients with genetically confirmed Dent’s disease for vitamin A status and RBP in serum and urine.
Patients and methods
Patient 1
A boy born to healthy nonconsanguineous parents of North African–Jewish descent presented with massive proteinuria at the age of 4 years [urine total protein/creatinine (TP/Cr) 12.2 mg/mg]. Clinical findings included LMW proteinuria, hypercalciuria, and nephrocalcinosis with normal renal function and serum proteins. In addition, hypokalemia and hypophosphatemia were found, with mild rickets and tibial bowing on X-ray. Genetic analysis demonstrated the known R28X mutation in the CLCN5 gene, confirming the diagnosis of Dent’s disease. At the age of 7 years, the child complained of night blindness. A full ophthalmologic examination was normal. Serum retinol concentration was severely abnormal at 3 and 6 mcg/dl on repeated tests (normal 25–100 mcg/dl). Assessment of his nutritional intake was normal for age. There was no history of diarrhea or other gastrointestinal illness; liver enzymes and prothrombin time were normal, as were thyroid function and serum concentrations of vitamin D and E. He had a recent complaint of dry itchy skin. Treatment with 10,000 U vitamin A orally three times a day was started. Three days after starting treatment, he complained of headaches and recurrent vomiting and was admitted to the hospital. Ophthalmologic examination showed bilateral papilledema, and computed tomography (CT) of the brain showed mild Chiari I malformation. Vitamin A treatment was stopped and acetazolamide was given to lower increased intracranial pressure. The symptoms and papilledema gradually resolved and subsequently low dose vitamin A supplementation (1,250 U daily) was resumed without further complications. However, retinol concentrations increased only minimally, so to avoid side effects, treatment was changed to a beta carotene supplement (Now foods, Bloomingdale, IL, USA) 25,000 U four times daily. Retinol concentrations gradually increased to 19.6 mcg/dl over 6 months; visual complaints and skin condition resolved completely. He is maintained on this dose of beta carotene, with serum retinol concentrations remaining slightly low at 17–22.5 mcg/ dl.
Patient 2
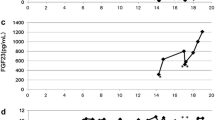
Patient 1’s younger brother was diagnosed with Dent’s disease at the age of 2 years. Initial presentation was hypophosphatemic rickets, and laboratory evaluation showed heavy proteinuria (TP/Cr 5.7 mg/mg), LMW proteinuria, hypercalciuria and nephrocalcinosis, hypokalemia, and normal renal function. Dent’s disease was confirmed by genetic analysis. Hypothyroidism was found at age 5 years and treated with levothyroxine. At age 5 years, around the same time as his brother’s visual symptoms occurred, the parents expressed concerns about his night vision, though it was not as obvious as in his older brother. Ophthalmologic evaluation was normal, but serum retinol concentration was 2–3 mcg/dl. Because of his brother’s adverse reaction to vitamin A therapy, treatment was started at 1,250 U daily and slowly increased to 10,000 U three times daily. There was a gradual increase of serum retinol concentrations to 33.3 mcg/dl over 6 months, visual symptoms resolved, and no adverse effects were seen. He has been maintained on this dose of vitamin A, with serum retinol concentrations monitored every 2–3 months, ranging from 21–37 mcg/dl.
Patient 3
A 10-year-old boy born to healthy nonconsanguineous Arab parents was referred to our service for further evaluation of heavy proteinuria, which was first detected by another institution 2 years earlier. He had undergone renal biopsy, and results were consistent with focal segmental glomerulosclerosis. He received immunosuppressive therapy with orally and intravenously administered corticosteroids, cyclosporine, and mycophenolate mofetil. On admission to our service, we noted that he had never had hypoalbuminemia or edema and the possibility that he may have tubular proteinuria was therefore evaluated. He was found to have heavy proteinuria (TP/Cr 2.9 mg/mg) with beta-2 microglobulin of 74.4 mg/L (n < 0.2), leading to a diagnosis of Dent’s disease, which was confirmed by mutational analysis showing a nonsense mutation c.366G > A in the CLCN5 gene. The mutation was found in the boy and his asymptomatic mother. Immunosuppression was tapered and stopped. At the time of referral, the patient complained of dry eyes. Ophthalmologic examination showed Bitot’s spots in the cornea and normal fundi. Serum retinol concentration was 20 mcg/dl. Oral treatment with vitamin A 10,000 U twice daily resulted in rapid resolution of visual disturbances and ophthalmologic findings, with no adverse effects. Serum retinol increased to 50 mcg/dl after 4 months of treatment, and vitamin A supplementation was decreased and then stopped, with continued clinical and laboratory follow-up. Serum retinol concentrations remained within the normal range of 27–43.5 mcg/dl without supplementation over the following year.
Methods
We investigated vitamin A status and serum and urine RBP in patients with genetically confirmed Dent’s disease. After obtaining informed consent from the participants or their parents, blood and urine samples were taken after an overnight fast. Serum and urine creatinine concentrations were determined by Vitros analyzer (Raritan, NJ, USA). Serum for retinol and RBP were frozen at −20°C. Retinol and vitamin E (tocopherol) were determined by high-performance liquid chromatography (HPLC) (Young Lin 9100). Urine was adjusted to pH 6–8 with sodium hydroxide (NaOH) before freezing, in accordance with the manufacturer’s recommendations. RBP was determined by enzyme-linked immunosorbent assay (ELISA) (Immundiagnostik, Bensheim, Germany). To maximize precision, urine RBP was measured at 10-, 100-, and 1,000-fold dilutions. Urinary total protein was measured by pyrogallol red direct calorimetric method (Sentinel Diagnostics, Milan, Italy) and beta-2 microglobulin by Behring nephelometer II (Siemens Healthcare, Marburg, Germany). Student’s t test was employed to compare glomerular filtration rate (GFR), serum retinol RBP concentrations, and urinary RBP between patients with Dent’s disease and control groups. Results are given as mean ± standard deviation (SD).
Results
Eight patients with Dent’s disease were evaluated. Other than the three patients described, none had symptoms of decreased visual acuity or night blindness and had a normal ophthalmologic examination. Patient characteristics are summarized in Table 1. Patients 4 and 5 are brothers who presented with asymptomatic proteinuria and were found to carry the R648X mutation in CLCN5. Patient 6 was found to carry the pathogenic c.2179delG mutation in CLCN5 [4]. Patient 7 is a young man with proteinuria and mild decrease in renal function who subsequently developed hypercalciuria. He was found to have a known mutation in OCRL1 c.901 C- > T [5]. Patient 8 is a young man with long-standing proteinuria and slowly progressive renal insufficiency who was recently found to have a long deletion of about 7,000 bp including the first 3 exons of CLCN5 and was therefore diagnosed as having Dent’s disease.
Serum retinol concentrations were low in six patients, including three who were asymptomatic (Table 2). The two older patients with impaired renal function had normal retinol concentrations. Serum RBP concentrations were greatly decreased in patients 1 and 2, low in the remaining patients with retinol deficiency, and normal in patients 7 and 8. Urinary RBP concentration was very high in all patients with Dent’s disease irrespective of renal function. When this value was corrected to urine creatinine concentration, the three symptomatic patients had somewhat higher values compared with the other patients, though the numbers were too small to achieve statistical significance. Fractional excretion of RBP was grossly elevated in all patients but was highest in patients 1 and 2, in whom serum retinol concentrations were particularly low; these two patients also had low serum RBP. All patients reported adequate dietary intake. Serum vitamin E and prothrombin time were measured to rule out the possibility of gastrointestinal malabsorption of fat-soluble vitamins and were normal in all patients. Thyroid function was normal in all but patient 2.
Mean values of serum retinol, serum RBP, and urine RBP concentrations in patients with Dent’s disease were compared with five children with nephropathic cystinosis, another proximal tubulopathy, and five with chronic glomerular proteinuria. Patients with glomerular disease had chronic nephrotic range proteinuria of 2.5–10 g/day, three patients had biopsy findings of focal segmental glomerulosclerosis, and two had membranoproliferative glomerulonephritis. Patients with cystinosis had a lower mean estimated GFR than those in the other two groups but had signs of severe tubular disease, including elevated urinary beta 2 microglobulin and phosphate and potassium wasting. Serum retinol concentrations were normal in all patients with cystinosis and chronic glomerular proteinuria and significantly higher than in patients with Dent’s disease. Serum RBP was slightly decreased in one patient with cystinosis and normal renal function and was normal in the remaining patients with cystinosis or nephrotic syndrome. Urinary RBP concentrations were extremely elevated in patients with cystinosis, as was fractional excretion of RBP, with values comparable with those of patients with Dent’s disease. In patients with glomerular nephrotic-range proteinuria, urinary RBP was significantly lower, with values close to normal. Results are summarized in Table 3.
Patients with asymptomatic retinol deficiency (patients 4–6) were treated with vitamin A supplement 10,000 U daily, with an increase in serum retinol concentrations to normal after 3–4 months. After stopping supplementation, a gradual decline in serum retinol was observed, to 16–21 mcg/dl after 4–6 months, and treatment was resumed. No adverse effects were noted.
Discussion
In our cohort of patients with genetically confirmed Dent’s disease, vitamin A deficiency was present in six of eight, with clinical findings in half of these patients. In fact, vitamin A was depleted in all patients with preserved renal function. Serum retinol concentrations were significantly decreased, with an accompanying decrease of serum RBP and 400- to 900-fold increase in urinary RBP concentration compared with normal values. The two oldest patients with Dent’s disease had normal serum RBP and retinol despite urinary RBP wasting. This may be due to decreased GFR, although other causes, such as the specific mutation, cannot be excluded.
The main dietary sources of preformed vitamin A (retinol) are animal products such as liver, eggs, dairy products, and fish oil. Provitamin A (carotenoids) is found in plants, such as dark-green leafy vegetables, and orange and red vegetables and fruits and is converted to retinol in the liver [6]. Nutritional vitamin A deficiency is virtually unheard of in developed countries, including Israel. All our patients reported adequate dietary intake and exhibited no clinical or biochemical signs of other nutritional deficits. Retinol, as well as RBP, is synthesized in the liver. It has a molecular weight of 21 kD and circulates in the blood bound to RBP and transthyretin. Retinol complexed with RBP is filtered at the glomerulus and then reabsorbed in the proximal tubules by binding of the retinol-RBP complex to megalin and endocytosis [7, 8]. In this way, retinol is salvaged and recycled into the circulation. Retinol is also metabolized in renal tubular cells into retinoic acid [6, 7]. Retinol metabolism is reduced in renal failure, and together with decreased filtration of RBP results in increased concentration of both retinol and RBP in patients with chronic kidney disease. On the other hand, lower retinol concentrations in adult hemodialysis patients are associated with increased overall and cardiovascular mortality rates, even after adjusting for markers of malnutrition and inflammation [8]. Vitamin A deficiency in children is associated with poor growth, skin disease, anemia, increased susceptibility to infection, and lower survival rates. However, its most prominent effect is on the eye, causing decreased mucus secretion by goblet cells and night blindness due to decreased supply of retinol to the retinal pigment epithelium and an increase in rod illumination threshold. Left untreated, xerophthalmia develops and can lead to irreversible blindness [9].
Mutations in the CLCN5 gene, which are found in most patients with Dent’s disease, may cause truncation of the ClC-5 protein and complete loss of function or missense mutations, which result in its degradation or abnormal distribution [1]. ClC-5 is a chloride–proton exchanger, mostly found in the proximal tubular cells but also in the thick ascending limb of Henle and the alpha-intercalated cells of the collecting duct [10]. In the proximal tubule it is located in the membrane of the early endosomes and is important in endosome acidification. Patients with CLCN5 mutations have reduced or absent chloride transport activity of ClC-5 and abnormal endosomal function. Progression along the endocytic path requires endosomal acidification, via vacuolar hydrogen adenosine triphosphatase (ATPase) with ClC5 necessary to dissipate positive charge. Acidification causes dissociation of ligand–receptor and recycling of megalin and cubilin. Impaired endocytosis results in defective trafficking of megalin and cubilin to the apical membrane, with consequent loss of their ligands, such as RBP-retinol complex, in the urine [11, 12]. In one study, mice with a kidney-specific megalin defect excreted large amounts of retinol and RBP, although serum concentrations were not affected [8]. LMW proteinuria also has downstream effects in the nephron, for example, PTH, because it is not reabsorbed in the early proximal tubule, is present in excess in the distal proximal tubule, and stimulates internalization of sodium–inorganic phosphate cotransporter (NaPi-IIa), causing phosphate wasting [1]. The OCRL1 gene encodes OCRL1, which is a lipid phosphatase expressed in most cell types. Some mutations in the gene cause Lowe’s syndrome, whereas others result in Dent’s disease; the mechanism for this is not fully understood [3].
We compared our patients with Dent’s disease to two groups of patients with persistent severe proteinuria: children with cystinosis, whose proteinuria is predominantly tubular, and children with chronic nephrotic-range glomerular proteinuria. The children with glomerular proteinuria had only a minimal increase in urinary RBP, similar to previously described findings [13], whereas children with cystinosis had a large increase in urinary RBP excretion (200-fold). Despite high urinary loss of RBP in the cystinosis patients, serum RBP and retinol concentrations remained normal. This may be partially due to the significantly lower eGFR in the group of patients with cystinosis compared with those with Dent’s disease. Reduced metabolism of retinol in chronic kidney disease may partially compensate for its urinary loss. On the other hand, megalin and cubilin expression is not decreased in proximal tubules of cystinotic kidney, suggesting that the mechanism producing low-molecular weight proteinuria in the two diseases might be different [14].
Urinary loss of retinol and RBP is also seen in premature infants, presumably a sign of tubular dysfunction or immaturity [15]. RBP concentrations in serum are tightly correlated with retinol concentrations; this allows RBP to be used as a surrogate marker for retinol in detecting nutritional vitamin A deficiency in low-resource settings [16]. A genetic defect in RBP synthesis was described in two sisters, which resulted in undetectable RBP concentrations, with severely decreased serum retinol concentrations but normal retinyl esters in serum. Ophthalmological disease manifested as night blindness and progressive atrophy of the retinal pigment epithelium but no other symptoms [17].
There has been one report of vitamin-A-responsive night blindness in three patients with Dent’s disease. These patients presented with early-onset rickets due to phosphate wasting, hypokalemia, and normal renal function, similar to our first two patients with severe vitamin A deficiency. They experienced episodes of night blindness with a normal ophthalmological examination; in two patients, the episodes were recurrent. A diagnosis of Dent’s disease was made based on mutations found in the CLCN5 gene. They were treated orally with a vitamin A regimen used in areas with endemic nutritional vitamin A deficiency: 200,000 U on two consecutive days, with clinical improvement. However, serum retinol concentrations were not monitored before or after treatment, and RBP status was not studied [18]. We describe a cohort of eight patients with genetically confirmed Dent’s disease who exhibited a high rate of retinol deficiency (75%), which was clinically apparent in half of them. Urinary RBP wasting resulted in depleted serum RBP concentrations in patients with normal renal function. Vitamin-A-deficient patients responded within 2–6 months to oral supplementation, though retinol concentrations decreased gradually after stopping treatment.
In our study, patient 1 had a severe adverse reaction at the start of vitamin A therapy. This may be related to the finding of Chiari malformation of the brain. On the other hand, severe retinol deficiency may cause symptoms of increased intracranial pressure, as does hypervitaminosis A. The patient’s symptoms occurred at the beginning of therapy with a relatively low dose, whereas the patient had very low serum retinol concentrations, making vitamin A toxicity unlikely. However, the possibility of an acute increase in retinyl esters (which were not measured) during initial treatment causing symptoms, could not be ruled out. Patient 3 had clinical symptoms (dry eyes) and signs (Bitot’s spots) of vitamin A deficiency, despite only slightly low serum retinol concentrations of 20 mcg/dl. However, his rapid clinical response to vitamin A therapy, which corresponded to an increase in serum retinol, suggested that the cause of the symptoms was indeed vitamin A deficiency.
Given our findings, we recommend that clinicians inquire about visual symptoms and implement surveillance measurements of serum retinol concentrations as well as periodic ophthalmological examinations in patients with Dent’s disease. Retinol deficiency should be treated with oral supplementation and followed clinically and by serial laboratory monitoring. RBP has long been an established marker for tubular proteinuria; however, urinary RBP loss may have clinical implications in patients with proximal tubulopathies, including early-stage cystinosis with Fanconi syndrome, although renal function is still near normal. The status of retinol in patients with other proximal tubular diseases warrants further investigation.
References
Claverie-Martin F, Ramos-Trujillo E, Garcia-Nieto V (2011) Dent’s disease: clinical features and molecular basis. Pediatr Nephrol 26:693–704
Wrong OM, Norden AGW, Feest TG (1994) Dent’s disease: a familial renal tubular syndrome with low molecular weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Q J Med 87:473–93
Bockenhauer D, Bokenkamp A, van’t Hoff W, Levchenko E, Kist-van Holthe JE, Tasic V, Ludwig M (2008) Renal phenotype in Lowe syndrome: A selective proximal tubular dysfunction. Clin J Am Soc Nephrol 3:1430–36
Frishberg Y, Dinour D, Belostotsky R, Becker-Cohen R, Rinat C, Feinstein S, Navon-Elkan P, Ben-Shalom E (2009) Dent’s disease manifesting as focal glomerulosclerosis. Is it the tip of the iceberg? Pediatr Nephrol 24:2369–73
Hoopes RR, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ (2005) Dent disease with mutations in OCRL1. Am J Hum Genet 76:260–267
Tanumihardjo SA (2004) Assessing vitamin A: Past present and future. J Nutr 134:290S–293S
Raila J, Willnow TE, Schweigert FJ (2005) Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr 135:2512–16
Kalousova M, Kubena AA, Kostirova M, Vinglerova M, Mestek O, Dusilova-Sulkova S, Tesar V, Zima T (2010) Lower retinol levels as an independent predictor of mortality in long-term hemodialysis patients: A prospective observational cohort study. Am J Kidney Dis 3:513–21
Ross AC (1999) Mutations in the gene encoding retinol binding protein and retinol deficiency: is there compensation by retinyl esters and retinoic acid? Am J Clin Nutr 69(5):829–30
Monnens LA, Levtchenko E (2008) Evaluation of the proximal tubular function in hereditary renal Fanconi syndrome. Nephrol Dial Transplant 23:2719–22
Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK (1999) Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 10:685–95
Christensen EI, Devuyst O, Dom G, Nielsen R, Van Der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ (2003) Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci 100(14):8472–77
Norden AGW, Scheinman SJ, Deschodt-Lanckman MM, Lapsley M, Nortier JL, Thakker RV, Unwin RJ, Wrong O (2000) Tubular proteinuria defined by a study of Dent’s (CLCN5 mutation) and other tubular diseases. Kidney Int 57:240–9
Wilmer MJ, Christensen EI, van den Heuvel HP, Monnens LA, Levchenko EN (2008) Urinary protein excretion pattern and renal expression of megalin and cubilin in nephropathic cystinosis. Am J Kidney Dis 51(6):893–903
Nagl B, Loui A, Raila J, Felderhoff-Mueser U, Obladen M, Schweigert FJ (2009) Urinary vitamin A excretion in very low birth weight infants. Pediatr Nephrol 24:61–66
Gorstein JL, Dary O, Pongtorn Shell-Duncan B, Quick T, Wasanwisut E (2007) Feasibility of using retinol-binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low-resource settings. Public Health Nutr 11(5):513–20
Biesalski HK, Frank J, Beck SC, Heinrich F, Illek B, Reifen R, Gollnick H, Seeliger MW, Wissinger B, Zrenner E (1999) Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am J Clin Nutr 69(5):931–6
Sethi SK, Ludwig M, Kabra M, Hari P, Bagga A (2009) Vitamin A responsive night blindness in Dent’s disease. Pediatr Nephrol 24:1765–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becker-Cohen, R., Rinat, C., Ben-Shalom, E. et al. Vitamin A deficiency associated with urinary retinol binding protein wasting in Dent’s disease. Pediatr Nephrol 27, 1097–1102 (2012). https://doi.org/10.1007/s00467-012-2121-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2121-0