Abstract
Background
The presence of circulating donor-specific human leukocyte antigen antibodies (HLA-DSA) has been associated with chronic antibody-mediated rejection, leading to progressive graft dysfunction and poor graft survival. The aim of this study was to investigate the incidence and significance of HLA-DSA in paediatric renal transplantation (RTx) patients.
Methods
A total of 294 post-transplant serum samples from 123 RTx patients were retrospectively analysed for HLA antibodies. Positive samples were further tested for HLA-DSA by a Luminex Single Antigen bead assay. The antibody findings were correlated to measured glomerular filtration rate (GFR) and clinical outcome.
Results
HLA antibodies were detected in half of the routine samples (140/294) taken 1 month to 10 years after RTx, and 40% (62/140) of these were HLA-DSA. Overall, one-third (42/123) of the patients had HLA-DSA, which mostly (65%) reacted against class II antigens. Detection of HLA-DSA was not associated with poor GFR at the time of sampling, and no exceptional deterioration of GFR after the HLA-DSA detection was noted in individual patients regardless of the antibody level. The presence of HLA-DSA in the first 2 years posttransplantation was not associated with poorer graft function later on.
Conclusion
Detection of HLA antibodies is common in children after RTx, and this finding, as such, does not predict any deterioration of graft function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal transplantation (RTx) is a successful treatment modality for terminal renal failure both in adults and children. The patient and graft survivals 1 year after transplantation are over 90%. However, the annual rate of allograft failure beyond the first year has remained relatively constant [1, 2]. This is attributed to chronic allograft dysfunction, which is a nonspecific entity reflecting the dual impact of both immunologic and nonimmunologic injury [3].
During the past few years the significance of chronic antibody-mediated rejection (CAMR) in progressive graft dysfunction and poor graft survival has been emphasized [4–6]. The presence of circulating donor-specific human leukocyte antigen antibodies (HLA-DSA) and complement factor C4d accumulation in the graft has been associated with CAMR. Techniques for the detection of HLA antibodies (HLAab) have become more sensitive with the introduction of solid phase assays, such as the enzyme-linked immunosorbent assay (ELISA) and Luminex assay [7, 8]. Although there have been several studies showing that the presence of HLA-DSA after transplantation is associated with chronic graft failure [9–12], many adult patients with circulating HLA-DSA and stable graft function have also been described [10, 13, 14], and the clinical relevance of HLAab has been questioned [12, 15–18]. Despite these somewhat controversial results, HLAab monitoring and adaptation of the immunosuppressive treatment in RTx patients with DSA has become a common policy in many transplant centres.
Data on the role of HLAab in children with RTx are so far very limited [19]. The significance of HLA-DSA before RTx has been reported in two studies [20, 21], and one study has examined the role of posttransplant HLA-DSA on graft survival [22]. The aim of our study was to examine how commonly HLAab are detected in paediatric RTx patients posttransplant by analysing routine serum samples taken at various times after RTx. An additional objective was to determine whether the detection of HLA-DSA was associated with any deterioration of the renal graft function as studied by glomerular filtration rate (GFR) measurements.
Materials and methods
Patients
A total of 142 paediatric patients underwent RTx between 1989 and 2004 at the Hospital for Children and Adolescents, Helsinki University Central Hospital. Of these, 123 patients were included in this study, 74% of whom received the graft from a deceased donor. Nineteen patients were excluded because of insufficient follow-up data (lack of adequate posttransplant serum samples or GFR measurements) (Table 1). The patients had postoperative follow-up visits at our centre every 3–6 months during the first 2 years, and annually thereafter.
All patients not previously transplanted or transfused received three non-filtered HLA AB-matched red blood cell transfusions prior to the operation. A negative complement-dependent lymphocytotoxicity crossmatch (CDC) against donor spleen lymphocytes was also required before the transplantation. All patients received triple drug immunosuppressive medication, including cyclosporine A, azathioprine and methylprednisolone, as previously described [23]. The switch from cyclosporine A to tacrolimus and from azathioprine to mycophenolate were performed on individual basis if clinically indicated. Induction therapy with basiliximab has been used since 1999. Rejection episodes were treated with an increased oral or intravenous methylprednisolone dosing. Graft failure was defined as a return to dialysis.
HLA typing
Patients and their donors were initially as HLA-A, -B or -DR typed using a serological assay and low-resolution sequence specific primers (PCR-SSP). In situations where extended typing was needed to confirm a donor specificity of an antibody, additional typing was performed with sequence-specific oligonucleotide probes (PCR-SSO) (LABType;, One Lambda, Canoga Park, CA).
Data collection
Clinical data were collected retrospectively from the patient charts. GFR was measured by 51Cr-labelled EDTA (ethylenediaminetetraacetic acid) clearance and correlated with the modified Brochner–Mortensen equation every 3–6 months and then annually during the follow-up [24]. The values were corrected for a standard body area of 1.73 m2, and only measurements with a marker distribution of between 15 and 35% of body volume were included. The GFR measurements and serum sampling for antibody measurements took place during the same 1- to 3-day visit on the ward. A total of 1,152 GFR measurements were included in this study (average of 9 measurements per patient).
Measurement of HLA antibodies
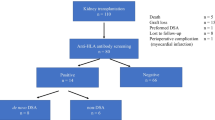
A total of 294 posttransplant serum samples from the 123 kidney transplant patients were retrospectively screened for HLAab, and positive samples were further tested for HLA-DSA. More specifically, 144 samples from 90 patients were taken during the first 2 years after transplantation, 89 samples from 75 patients were taken between 3 and 5 years after transplantation and 61 samples were taken from 50 patients 6–10 years after transplantation. The serum samples were collected by standard protocol independently of clinical findings. The samples were first screened with the LabScreen Mixed screening assay according the manufacturer’s instructions (One Lambda). If the screening was positive, the samples were then analysed with LabScreen single antigen kits to identify antibody specificities. All sera were tested for HLA class I (HLA-A, B, Cw) and class II (HLA-DR, DQ, DP) antibodies. Antibodies were assigned using HLA Visual software (One Lambda). HLA-DSA was assigned by comparing the assigned antibodies to the serological equivalent of the donor’s HLA-type. The strength of the identified donor-specific antibodies was determined using mean fluorescence intensity (MFI) values. HLA-DSA with MFI of >500 were taken into account, as suggested by the analysis software.
Statistical analysis
Descriptive data are reported as the mean ± standard deviation (SD) or as the median and ranges. Continuous variables were compared using independent-samples t test or analysis of variance (ANOVA) and categorical variables were compared using the chi-square test. SPSS ver. 16.0 was used for analysis. P values of <0.05 were considered to be statistically significant.
Ethics
This study was approved by the Ethics Committee for Paediatrics, Adolescent Medicine and Psychiatry of the Hospital District of Helsinki.
Results
The postoperative HLAab were retrospectively studied in 123 RTx children and adolescents. The findings of the HLA antibody assay were correlated to clinical outcome and measured GFR. The patients were transplanted at a median age of 4.3 years (range 0.7–18.2 years) and were followed for a median of 10.7 years (range 4.2–19.7 years) (Table 1). The median number of HLA class I and II antigen mismatches were 1.47 and 0.72, respectively. Seven graft losses occurred in six patients.
HLA antibody findings
Human leukocyte antigen antibodies were analysed by the Luminex assay in 294 post-RTx serum samples (average of 2.4 measurements/patient) taken at routine (mostly annual) visits after RTx (Fig. 1). HLAab were detected in 48% of the samples (140/294), and the proportion of HLAab-positive samples (36–59%) was quite similar at different time points posttransplant (Fig. 1). Forty-four percent (62/140) of the HLAab-positive samples contained antibodies against the donor HLA antigens (HLA-DSA), while the other half (56%, 78/140) contained antibodies against third-party HLA antigens (HLA-nonDSA) only. Of the patients, 64% (79/123) had HLAab in at least in one sample, while no HLAab were detected in 36% of the patients (44/123) (Table 2).
Patients with HLA-DSA
Donor-specific HLA were detected in 62 serum samples taken from 42 patients. A third (23/62) of the HLA-DSA-positive samples had MFI values of >10,000 (median 5,208, range 500–22,017) (Table 2). The frequency of HLA-DSA-positive samples increased somewhat with time from 15% (22/144) in the first 2 years to 20% (18/89) in 3–5 years and 36% (22/61) in 6–10 years (Fig. 1) (1–24 vs. 36–60 months, p = 0.33; 36–60 vs. 72–120 months, p = 0.032). Fifty-five percent (23/42) of the HLA-DSA-positive patients had antibodies against class II antigens, 29% (12/42) had antibodies against class I antigens and 17% (7/42) had HLA-DSA against both class I and II antigens. HLA-DSA against HLA-Cw or HLA-DQ antigens were only present in 16 patients. Only one HLA-DSA positive sample was detected in 62% (26/42) patients, while 16 patients had two to three HLA-DSA samples positive for the same donor antigen specificity. In a few patients (9/42, 21%) one to two negative DSA findings were observed after a DSA-positive sample.
Patients with and without HLA-DSA, showed no difference in terms of age at the RTx (mean age 6.0 vs. 7.0 years, respectively), HLA class I mismatches (1.6 vs. 1.4), HLA class II mismatches (0.8 vs. 0.7), cold ischemia time (18.0 vs. 14.7 h) and positive cytomegalovirus (CMV) status at the time of RTx (44 vs. 53%). HLA-DSA were observed in 22% of patients receiving a living related donor kidney transplant and 39% of patients receiving a deceased donor kidney transplant (p = 0.82).
Renal function at the time of HLAab measurement
The GFR was measured by 51Cr-EDTA clearance at the time of HLAab measurement on 283 occasions. The distribution of GFR values was quite similar in HLAab-negative patients and those positive for HLA-nonDSA or HLA-DSA, regardless of the antibody level (Fig. 2). Similarly, the mean GFR values during the time periods of 0–2, 3–5 and 6–10 years were not different in the three patient cohorts (Table 3). Patients with HLA-DSA class I antibodies had a lower mean GFR value at 0–2 years as compared to those with no antibodies (p < 0.05) (Table 3).
Renal function after HLAab detection
The important question is what happens to renal function after HLAab detection. Analysis of the serum samples taken during the first 2 years post-RTx revealed HLA-DSA in 22 patients, HLA-nonDSA in 31 patients and no HLAab in 38 patients. The GFR values at 3 years post-RTx and thereafter did not significantly differ in these three groups, as shown in Table 4, indicating that early HLA-DSA, even with high MFI-levels, were not associated with poorer graft function later on.
This finding was also evident when the annual decline of GFR was associated with the HLAab findings at an individual level (Fig. 3, Table 5). After a negative HLAab finding, the mean GFR decreased 2.6 ml/min/1.73 m2 per year. After HLA-DSA and HLA-nonDSA detection, the mean decline was 1.3 and 2.8 ml/min/1.73 m2 per year, respectively (Table 5). Also, GFR decline after the detection of HLA-DSA was not more pronounced than the drop observed before the sample was taken (1.3 vs. 6.7 ml/min/1.73 m2). Finally, the proportion of patients showing an exceptionally marked annual decline of GFR (>20 ml/min/1.73 m2) was not different in the HLA-DSA, HLA-nonDSA and HLAab-negative groups during the first 4 years after antibody measurement (10, 24, and 17%, respectively; p = 0.077).
Individual glomerular filtration rate (GFR) measurements (51Cr-EDTA, ml/min/1.73 m2) 2 years before and 4 years after the detection of HLA-DSA in 42 patients (grey lines). Black lines Mean GFR of human leukocyte antigen antibodies (HLAab) in the negative control group (n = 44). The findings were separated in three time periods for clarity. 0 on the x-axis Time of first antibody detection
Graft survival
Seven grafts in six patients failed during the follow-up period. Three grafts in two patients with congenital nephrotic syndrome (CNF) were lost due to recurrent nephrotic syndrome caused by anti-nephrin antibodies. Four other grafts were lost 1–14 years after RTx: three of the patients had a severe vascular rejection (with a positive posttransplant CDC) early after RTx operation, and one graft was lost due to chronic allograft dysfunction. All four patients had HLA-DSA in solitary samples with MFI values ranging from 1,040 to 1,3273.
Discussion
We measured HLAab in serum samples taken at various times after paediatric RTx in order to evaluate whether the detection of donor-specific HLAab was associated with deterioration of the renal graft function. The analyses were performed retrospectively from protocol serum samples, and since the results were not available at the time of sampling, no adaptation in the immunosuppressive medication occurred. Both DSA-HLA and HLA-nonDSA were commonly observed in RTx children and, as such, their appearance did not indicate or precede poor graft function. To the best of our knowledge, this is the first time HLAab findings have been correlated to adequately measured graft function.
The Luminex assay revealed HLAab in one-half of the 294 serum samples and in two-thirds of the 123 children and adolescents after RTx. This frequency of antibody detection was clearly higher than that reported previously in adults. In a large cohort of about 2,600 adults, HLAab were documented in 20% of the patients with functioning graft [14]. Similarly, in a recent report of 1,014 adult recipients from a single centre, 30% of the patients showed HLAab [10]. These antibodies appeared at various time points posttransplant, as was the case in our study. Interestingly, half of the HLAab-positive samples in our study were donor specific, which is a higher proportion than that found in adults [10]. The high frequency of antibodies in our patients may partly be explained by the fact that donor-specific and random red cell transfusions were used in our centre until year 2006.
Both class I and II HLAab have been associated with acute and chronic allograft rejection in kidney transplantation [4]. However, class I antibodies have been detected more in patients with acute rejection and class II antibodies more in patients with chronic rejection [8, 25]. In our study, patients with HLA-DSA had mostly class II antibodies and patients with HLA-nonDSA had mainly class I antibodies. The same was recently reported in adults [10]. Interestingly, the seven patients with HLA-DSA class I during the first 2 years after RTx had poorer GFR values than patients with HLA-DSA class II or no HLAab. The small number of these patients did not allow a more thorough analysis.
Data on the role of HLAab as detected by the solid-phase assays are currently scarce in children with RTx. The significance of pretransplant HLA-DSA has been examined in two studies. Roberti et al. [20] saw an excellent outcome of six children with HLA-DSA before living donor RTx, and Verghese et al. [21] found impaired renal allograft survival in ten children with pretransplant HLA-DSA. The significance of posttransplant HLA-DSA has been addressed in only one paediatric study: Emonds et al. analysed retrospectively postoperative HLA-DSA in ten children who had lost their deceased donor kidney grafts due to rejection 2–68 months after RTx. These investigators found class I and/or class II HLA-DSA antibodies in all ten patients [22]. In contrast, all patients without HLA-DSA had a good graft outcome.
The major finding in our study was that the presence of HLA-DSA after RTx was not associated with poor graft function at the time of antibody detection or thereafter. In our patients, GFR was followed by 51Cr-EDTA clearance, which is a sensitive and reliable method to reveal deterioration of the graft function already at an early stage. The distribution of GFR values in patients with HLA-DSA patients was not different from the others. Therefore, patients with HLA-DSA had variable GFR at the time of antibody detection. This was the case even in patients having high (MFI >10,000) antibody levels.
According to “the humoral theory of rejection”, HLAab are the major cause of chronic graft loss but it takes time before their detrimental effect becomes apparent [5]. Some investigators have observed that a graft failure can be detected by a rise in serum creatinine on average 29 months after the emergence of HLAab and that the graft is completely lost after a mean of 44 months [9, 26]. In our study, individual GFR levels before and after HLAab detection were followed for several years, but the decline of GFR in the HLA-DSA- and HLA-nonDSA-positive patients was not different from that observed in children without detectable antibodies. The detection of HLA-DSA did not predict an exceptionally marked decline in GFR. In fact, the annual decrease of GFR after the detection of HLA-DSA was on average less than that after a negative HLAab finding (1.3 vs. 2.6 ml/min/1.73 m2 per year). The presence of high levels of HLA-DSA in many of the serum samples underscores this finding.
Only a few serum samples per patient were tested for HLAab, which is a limitation of our study, as has been the case with most previous studies in adults. In spite of the fact that the detection rate of HLAab in our children was higher than that observed in adults, measurement of more samples would probably have revealed more HLAab-positive patients. This is especially so as the HLAab levels in an individual seem to fluctuate with time. Also, we were not able to analyse pretransplant HLAab due to a lack of samples. All patients had negative CDC crossmatch on the day of transplantation, but it is obvious that the Luminex assay would have detected pretransplant HLA-DSA in a number of patients due to its high sensitivity [18, 24]. Lymphocyte CDC or flow crossmatch is still the key analysis for donor- recipient selection in the majority of transplantation centres. The clinical relevance of pretransplant HLA-DSA detected by Luminex with simultaneous negative CDC or flow crossmatch is still under debate, and more data are required to assess its clinical use in children and adults [27, 28].
The importance of antibody-mediated acute and chronic rejection has been clearly shown in RTx [5, 6]. Accordingly, three of the 123 children with HLA-DSA in our patient cohort showed positive cross-match after transplantation and developed severe vascular rejection and graft loss in late 1990s. The harmful effect of cytotoxic antibodies in paediatric RTx was reported as early as the early 1990s [29, 30]. Thus, the frequent finding of HLAab and the lack of association of the HLA-DSA findings with the renal function was somewhat surprising in our study. The search for HLA-DSA seems to be important in RTx children with signs of rejection or deteriorating graft function, but in patients without clinical problems the Luminex assay may “overdetect” HLA-DSA which do not have functional significance. Whether C1q assays to detect complement-fixing antibodies with Luminex-based assays will give more clinically relevant information will be resolved in the future [31, 32].
Abbreviations
- CAMR:
-
Chronic antibody mediated rejection
- CDC:
-
Complement-dependent lymphocytotoxicity crossmatch
- CMV:
-
Cytomegalovirus
- CNF:
-
Congenital nephrotic syndrome
- 51Cr-EDTA:
-
51Chromium-labeled ethylenediaminetetraacetic acid
- HLAab:
-
Human leukocyte antigen antibodies
- HLA-DSA:
-
Donor-specific HLA antibodies
- HLA-nonDSA:
-
HLA antibodies not specific against donor antigens
- MFI:
-
Mean fluorescence intensity
- PCR-SSP:
-
Sequence specific primers and PCR
- RTx:
-
Renal transplantation
References
Meier-Kriesche HU, Schold JD, Kaplan B (2004) Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4:1289–1295
Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ (2010) Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90:68–74
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR (2003) The natural history of chronic allograft nephropathy. N Engl J Med 349:2326–2333
Akalin E, Pascual M (2006) Sensitization after kidney transplantation. Clin J Am Soc Nephrol 1:433–440
Terasaki PI, Cai J (2008) Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation 86:377–383
Böhmig GA, Bartel G, Regele H, Wahrmann M (2009) Prospects and limitations of post-transplantation alloantibody detection in renal transplantation. Hum Immunol 70:640–644
Jordan SC, Reinsmoen N, Peng A, Lai CH, Cao K, Villicana R, Toyoda M, Kahwaji J, Vo AA (2010) Advances in diagnosing and managing antibody-mediated rejection. Pediatr Nephrol 25:2035–2045
Riethmüller S, Ferrari-Lacraz S, Müller MK, Raptis DA, Hadaya K, Rüsi B, Laube G, Schneiter G, Fehr T, Villard J (2010) Donor-specific antibody levels and three generations of crossmatches to predict antibody-mediated rejection in kidney transplantation. Transplantation 90:160–167
Cai J, Terasaki PI (2008) Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol 20:602–606
Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, Pratschke J, Rudolph B, Schmidt D, Salama A, Schönemann C (2009) Anti-human leukocyte antigen and donor-specific antibodies detected by Luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 87:1505–1513
Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD (2005) Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 16:2804–2812
Claas FH (2010) Clinical relevance of circulating donor-specific HLA antibodies. Curr Opin Organ Transplant 15:462–466
Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, Tsai A, Lei HY (2002) All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation 74:1192–1194
Terasaki PI, Ozawa M, Castro R (2007) Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant 7:408–415
Phelan D, Mohanakumar T, Ramachandran S, Jendrisak MD (2009) Living donor renal transplantation in the presence of donor-specific human leukocyte antigen antibody detected by solid-phase assay. Hum Immunol 70:584–588
Aubert V, Venetz JP, Pantaleo G, Pascual M (2009) Are all donor-specific antibodies detected by solid-phase assay before transplantation clinically relevant? Transplantation 87:1897–1898
Bartel G, Regele H, Wahrmann M, Huttary N, Exner M, Hörl WH, Böhmig GA (2008) Posttransplant HLA alloreactivity in stable kidney transplant recipients-incidences and impact on long-term allograft outcomes. Am J Transplant 8:2652–2660
Süsal C, Ovens J, Mahmoud K, Döhler B, Scherer S, Ruhenstroth A, Tran TH, Heinoid A, Opelz G (2011) No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: a Collaborative Transplant Study report. Transplantation 91:883–887
Girnita AL, Webber SA, Zeevi A (2006) Anti-HLA alloantibodies in pediatric solid organ transplantation. Pediatr Transplant 10:146–153
Roberti I, Vyas S, Pancoska C (2007) Donor-specific antibodies by flow single antigen beads in pediatric living donor kidney transplants: single center experience. Pediatr Transplant 11:901–905
Verghese PS, Smith JM, McDonald RA, Schwartz SM, Nelson KA, Warner PR (2010) Impaired graft survival in pediatric renal transplant recipients with donor-specific antibodies detected by solid-phase assays. Pediatr Transplant 14:730–734
Emonds MP, Herman J, Dendievel J, Waer M, Van Damme-Lombaerts R (2000) Evaluation of anti-human leukocyte antigen allo-immunization in pediatric cadaveric kidney transplantation. Pediatr Transplant 4:6–11
Valta H, Jalanko H, Holmberg C, Helenius I, Mäkitie O (2008) Impaired bone health in adolescents after liver transplantation. Am J Transplant 8:150–157
Jodal L, Brochner-Mortensen J (2009) Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part I: Analytically correct relationship between total and one-pool clearance. Scand J Clin Lab Invest 69:305–313
Eng HS, Bennett G, Bardy P, Coghlan P, Russ GR, Coates PT (2009) Clinical significance of anti-HLA antibodies detected by Luminex: enhancing the interpretation of CDC-BXM and important post-transplantation monitoring tools. Hum Immunol 70:595–599
Terasaki P, Lachmann N, Cai J (2006) Summary of the effect of de novo HLA antibodies on chronic kidney graft failure. Clin Transpl 455–462
Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S (2009) Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 87:1681–1688
Gupta A, Sinnott P (2009) Clinical relevance of pretransplant human leukocyte antigen donor-specific antibodies in renal patients waiting for a transplant: a risk factor. Hum Immunol 70:618–622
Fine RN, Malekzadeh MH, Pennisi AJ, Ettenger RB, Uittenbogaart CH, Korsch BM (1979) Renal transplantation in children. J Pediatr 95:244–248
Davenport A, Younie ME, Parsons JE, Klouda PT (1994) Development of cytotoxic antibodies following renal allograft transplantation is associated with reduced graft survival due to chronic vascular rejection. Nephrol Dial Transplant 9:1315–1319
Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyran DB (2011) C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 91:342–347
Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, Rosenthal D, Reinhartz O, Tyan D (2011) Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant 30:158–163
Author information
Authors and Affiliations
Corresponding author
Additional information
Jenni Miettinen and Juha Peräsaari contributed equally to this work.
Rights and permissions
About this article
Cite this article
Miettinen, J., Peräsaari, J., Lauronen, J. et al. Donor-specific HLA antibodies and graft function in children after renal transplantation. Pediatr Nephrol 27, 1011–1019 (2012). https://doi.org/10.1007/s00467-012-2101-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2101-4