Abstract
Several epidemiologic studies have clearly demonstrated that obesity increases the risk of kidney diseases. We have attempted to evaluate the association of obesity with albuminuria, an early marker of kidney disease, among obese children and its relation to metabolic syndrome. This study included 150 obese children. Blood pressure, fasting blood glucose, plasma insulin and the lipid profile were assessed. The homeostasis model assessment of insulin resistance (HOMA-IR) was used to calculate in vivo insulin resistance. Urinary albumin and creatinine were estimated. Microalbuminuria was detected in 22 (14.7%) of the obese children. Waist circumference, blood pressure, triglyceride, low-density lipoprotein (LDL), insulin resistance and fasting blood glucose were significantly higher in obese children with microalbuminuria than in those with normoalbuminuria and showed significant positive correlations with microalbuminuria. High-density lipoprotein (HDL) was significantly lower in obese children with microalbuminuria than in those with normoalbuminuria, with a significant negative correlation with microalbuminuria. We found that body mass index, abdominal obesity, hypertension, impaired fasting glucose level and insulin resistance significantly increased the odds of microalbuminuria in the obese children enrolled in this study. Moreover, high triglyceride, high LDL and low HDL were significantly associated with microalbuminuria. In our patient group, childhood obesity was a risk factor for the development of microalbuminuria, which in turn was significantly associated with metabolic syndrome and its different constituents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Childhood obesity is fast becoming a worldwide epidemic [1] and has reached an average prevalence of approximately 10% in a large number of developing countries. Several epidemiologic studies have clearly demonstrated that obesity increases the risk of kidney diseases [2], as well as its progression among diagnosed kidney disease patients [2, 3]. Obesity is strongly associated with the two most common causes of end-stage renal disease (ESRD), namely diabetes and hypertension; moreover, the metabolic syndrome (MS), a major consequence of obesity, also seems to be an independent risk factor for both chronic kidney disease (CKD) and ESRD [4]. Reduced insulin sensitivity and hyperinsulinemia are among the most important factors of MS contributing to renal injury [5]. The first association between massive obesity and nephrotic-range proteinuria was reported in 1974 [6, 7]. One year later, Cohen [8] described the presence of significant glomerular enlargement, mild hypercellularity and variable widening of mesangial regions in severely obese patients with normal renal function; of particular concern, these features had been observed even in children as young as 3 years of age. Albuminuria is an early marker of CKD and is a potential target of primary prevention measures [9]. Microalbuminuria is now considered to be an early marker of renal damage in nondiabetic patients [10]. In the study reported here, we attempted to evaluate the association of obesity with albuminuria, an early marker of kidney disease, among obese children and determine its relation to metabolic syndrome.
Patients and methods
This was a cross-sectional study comprising 150 obese children selected from those attending the Pediatric Department and Outpatient Clinic of Zagazig University Hospital for routine medical examination or for obesity-related complaints without any manifestation of endocrine disorder during the period from January 2008 to December 2010. All enrolled children were prepubertal with a mean age of 7 ± 2.4 years. Parental consent was required to be eligible for enrollment, and the medical ethics committee of the hospital approved the protocol. Demographic and clinical data of these children were recorded. Body mass index (BMI) was calculated from the height and weight data using the formula BMI = weight (kg)/height (m2). Children were included in the study if the BMI was ≥95th centile for age and sex, as determined from the U.S. Centers for Disease Control and Prevention (CDC) 2000 reference values [11]. Patients excluded from the study were those with fever, infections, renal diseases, endocrine disorders, systemic lupus erythematosus and those who on vigorous exercise programs. Patients with albuminuria associated with urinary tract infections were also excluded.
All patients were subjected to a full medical history that included clinical examination, routine laboratory investigations, X-ray, and ultrasonographic studies of the abdomen and pelvis. Blood urea, serum creatinine, fasting blood glucose, plasma insulin levels were determined, and lipid profiling was performed. Urinalysis and culture sensitivity were also performed.
Sample collection
A urine sample was taken in the morning with the child in supine position; instructions were given to the patient and his/her parents for the former to avoid exercise and hyperactivity 24 h prior to sampling. Urinalysis and culture sensitivity were performed on part of the sample; the remaining urine was stored at −70°C for measurement of albuminuria.
Fasting venous blood samples were taken for determination of plasma glucose, plasma insulin concentration and lipid profiling.
Laboratory analyses
Urinary albumin concentrations were measured with a solid-phase fluorescent immunoassay, and urinary creatinine levels were measured with the Jaffe rate reaction method, using a CX3 analyzer (Beckman Instruments, Brea, CA). The urinary albumin/creatinine ratio was expressed as milligrams of albumin per gram of creatinine.
Fasting plasma glucose levels were measured with the modified hexokinase enzymatic method.
Plasma insulin levels were measured using a commercially available radioimmunoassay kit (Coat-A-Count Insulin; Diagnostic Products, Los Angeles, CA). The homeostasis model assessment for insulin resistance (HOMA-IR) was used to calculate in vivo insulin sensitivity according to the formula: fasting blood glucose (mmol/l) × fasting insulin (mU/l)/22.5.
Fasting serum triglyceride levels were measured enzymatically. High-density lipoprotein (HDL) cholesterol levels were measured by using the heparin-manganese precipitation method.
The MS was defined in accordance with the recommendations of the National Cholesterol Education Program Adult Treatment Panel III, modified for age. Participants with three or more of the following criteria met the definition of the MS: abdominal obesity, hypertension, high triglyceride level, low HDL cholesterol level, impaired fasting glucose or diabetes mellitus [12]. Abdominal obesity was defined as a waist circumference of ≥90th percentile for age and gender [13]. Hypertension was defined as an average systolic or diastolic blood pressure of ≥95th percentile for age, gender and height [14]. A high triglyceride level was defined as a fasting serum triglyceride level of ≥95th percentile for age and gender [15]. A low HDL cholesterol level was defined as a HDL cholesterol level of <5th percentile for age and gender [15]. Impaired fasting glucose was defined as a fasting plasma glucose level between 100 and 125 mg/dl without a history of diabetes mellitus [16]. Diabetes mellitus (DM) was defined as a fasting glucose level of ≥126 mg/dl, a self-reported history of DM or history of diabetes medication usage. Insulin resistance was defined as a HOMA-IR score of >4.34 without a history of DM or diabetes medication usage [16].
Normal urinary albumin excretion was defined as an albumin/creatinine ratio of <30 mg/g. Microalbuminuria was defined as an albumin/creatinine ratio of ≥30 mg/g and <300 mg/g [17, 18].
Statistical analysis
SPSS for Windows ver. 11 (SPSS, Chicago, IL) was used for the analysis. All values are given as the mean ± standard deviation (SD). The BMI z-score was determined. The Student t test and chi-square test were used to compare data. Logistic regression models were developed to analyze associations with microalbuminuria using clinical and laboratory data as independent variables and adjusting for age, gender, BMI, MS and all of its different constituents. Correlation analysis was used to detect the relation between microalbuminuria and other variables.
Results
The full medical examination and laboratory tests revealed that there were 140 cases (93.3%) of abdominal obesity, 24 cases (16%) of hypertension, 22 cases (14.7%) of microalbuminuria, 45 cases (30%) of high triglyceride, 37 cases (24.7%) of high LDL, 31 cases (20.7%) of low HDL, 30 cases (20%) of impaired fasting glucose level, 48 cases (32%) of insulin resistance and 23 cases (15.3%) of metabolic syndrome (Table 1).
The children were stratified according to presence of microalbuminuria or normoalbuminuria. There were no significant differences between obese children with microalbuminuria and those with normoalbuminuria in terms of age, gender BMI and BMI z-score (P > 0.05) (Table 2).
Values for waist circumference and blood pressure were significantly higher in obese children with microalbuminuria than in those with normoalbuminuria (P < 0.05) (Table 2).
Triglyceride and LDL levels were significantly higher in obese children with microalbuminuria than in those with normoalbuminuria (P < 0.01) (Table 2), while HDL level was significantly lower in obese children with microalbuminuria compared to those with normoalbuminuria (P < 0.01) (Table 2).
Insulin resistance and fasting glucose level were significantly higher in obese children with microalbuminuria than in those with normoalbuminuria (P < 0.01) (Table 2).
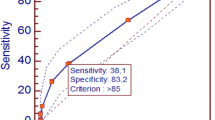
Obese children with microalbuminuria had a significant higher incidence of hypertension (36 vs. 14%; P < 0.01), high triglyceride levels (59 vs. 25%; P < 0.01), high LDL levels (37 vs. 23%; P < 0.01), low HDL levels (64 vs. 21%; P < 0.01), insulin resistance (68 vs. 26%; P< 0.01), impaired fasting glucose (41 vs. 16%; P < 0.01) and MS (59 vs. 8%; P< 0.01) than obese children with normoalbuminuria (Fig. 1).
Logistic regression analyses were performed to assess the association between different variables and the presence of microalbuminuria in obese children (Table 3). In the adjusted model, BMI, abdominal obesity, hypertension, impaired fasting glucose level, insulin resistance and MS significantly increased the odds of microalbuminuria in obese children; the respective adjusted odds ratios and 95% confidence intervals were 1.15, 1.46, 1.82, 2.41, 2.14 and 2.35, and 1.02–4.55, 1.24–3.98, 1.19–4.98, 1.92–3.55, 1.15–5.53 and 1.89–4.91; P < 0.01 for all previous variables (Table 3). Moreover, high triglyceride, high LDL and low HDL levels were significantly associated with microalbuminuria (P < 0.05) (Table 3). Age and gender were not significantly related to microalbuminuria in obese children (P > 0.05) (Table 3).
There were significant positive correlations between microalbuminuria and BMI, waist circumference, systolic and diastolic blood pressure, triglyceride level, LDL level, insulin resistance and fasting glucose level, respectively. In contrast, there was significant negative correlation between microalbuminuria and HDL level (P < 0.01) (Table 4). There were no significant correlations between microalbuminuria and age in obese children (P > 0.05) (Table 4).
There were significant positive correlations between BMI and age, BMI z-score, waist circumference, systolic and diastolic blood pressure triglyceride level, LDL level, insulin resistance and fasting glucose level, respectively. In contrast, there was a significant negative correlation between BMI and HDL (P < 0.01) (Table 4).
Discussion
Renal dysfunction may start long before the appearance of hypertension or diabetes in adulthood [4]. The long-term cardiovascular and renal impact of obesity, although deferred to adult life, has its origin in childhood [19].
In our study, we detected abdominal obesity in 140 children (93.3%), hypertension in 24 children (16%), microalbuminuria in 22 children (14.7%), high triglyceride levels in 45 children (30%), high LDL levels in 37 children (24.7%), low HDL levels in 31 children (20.7%), impaired fasting glucose level in 30 children (20%), insulin resistance in 48 children (32%) and MS in 23 children (15.3%).
Nguyen et al. [18] found that obese adolescents had prevalence rates of 96.6, 16.5, 62.5, 10.7, 26.4, 24.7 and 22% for abdominal obesity, impaired fasting glucose, insulin resistance, hypertension, high triglyceride levels, low HDL levels, and MS, respectively. Excess weight is thought to increase intraglomerular capillary pressure, resulting in glomerular hyperfiltration, a permissive environment or condition for end-organ damage. In this setting, hypertension, impaired fasting glucose or DM may provide a second “hit”, causing endothelial dysfunction that leads to microalbuminuria [20]. Hyperinsulinemia, a marker of reduced insulin sensitivity, seems to play a pivotal role in the pathogenesis of renal hemodynamic abnormalities. At the tubular level, insulin has an antinatriuretic effect, increasing sodium reabsorption, without affecting glomerular filtration rate (GFR), renal plasma flow, filtered load of glucose and plasma aldosterone levels. At the glomerular level, insulin is related to contradicting effects on GFR [6].
In our study, obese children with microalbuminuria had significantly higher values for waist circumference than obese children with normoalbuminuria. There is strong evidence that obesity, in particular central body fat distribution, is an important risk factor for renal function abnormalities [21].
We also found that obese children presenting with microalbuminuria had significantly higher blood pressure, triglyceride level and LDL level than those without microalbuminuria. There was a significantly higher prevalence of insulin resistance, impaired fasting glucose level and MS among obese children with microalbuminuria than among obese children with normoalbuminuria.
Nguyen et al. [18] found that obese adolescents with impaired glucose tolerance and insulin resistance had significant microalbuminuria. Prolonged exposure to hyperglycemia or unrecognized DM contributes to the early development of microvascular disease in patients with type 2 DM [22]. Obesity and its metabolic consequences lead to endothelial dysfunction and cardiovascular diseases [23]. Microalbuminuria is a marker of endothelial dysfunction and reflects renal and systemic endovascular damage [24].
Among our patient cohort, BMI, abdominal obesity, hypertension, impaired fasting glucose level and insulin resistance significantly increased the odds of microalbuminuria in obese children. High triglyceride, high LDL and low HDL levels were also significantly associated with microalbuminuria. Nguyen et al [18] found that within their subgroup of obese adolescents, cardiovascular risk factors, such as impaired fasting glucose, insulin resistance and hypertension, were strongly associated with microalbuminuria.
Reduced insulin sensitivity represents the most important link between obesity and other metabolic complications leading to renal injury [5]. Insulin interferes at several points in the systemic renin–angiotensin–aldosterone system, increasing its activity despite a state of sodium retention and volume expansion [25]. Insulin also increases the effects of angiotensin II on mesangial cells [26], thus contributing to hypertension, raised intraglomerular pressure, exacerbation of proteinuria, induction of intrarenal inflammatory cytokines and growth factors and apoptosis [27].
Another unifying explanation for our findings may stem from the recently elucidated role of adipokines in influencing body weight and glucose and via the effect of adiponectin on lipid metabolism in adipocytes [28]. Adiponectin levels have been found to decrease in hyperlipidemic rats as well as in rats having both DM and hyperlipidemia, possibly predicting the presence of hyperlipidemia and/or DM in an animal model [29]. Serum adiponectin levels are negatively associated with the BMI and may serve as a useful marker to evaluate glucose metabolism and obesity as well as insulin resistance and the MS [28]. The adiponectin null mouse exhibits albuminuria, and pathologic evaluation demonstrated foot process effacement of podocytes under baseline conditions [30]. Podocytes express the AdipoR1 receptor, and treatment with adiponectin normalizes albuminuria and restores foot process architecture. This effect is mediated through adiponectin stimulation of the AMPK pathway, a key regulator of intracellular energy status with potent antiproliferative effects [31].
Our data is in agreement with those of Nguyen et al. [18] who found that neither race nor age was significantly associated with microalbuminuria among nonobese and obese adolescents.
In our study, microalbuminuria showed a significant positive correlation with BMI, waist circumference, systolic and diastolic blood Pressure, triglyceride level, LDL level, insulin resistance and fasting glucose level. However, it showed a significant negative correlation with HDL.
Microalbuminuria is positively correlated with the severity of obesity [6]. Measures that enhance insulin sensitivity, reduce blood pressure and improve glycemic control have all been shown to reduce microalbuminuria [25]. Efforts to prevent and treat obesity early in life can be expected to have a major impact on the incidence, progression, costs and comorbidities of kidney disease [6]. Identification of modifiable risk factors for ESRD is critical to the development of effective, population-based preventive strategies [2]. One of the limitations of our study was the use of a single urine sample to estimate microalbuminuria, which might have led to an overestimation because albuminuria can be transient. However, we attempted to avoid orthostatic proteinuria by taking samples in the early morning with the children in supine position and to decrease the overestimation of albuminuria induced by exercise via instructions to limit strenuous (hyper) activity for 24 h prior to sampling.
Conclusion
Obesity in children is a risk factor for the development of microalbuminuria, which is associated significantly with metabolic syndrome and its different constituents. These findings raise the issue as to whether it would be beneficial to propose microalbuminuria as a qualifying item in the diagnosis of metabolic syndrome in children.
References
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295(13):1549–1555
Wang Y, Chen X, Klag MJ, Caballero B (2006) Epidemic of childhood obesity: implications for kidney disease. Adv Chronic Kidney Dis 13(4):336–351
Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ (2008) Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73(1):19–33
Wahba IM, Mak RH (2007) Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2(3):550–562
Sarafidis PA, Ruilope LM (2006) Insulin resistance, hyperinsulinemia and renal injury: mechanisms and implications. Am J Nephrol 26(3):232–244
Savino A, Pelliccia P, Chiarelli F, Mohn A (2010) Obesity-related renal injury in childhood. Horm Res Paediatr 73(5):303–311
Weisinger JR, Kempson RL, Elridge L, Swenson RS (1974) The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 81(4):440–447
Cohen AH (1975) Massive obesity and the kidney. A morphologic and statistical study. Am J Pathol 81(1):117–130
de Jong PE, Curhan GC (2006) Screening, monitoring, and treatment of albuminuria. Public health perspectives. J Am Soc Nephrol 17(8):2120–2126
Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE (2004) An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl 92:S18–S21
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL (2000) CDC growth charts: United States. National Center for Health Statistics, Hyattsville
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ, National Heart, Lung, and Blood Institute, American College of Cardiology Foundation, American Heart Association (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110(2):227–39
Fernandez JR, Redden DT, Pietrobelli A, Allison DB (2004) Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 145(4):439–444
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]:555–576
American Academy of Pediatrics (1992) National cholesterol education program: report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics 89(3Pt 2):525–84
American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care 27[Suppl 1]:S5–S10
Basi S, Lewis JB (2006) Microalbuminuria as a target to improve cardiovascular and renal outcomes. Am J Kidney Dis 47(6):927–946
Nguyen S, McCulloch C, Brakeman P, Portale A, Hsu CY (2008) Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics 121(1):37–45
Srivastava T (2006) Non diabetic consequences of obesity on kidney. Pediatr Nephrol 21(4):463–470
Nenov VD, Taal MW, Sakharova OV, Brenner BM (2000) Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 9(2):85–97
Pinto-Sietsma SJ, Navis G, Janssen WMT, de Zeeuw D, Gans ROB, de Jong PE, for the PREVEND Study Group (2003) A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41(4):733–741
Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, Silink M, Donaghue KC (2006) Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 29(6):1300–1306
Caballero AE (2003) Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res 11(11):1278–1289
Ritz E (2003) Albuminuria and vascular damage: the vicious twins. N Engl J Med 348(23):2349–2352
El-Atat FA, Stas SN, McFarlane SI, Sowers JR (2004) The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 15(11):2816–2827
Kreisberg JI (1982) Insulin requirement for contraction of cultured rat glomerular mesangial cells in response to angiotensin. II: possible role for insulin in modulating glomerular hemodynamics. Proc Nat Acad Sci USA 79(13):4190–4192
Ruster C, Wolf G (2006) Renin–angiotensin–aldosterone system and progression of renal disease. J Am Soc Nephrol 17(11):2985–2991
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116(7):1784–92
Margoni A, Perrea DN, Vlachos I, Prokopaki G, Pantopoulou A, Fotis L, KostakiM PAG (2011) Serum leptin, adiponectin and tumor necrosis factor in hyperlipidemic rats with/without concomitant diabetes mellitus. Mol Med 17(1–2):36–40
Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ (2008) Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118(5):1645–1656
Joachim H, Sharma K (2010) Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol 21(3):406–412
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanad, M., Gharib, A. Evaluation of microalbuminuria in obese children and its relation to metabolic syndrome. Pediatr Nephrol 26, 2193–2199 (2011). https://doi.org/10.1007/s00467-011-1931-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1931-9