Abstract
We conducted a retrospective study on children with primary nephrotic syndrome (NS) to evaluate the clinical course and outcome of children with steroid-sensitive NS (SSNS). The medical records of 226 children, median 3.46 years (min 1.00, max 15.08) who referred to our clinics with SSNS between January 1978 and September 2005 were reviewed and entered into the study. Minimum duration of follow-up was 5 years and maximum 20 years (median 7.25 years). Of 226 patients who were treated with corticosteroids, 38 (16.8%) had no relapse but the remaining 188 (83.2%) patients experienced several relapses of which 128 patients (56.6%) required additional immunosuppressive agents for the remission. Of these, 122 (95%) were treated with levamisole, 22 (17%) with cyclosporine, 36 (28%) with cyclophosphamide, and ten (7.8 %) treated with mycophenolate mofetil. Several patients had to switch from one medication to others due to lack of response. On the last follow-up visit, 64(28.3%) patients were still under treatment, some patients had taken all of the above-mentioned drugs but still had multiple recurrences. Only 103 (45.5%) patients were in remission off the drug more than 3 years. This study shows that nearly one-third of pediatric patients with SSNS experience frequent relapses despite the combination of multiple immunosuppressive medications, which may continue until adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroid-sensitive nephrotic syndrome (SSNS) is the most common form of childhood nephrotic syndrome (NS). Although children with SSNS respond to corticosteroids, however, 80–90% have relapses and need repeated courses of steroid therapy [1]. Although corticosteroids have reduced mortality of SSNS to less than 5% [2], repeated courses of steroid therapy have potential serious adverse effects such as obesity, poor growth, hypertension, osteoporosis, and diabetes mellitus. Thus, immunosuppressant agents including cyclosporine, levamisole, cyclophosphamide, and mycophenolate mofetil are used to treat these children. There still exist a large number of children in whom combination of these drug therapies failed to control NS satisfactorily.
There are few published studies regarding the long-term outcome of children with SSNS. In earlier studies reported by Trompeter et al. [3] and Koskimies et al. [4], long-term remission was observed in more than 90% of children with minimal change primary NS, so SSNS is considered a benign disorder with a very good prognosis. However, recent studies show that 33–42% of children have relapses until adolescence and adulthood [5, 6].
The present study was undertaken to add more information regarding the clinical course and outcome of children with SSNS from early childhood to adulthood treated with corticosteroids alone or combination with other immunosuppressive agents.
Patients and methods
We reviewed the medical records of all children with SSNS who were referred to the Pediatric Nephrology Clinics of our university between January 1978 and September 2005. Pertinent data including age of the disease onset, gender, and number of relapses, type of prescribed medications, and the disease outcome for a minimum of 5 years follow-up were obtained in all patients.
NS was defined by:
-
(1)
Urine protein excretion of more than 40 mg/m2/h or urinary protein to creatinine ratio more than 2 mg/mg
-
(2)
Hypoalbuminemia (serum albumin < 2.5 g/l).
Remission of NS was characterized by reduction of urine protein excretion to normal level (less than 4 mg/m2/h or urine albumin dipstick of 0 to trace for three consecutive days), normalization of serum albumin level, and resolution of edema. Patients who entered remission in response to corticosteroid treatment alone were referred to as having SSNS. Only children with steroid-sensitive nephrotic syndrome who were treated from the beginning of their disease and followed for at least 5 years were entered into the study. Children who had been treated in other centers and had referred to our clinics because of NS recurrence were excluded from the study. Children who were steroid-resistant in their first episode of NS and children with clinical or laboratory features incompatible with idiopathic primary NS were excluded from the study.
Relapsing NS was defined as urinary protein excretion more than 40 mg/m2/h or urine albumin 3+ to 4+ lasting for three consecutive days.
All patients received prednisolone 2 mg/kg/day or 60 mg /m2/day until proteinuria had disappeared for 4–5 days, and then prednisolone dose was switched to alternate day and tapered during 8–12 weeks. In steroid-dependent and frequently relapsing NS, if recurrence had occurred with steroid dose less than 0.5 mg/kg, after remission induction with daily prednisolone as mentioned above, the alternate day prednisolone prescribed and tapered gradually to a dose slightly above the threshold dose at which the proteinuria had occurred and with this dose it was continued for a period of 6–12 months and then gradually discontinued. If NS had recurred with an alternate dose above 0.5 mg/kg or patient manifested steroid toxicity (signs of Cushing's syndrome, hypertension, obesity, cataract, osteoporosis, etc.), the patient was treated with additional immunosuppressive agents.
Levamisole was used as a steroid-sparing agent with a dosage of 2–2.5 mg/kg on alternate days. In the patients that were to be treated with levamisole, concurrent with levamisole, prednisolone was prescribed as above until remission, and then the prednisolone dose was switched to the alternate day and tapered gradually to less than 0.5 mg/kg on the alternate day and continued for 6–12 months and then discontinued. If NS recurrence was seen with previous prednisolone dose, levamisole was considered ineffective for lowering prednisolone dose and other immunosuppressive drugs such as cyclosporine A, 5 mg/kg/day for 6 months or longer, cyclophosphamide, 2.5 mg/kg/day for 8 weeks, or mycophenolate mofetil 1,200 mg/m2/day in two divided doses prescribed for 6 months.
Cyclosporine dose was adjusted to maintain a blood cyclosporine trough level between 80 and 120 and 50 and 100 ng/ml, before and after remission periods, respectively. Cyclosporine with a trough level of 50–100 ng/ml continued for at least 6 months, and then tapered gradually for an additional 6 months and then discontinued. If NS relapsed during cyclosporine treatment with a trough level between 50 and 100 ng/ml, it considered ineffective as a steroid-sparing drug, but if NS relapsed during tapering, or after withdrawal, we defined it as cyclosporine dependency. Prednisolone was administered concurrently (1–1.5 mg/kg/day) with other immunosuppressive agents during the period of overt proteinuria and switched to the alternate day after the remission induction, then tapered gradually to less than 0.5 mg/kg alternate day and continued for 6–12 months.
A failure to respond to any one of these regimens justified the use of other immunosuppressive medications.
Our physicians have different opinions regarding performing renal biopsy in children with SSNS, and kidney biopsy was performed in only 35 patients. The indications for performing a kidney biopsy were determining any histologic feature of SSNS before starting second-line drugs in some patients, late resistance to steroid treatment, and evaluation of cyclosporine nephrotoxicity in children who had received cyclosporine for 12 months or more.
Statistical analysis was done with SPSS software version 16.02(SPSS Inc., Chicago, IL). Student's t test was used to compare between groups. Differences with p values <0.05 were regarded as significant.
Results
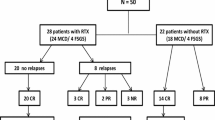
During the study period, 339 children with onset of NS were referred to our clinics. Of these, 311 were steroid-sensitive and 28 were steroid-resistant. Eighty-five patients were lost to follow-up; some after symptomatic improvement and others after one or two recurrences. Out of 311 children, 226 were followed more than 5 years and were entered into the study. There were 157(69.5%) males and 69(30.5%) females. The median age of the patients at first attack of NS was 3.46 years (min 1.00, max 15.08). Minimum duration of follow-up was 5 years and maximum 20.25 years (median 7.25). The age at last visit was median 11.50 years (min 6, max 25.6). Five patients at last visit were older than 18.
Of 226 patients who were treated with corticosteroids, 38 (16.8%) had no relapse but the remaining 188 (83.2%) patients experienced several relapses of which 128 patients (56.6%) required additional immunosuppressive agents for satisfactory control of NS. Of these, 124 (96.8%) were treated with levamisole, 22 (17%) with cyclosporine, 36 (28.1%) with cyclophosphamide, and ten (7.8 %) were treated with mycophenolate mofetil. Levamisole was effective as a steroid-sparing drug in 103 (83%) of patients, but only 43 (35%) remained in long-term remission (remissions without any treatment for more than 3 years) after the drug cessation. Long-term remissions were observed in 13 patients (36.1%) receiving cyclophosphamide. Cyclosporine was effective as a steroid-sparing drug in 21(95.4%) of patients who received this drug, but cyclosporine therapy caused permanent remissions in only two patients (9%), 19 (86%) relapsed after cessation of the drug and 16 (72.7%) became cyclosporine dependent. Therapy with mycophenolate mofetil resulted in complete remission in only two patients (20%), eight patients (80%) had relapses either during the treatment or after the drug cessation.
On the last follow-up visit, 64(28.3%) of patients were still under treatment, some patients had taken all of the above-mentioned drugs but still had multiple recurrences. A total of 161 (71.2%) patients out of 226 were on remission off treatment, lasting either for more than 3 years (103, 45.5%) or less than 3 years 58 (25.6%). In the group still receiving treatment after 5 years, the age at disease onset was 3.50 ± 2.31 and in the group still in remission more than 3 years, the age at disease onset was 4.21 ± 2.29. Also, t test showed there was no significant difference between the age of two groups (p = 0.15). One patient died because of pulmonary thromboembolic complications (Table 1).
Of 35 patients who underwent kidney biopsy, 24 (69%) had MCNC, 4 (11%) focal segmental glomerulosclerosis (FSGS), and 7 (20 %) diffuse mesangial proliferation,
Discussion
Corticosteroids have been used to treat idiopathic nephrotic syndrome since the early 1950s [7] and in fact have been the cornerstone of SSNS therapy. The International Study of Kidney Disease in Children (ISKDC) in 1981 suggested a regimen of prednisolone for treatment of nephrotic syndrome [8]. The characteristic feature of SSNS is a tendency to relapse frequently and with the ISKDC recommended drug therapy the relapse rate is high. Later, other studies showed that prolongation of initial daily prednisolone or longer duration of alternate-day prednisolone therapy decreases the relapse rate [9–15]. The Cochrane renal group on systematic analysis of the literature recommends that the duration of the initial prednisolone therapy should be for a minimum of 12 weeks. It also suggests that the alternate-day treatment should not be stopped abruptly at 12 weeks, but instead tapered over the next 2–4 months to decrease the relapse rate [16]. However, even with new corticosteroid regimens, 80–90% of children with SSNS have relapses, and nearly 50% relapse frequently [1]. Those children who receive repeated courses of steroid are at risk of the adverse effects of steroids. Some of them, such as cushingoid feature, obesity, hirsutism, hypertension, and psychological disturbances, are usually reversible after cessation of steroid therapy, but striae and cataract are not reversible [12]. Children with frequently relapsing or steroid-dependent NS who show adverse effects of corticosteroids are candidate for treatment with non-corticosteroid agents. These drugs are used to prolong the period of remission in these children. However, these agents have significant potential adverse effects. Currently, there is no consensus as to the most appropriate second-line agent in children with SSNS who continue to relapse with corticosteroids [1].
In the present study, more than 83% of patients had at least one relapse after the initial course of steroid therapy. Of our patients, 43% responded satisfactorily to steroid monotherapy, 57% had relapses with high doses of steroid or showed side-effects of steroids, requiring additional immunosuppressive therapy.
Levamisole has been used for the treatment of SSNS [17, 18]. A meta-analysis of six clinical trials has shown that levamisole significantly reduces the relapse rate compared to placebo or prednisolone alone [1]. Levamisole effectively reduces the need for corticosteroids, however, many patients experience relapse after stopping the treatment [19]. We used levamisole as a steroid-sparing drug for our patients who had relapses with high doses of steroid. It was effective in lowering the steroid dose in about 83% of patients. Furthermore, with usage of levamisole, we could taper the steroid dose to lower than 0.5 mg/kg on alternate days and prevent major steroid side-effects. Unfortunately, most patients had recurrences after stopping this drug; only 35% had permanent remission after cessation of the drug. Side-effects of this drug are rare [20, 21]; we used levamisole for up to 2 years; only two patients complained of nausea and dizziness and one developed reversible neutropenia.
Cyclosporine has also been used effectively for reducing the relapse rate of SSNS in children, but its effect has not been sustained after cyclosporine was discontinued [1, 17, 19, 22–26]. Cyclosporine was also effective in remission induction in most of our patients, but only in 13% of patients we observed more than 3 years remission off treatment following cyclosporine usage and about 72% became drug-dependent and relapsed after the drug dose reduction or cessation. Side-effects of cyclosporine may include hypertension, gingival hypertrophy, hirsutism, nephrotoxicity, hypercholesterolemia, and elevated transaminases [27, 28].
Cyclophosphamide has been used for the treatment of NS for more than 30 years [29] and has been effective in reducing relapse rates. Barrat et al. prescribed cyclophosphamide orally in a dosage of 3 mg/kg/day for 8 weeks and reported that it induced sustained remission in 69% at one year and 44% at 5 years [29]. In another study, the long-term outcome following cyclophosphamide usage was examined in 93 children with SSNS. Only 35% experienced no relapses after cyclophosphamide administration [30]. In this study, only 36% of patients showed long-term remission after treatment with cyclophosphamide. Side-effects of cyclophosphamide are nausea, vomiting, leucopenia, infections, thrombocytopenia, hemorrhagic cystitis, and gonadal toxicity [26]. Total leukocyte counts should be monitored every 2 weeks during cyclophosphamide therapy and if the count falls below 4,000/mm3, the drug should be temporarily discontinued. For prevention of gonadal toxicity, the use of more than one course of cyclophosphamide therapy should be avoided [26].
The mycophenolate mofetil is a relatively new drug used for the treatment of SSNS. A study by Bagga and coworkers showed that mycophenolate mofetil treatment resulted in steroid sparing, but after discontinuation of mycophenolate mofetil, 68.4% of patients had an increased frequency of relapses and recurrence of steroid dependence, requiring treatment with other medications [31]. Dorresteijn and coworkers, with a randomized clinical trial, showed that in children with frequently relapsing MCNS, mycophenolate mofetil had a favorable effect, but there was a higher tendency to relapse in patients treated with mycophenolate mofetil than in patients treated with cyclosporine [32]. Eighty percent of our patients faced recurrence of NS after stopping the mycophenolate mofetil.
Earlier reports on the outcome of SSNS [3, 4] have suggested SSNS as a benign condition with the assumption that over 90% of children enter permanent remission in late childhood or after puberty. More-recent studies, however, show that none of the available immunosuppressive drugs has absolute curative effect on NS. All patients in the present study responded to steroid initially but the majority of them experienced one or more relapses, and some patients continued to have relapses until adulthood. The present study showed that after 5 years of follow-up, about 28.4% of patients were still under treatment with various immunosuppressive agents because of recurrences, and only about 45% of patients were in remission longer than 3 years. A study by Ruth and coworkers found that 33% children with NS relapsed in adulthood [5]. In another study by Fakhouri et al., 42.2% of patients experienced at least one relapse of nephrotic syndrome in adulthood [6].
In summary, children with SSNS enter remission with available immunosuppressive drugs and progression to chronic renal failure is rare, but most have recurrences, and contrary to the earlier reports, a considerable number (in our study 28.5%) have multiple recurrence episodes which may continue until adulthood. SSNS is a challenging problem for pediatric nephrologists.
References
Hodson EM, Willis NS, Craig JC (2008) Non-corticosteroid treatment for nephrotic syndrome in children. Cochran Database Syst Rev 23(1):CDOO2290
Report of the International Study of Kidney Disease in Children (1984) Minimal change nephrotic syndrome in children: deaths during the first 5 to 15 years' observation. Pediatrics 73(4):497–501
Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS (1985) Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 16(1(8425)):368–370
Koskimies O, Vilska J, Rapola J, Hallman N (1982) Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57:544–548
Ruth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ (2005) Children with steroid-sensitive nephrotic syndrome come of age: long–term outcome. J Pediatr 147(2):202–207
Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux MF, Landais P, Lesavre P, Chauveau D, Knebelmann B, Broyer M, Grünfeld JP, Niaudet P (2003) Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 41(3):550–557
Arneil GC (1956) Treatment of nephrosis with prednisolone. Lancet 1:409–411
(1981) International study of kidney Disease in children. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisolone, J Pediatr 98:561–564
(1979) Alternate–day versus intermittent prednisolone in frequently relapsing nephrotic syndrome. A report of Arbetsgemeinschaft für Pädiatrische Nephrologie: Lancet 1(8113):401–403
Bagga A, Hari P, Srivastava RN (1999) Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 13:824–827
Brodehl J (1991) The treatment of minimal change nephritic syndrome: lessons learned from multicentre co-operative studies. Eur J Pediatr 150:380–387
Ehrich JH, Brodehl J (1993) Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children; Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 152(4):357–361
Ueda N, Chihara M, Kawaguchi S, Niinomi Y, Nonoda T, Matsumoto J, Ohnishi M, Yasaki T (1988) Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. J Pediatr 112(1):122–216
Ksiázek J, Wyszyńska T (1995) Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr 84(8):889–893
Jayantha UK (2004) Comparison of ISKDC regime with a 7 month regime in the first attack of nephrotic syndrome (Abstract). Pediatr Nephrol 19(9):C81
Hodson EM., Willis NS, Craig IC (2007) Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 17(4):CD001533
Abeyagunawardena A, Dillon MJ, Rees L, Van’t Hoff W, Trompeter RS (2003) The use of steroid-sparing agents in steroid-sensitive nephrotic syndrome. Pediatr Nephrol 18(9):919–924
Boyer O, Moulder JK, Grandin L, Somers MJ (2008) Short and long-term efficacy of levamisole as adjunctive therapy in childhood nephrotic syndrome. Pediatr Nephrol 23(4):575–580
Durkan AM, Hodson EM, Willis NS, Craig JC (2001) Immunosuppressive agents in childhood nephrotic syndrome: a meta- analysis of randomized controlled trials. Kidney Int 59(5):1919–1927
Sumegi V, Haszon I, Ivanyi B, Bereczki C, Papp F, Turi S (2004) long-term effects of levamisole treatment in childhood nephrotic syndrome. Pediatr Nephrol 19(12):1354–1360
Ginevri F, Trivelli A, Ciardi MR, Chiggeri CM, Parfumo F, Gusmano R (1996) Protracted levamisole in children with frequent relapse nephrotic syndrome. Pediatr Nephrol 10(4):550
El-Hosseini A, El-Basuony F, Mahmoud I, Sheashaa H, Sabry A, Hassan R, Taha N, Hassan N, Seyed-Ahmad N, Sobh M (2005) Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single- center experience. Nephrol Dial Transplant 20(11):2433–2438
Kemper MJ, Kuwertz-Broeking E, Bulla M, Mueller-Wiefel DE, Neuhaus TJ (2004) Recurrence of severe steroid dependency in cyclosporin A-treated childhood idiopathic nephrotic syndrome. Nephrol Dial Transplant 19(5):1136–1141
Ishikura K, Ikeda M, Hattori S, Yoshikawa N, Sasaki S, Iijima K, Nakanishi K, Yata N, Honda M (2008) Effective and safe treatment with cyclosporine in nephrotic children: a prospective, randomized multicenter trial. Kidney Int 73(10):1167–1173
Hino S, Takemura T, Okada M, Murakami K, Yagi K, Fukushima K, Yoshioka K (1998) Follow-up study of children with nephrotic syndrome treated with a long-term moderate dose of cyclosporine. Am J Kidney Dis 31(6):932–939
Bagga A (2008) Management of steroid-sensitive nephrotic syndrome: revised guidelines. Indian Pediatr 45:203–214
Iijima K, Hamahira K, Tanaka R, Kobayashi A, Nozu K, Nakamura H, Yoshikawa N (2002) Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 61(5):1801–1805
Magnasco A, Rossi A, Catarsi P, Gusmano R, Ginevri F, Perfumo F, Chiggeri GM (2008) Cyclosporine and organ specific toxicity: clinical aspects, pharmacogenetics and prospectives. Curr Clin Pharmacol 3(3):166–173
Barratt TM, Soothil JF (1970) controlled trials of cyclophosphamide in steroid-sensitive relapsing nephrotic syndrome of childhood. Lancet 2:479–482
Kyrieleis HA, levtchenko EN, Wetzels JF (2007) Long–term outcome after cyclophosphamide treatment in children with steroid dependent and frequently relapsing minimal change nephrotic syndrome. Am J Kidney Dis 49:592–597
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42(6):1114–2110
Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ (2008) Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 23(11):2013–2020
Acknowledgements
The authors thank professor Farahnak Assadi for his thoughtful reviewing of the manuscript and his very helpful advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esfahani, S.T., Madani, A., Asgharian, F. et al. Clinical course and outcome of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 26, 1089–1093 (2011). https://doi.org/10.1007/s00467-011-1837-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1837-6