Abstract
Experimental findings indicate that sirolimus (SRL) inhibits longitudinal growth by mechanisms potentially related to its inhibitory effects on both cell proliferation and expression of vascular endothelial growth factor (VEGF). The aim of this study was to investigate the growth pattern of kidney-transplanted children treated with SRL in a multicenter observational clinical study. Height, change in height SD (Δ height) and growth velocity of pediatric patients with renal transplant were calculated at 0, 6, 12, and 24 months after starting SRL. Controls of kidney-transplanted children not treated with SRL were matched by age, gender, renal function, and dose of corticosteroids. Sixty-eight children (34 SRL, 34 controls) were enrolled in the study. Nephrotoxicity was the most frequent indication to start therapy with SRL. SRL exerted an adverse effect on growth as demonstrated by significantly lower (p < 0.05) growth velocity (cm/year) and smaller change in height SD in the SRL group after 6 (4.08 vs. 6.56 and –0.05 vs. 0.14), 12 (4.44 vs. 6.11 and –0.03 vs. 0.28) and 24 (4.53 vs. 6.03 and –0.04 vs. 0.53) months of treatment. This study suggests that SRL therapy may interfere with growth of kidney-transplanted children. This undesirable effect needs to be taken into account when considering a switch to SRL and confirmed in further prospective trials including larger number of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sirolimus (SRL) is a potent immunosuppressive agent derived from macrolide antibiotics. SRL decreases cell proliferation [1] by inhibition of the mammalian target of rapamycin (mTOR), a key regulator of cell growth [2]. In addition, SRL has been demonstrated to exert anti-angiogenic properties by reduction in vascular endothelial growth factor (VEGF) expression [3].
Clinical studies have shown that the anti-proliferative and anti-angiogenic properties of SRL may be useful to prevent the intimal changes associated with chronic allograft nephropathy [4–8] and to reduce the incidence of post-transplant neoplasia [9, 11]. However, experimental findings indicate that administration of SRL to fast-growing rats impairs longitudinal growth and induces marked alterations in growth-plate structure by mechanisms likely related to its inhibitory effects on cell proliferation and VEGF local expression [12–14].
The above findings suggest that treatment with SRL in kidney-transplanted children might interfere with the longitudinal growth of these patients. This hypothesis has not been assessed in pediatric clinic trials and only a single case report analyzes growth and the response to growth hormone therapy in a child treated with SRL [15].
Accordingly, the objective of this study was to find out if SRL, given at doses usually used in clinical practice, adversely influences the growth of kidney-transplanted children.
Patients and methods
Study subjects
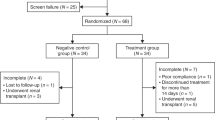
Multicenter observational clinical study. Data on growth and renal function of 34 kidney-transplanted children treated with SRL were retrospectively obtained from the clinical records of the participating renal transplant units. Transplanted children with either primary diseases (syndromic diseases, cystinosis, etc.) that may severely interfere with growth or unstable clinical condition during follow-up (intercurrent diseases, poor metabolic control, poor or dubious adherence to treatment) or multiorgan transplant patients were not included in the study.
Control group
Thirty-four kidney-transplanted children not treated with SRL served as controls. Each transplant unit recruited their controls. Controls were matched by chronological age, age at transplantation, gender, renal function, and corticosteroid dose with the SRL-treated patients so that no differences in these variables would exist between both groups of children.
Written informed consent was obtained from the parents of children enrolled in the study. Likewise, the study was approved by the corresponding ethics committees of each participating hospital.
Clinical studies
Anthropometric measurements, glomerular filtration rate (GFR), and steroid dose were obtained immediately before beginning treatment with SRL (time 0) and 6, 12, and 24 months after starting it.
Height Z scores (SDS) for each patient were calculated as follows: (mean normal height – patient’s height) / height SD. For age- and sex-matched reference values the National Health and Nutrition Examination Survey (NHANES) data were used. GFR was calculated by Schwartz’s formula [16] on the basis of serum creatinine concentrations measured by automatic analyzers in the clinical biochemistry laboratories of the participating hospitals. Bone age was determined by the Tanner Whitehouse method [17].
Height Z scores and growth velocity were compared between both groups of transplanted patients. A subgroup of prepubertal patients younger than 12 years of age with good renal function (GFR > 50 ml/min/1.73 m2), and low prednisone dose ( < 5 mg/day) beyond 6 months on SRL was specifically analyzed and compared with its matched control group.
Statistical analysis
Results are expressed as mean ± SD. Comparisons between groups were performed by ANOVA for variables with normal distribution and non-parametric Mann–Whitney U test was used for skewed data. p < 0.05 was considered statistically significant.
Results
Thirty-four children were included in each group. Prior to SRL, patients were receiving immunosuppressant treatment with prednisone, calcineurin inhibitors (CNIs), and mycophenolate. The most frequent indications to replace the CNIs by SRL were nephrotoxicity (26%), chronic allograft nephropathy (21%), malignancy (15%), acute rejection (12%), tacrolimus-induced glucose intolerance, or diabetes (6%).
Trough levels of SRL were 7.82 ± 3.92, 7.45 ± 3.71, 7.43 ± 3.38, and 6.31 ± 1.98 ng/ml at 0, 6, 12, and 24 months of follow-up, respectively.
According to the study’s design, there were no differences in gender, chronological age, primary nephropathy, age at transplantation, steroid dose, and GFR at the beginning of SRL therapy between both the study and control groups (Table 1). GFR and steroid dose remained similar between the two groups during the follow-up period. Table 1 also shows that GFR did not significantly change during the 24 months of follow-up in either of the two groups of children whereas prednisone dose decreased significantly (p < 0.001) in both.
There were no differences in height SDS between SRL and the control group at 0, 6, 12, and 24 months of follow-up (Table 1). However, Δ heights were significantly lower in the SRL group: –0.05 ± 0.30 versus 0.14 ± 0.28 SDS at 6 months (p = 0.014), -0.03 ± 0.44 versus 0.28 ± 0.46 SDS at 12 months (p = 0.006) and –0.04 ± 0.54 versus 0.53 ± 0.58 at 24 months (p = 0.001) of follow-up (Fig. 1a). Likewise, growth velocity expressed in cm/year was significantly reduced in the SRL group in comparison with the control group during the follow-up period: 4.08 ± 2.94 versus 6.56 ± 2.91 at 6 months (p = 0.001), 4.44 ± 2.84 versus 6.11 ± 2.63 (p = 0.015) at 12 months and 4.53 ± 2.44 versus 6.03 ± 2.36 at 12 months (p = 0.033) (Fig. 1b).
Evolution of ∆ height SDS (a) and growth velocity (b) during the 2-year study period in both groups of transplanted children. Columns and vertical bars represent mean and SD, respectively. Both anthropometric variables calculated with respect to the initial height at the moment of starting SRL treatment. Comparisons between groups: *p < 0.05; **p < 0.01
In addition, growth in two matched subgroups of prepuberal patients with GFR >50 ml/min/1.73 m2, and low prednisone dose (< 5 mg/day) selected from the SRL and the control populations was analyzed separately (Table 2). Both subgroups did not differ with respect to age, gender, GFR, and steroid dose. Again, Δ height and growth velocity were significantly worse in children treated with SRL.
Discussion
This study analyzes for the first time the longitudinal growth of a series of kidney-transplanted children treated with SRL. In spite of the methodological limitations resulting from the retrospective data collection and the relatively small number of patients included in the analysis, our study suggests that SRL has the potential to adversely affect the growth of these patients, at least during the first 2 years of treatment with SRL. This finding is in agreement with experimental studies showing that administration of pharmacological doses of SRL decreased the longitudinal growth rate in young rats due to mechanisms likely dependent on the inhibitory effects of SRL on cell proliferation and VEGF expression at long bone growth plate [12–14]. This deleterious effect on longitudinal growth was evident, in our study, by the lower growth velocity and the reduced increment in height SDS found in children treated with SRL in comparison to children not receiving this drug. The controls were matched for chronological age, age at transplantation, sex, renal function, and dose of corticosteroids, which is important as these factors are known to influence growth outcome of transplanted children [18, 19]. Moreover, the adverse influence of SRL on longitudinal growth was also observed in the selected subgroup of patients with younger age, optimal allograft function, and low prednisone dose, with a presumable better growth outcome. As shown in previous studies, the association of those three conditions facilitates the occurrence of catch-up growth in pediatric kidney transplant on immunosuppressive treatment with CNIs and mycophenolate [20–27]. In agreement with this assumption, our control group of patients with a mean age at the study entry of 4.7 years, mean corticosteroid dose during follow-up of approximately 2 mg/day and normal GFR exhibited a positive change in the height Z score whereas this effect was not found in the subgroup of SRL-treated patients with similar age, prednisone dose and renal function (Table 2). In 21 of our patients on SRL, growth rate could be compared during two sequential 12-month periods, before and after starting the drug, at an age of 11.2 ± 4.4 years. Growth velocity dropped from 5.43 ± 2.93 to 4.08 ± 2.78 cm/year (p < 0.05) but the change in height SD was not significantly different (–0.03 ± 0.40 vs. –0.07 ± 0.32). It should be pointed out that although the prednisone dose was similar in both periods (3.50 ± 3.59 vs. 3.85 ± 2.23 mg/day) the GFR significantly increased after the introduction of SRL (43.61 ± 27.85 vs. 74.68 ± 44.26 ml/min/1.73m2, p < 0.05). Thus, the patients were not well matched in both periods with regard to their renal function. However, the fact that the improvement in GFR was not associated with an acceleration of longitudinal growth rate further supports the inhibitory effect of SRL on growth.
It is of note that preliminary experimental data [28] and a single case report [15] indicate that treatment with recombinant human growth hormone (rhGH) may reverse, at least partially, the negative effect on longitudinal growth caused by SRL. However, as occurred in our study and in other studies [9–11], the development of a malignant tumor was one of the main causes for replacing CNIs by SRL in transplanted children, which may represent a contraindication to prescribe rhGH therapy in those patients.
Although it was not the goal of our study, the results presented here confirm the beneficial effect of SRL treatment on the renal function in pediatric kidney transplant recipients with chronic allograft dysfunction or CNIs related toxicity [4–8]. Despite lack of significance in our small population, the mean GFR tended to improve in the SRL group during the 24 months of follow-up, whereas it slightly declined in the control children not receiving SRL. This improvement of renal function might even counteract the potential inhibitory of SRL on growth, as suggested in a recent and preliminary study in which the mean height Z score improved similarly over a 24-month period in pediatric renal transplant patients treated with either SRL or CNIs [29].
In summary, this report analyzes for the first time the longitudinal growth profile in a series of kidney-transplanted children treated with SRL during a period of 2 years and highlights the potential adverse effect of this drug on the optimal achievement of height in those patients. Prospective clinical trials are necessary to confirm this effect, which needs to be taken into account at the time of prescribing SRL in pediatric transplanted patients.
Abbreviations
- SRL:
-
Sirolimus
- GFR:
-
Glomerular filtration rate
- mTOR:
-
Mammalian target of rapamycin
- VEGF:
-
Vascular endothelial growth factor
- Δ Height:
-
Change in height SD
- CNIs:
-
Calcineurin inhibitors
- rhGH:
-
Recombinant human growth hormone
References
Sehgal SN (2003) Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 35:7S–14S
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18:1926–1945
Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK (2002) Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 8:128–135
Ibáñez JP, Monteverde ML, Diaz MA, Goldberg J, Turconi AF (2007) Sirolimus in chronic allograft nephropathy in pediatric recipients. Pediatr Transplant 11:777–780
Höcker B, Feneberg R, Köpf S, Weber LT, Waldherr R, Wühl E, Tönshoff B (2006) SRL-based immunosuppression vs. CNI minimization in pediatric renal transplant recipients with chronic CNI nephrotoxicity. Pediatr Transplant 10:593–601
Ibáñez JP, Monteverde ML, Goldberg J, Diaz MA, Turconi A (2005) Sirolimus in pediatric renal transplantation. Transplant Proc 37:682–684
Mota A, Arias M, Taskinen EI, Paavonen T, Brault Y, Legendre C, Claesson K, Castagneto M, Campistol JM, Hutchison B, Burke JT, Yilmaz S, Häyry P, Neylan JF (2004) Sirolimus-based therapy following early Cyclosporine withdrawal provides significantly improved renal histology and function at 3 years. Am J Transplant 4:953–961
Falger JC, Mueller T, Arbeiter K, Boehm M, Regele H, Balzar E, Aufricht C (2006) Conversion from calcineurin inhibitor to sirolimus in pediatric chronic allograft nephropathy. Pediatr Transplant 10:565–569
Mathew T, Kreis H, Friend P (2004) Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 18:446–449
Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G (2005) Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 352:1317–1323
Sindhi R (2003) Sirolimus in pediatric transplant recipients. Transplant Proc 35:113S–114S
Alvarez-García O, García-López E, Loredo V, Gil-Peña H, Rodríguez-Suárez J, Ordóñez FA, Carbajo-Pérez E, Santos F (2010) Rapamycin induces growth retardation by disrupting angiogenesis in the growth plate. Kidney Int 78:561–568
Alvarez-Garcia O, Carbajo-Pérez E, Garcia E, Gil H, Molinos I, Rodriguez J, Ordoñez FA, Santos F (2007) Rapamycin retards growth and causes marked alterations in the growth plate of young rats. Pediatr Nephrol 22:954–961
Sanchez CP, He YZ (2009) Bone growth during rapamycin therapy in young rats. BMC Pediatr 9:3
Rangel GA, Ariceta G (2009) Growth failure associated with sirolimus: case report. Pediatr Nephrol 24:2047–2050
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Amst 34:571–590
Tanner JM, Marshall WA, Healy MJ, Goldstein H (1983) Assessment of skeletal maturity and prediction of adult height (tw2 method). 2nd edn. Academic Press, New York
Fine RN (2010) Etiology and treatment of growth retardation in children with chronic kidney disease and end-stage renal disease: a historical perspective. Pediatr Nephrol 25:725–732
Nissel R, Brázda I, Feneberg R, Wigger M, Greiner C, Querfeld U, Haffner D (2004) Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int 66:792–800
Grenda R, Watson A, Trompeter R, Tönshoff B, Jaray J, Fitzpatrick M, Murer L, Vondrak K, Maxwell H, van Damme-Lombaerts R, Loirat C, Mor E, Cochat P, Milford DV, Brown M, Webb NJ (2010) A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant 10:828–836
Ferraris JR, Pasqualini T, Alonso G, Legal S, Sorroche P, Galich A, Coccia P, Ghezzi L, Ferraris V, Karabatas L, Guida C, Jasper H (2010) A study on strategies for improving growth and body composition after renal transplantation. Pediatr Nephrol 25:753–762
Li L, Chang A, Naesens M, Kambham N, Waskerwitz J, Martin J, Wong C, Alexander S, Grimm P, Concepcion W, Salvatierra O, Sarwal MM (2009) Steroid-free immunosuppression since 1999: 129 pediatric renal transplants with sustained graft and patients benefits. Am J Transplant 9:1362–1372
Sarwal MM, Vidhun JR, Alexander SR, Satterwhite T, Millan M, Salvatierra O Jr (2003) Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation 76:1331–1339
Grenda R (2010) Effects of steroid avoidance and novel protocols on growth in paediatric renal transplant patients. Pediatr Nephrol 25:747–752
Tejani A, Fine R, Alexander S, Harmon W, Stablein D (1993) Factors predictive of sustained growth in children after renal transplantation. J Pediatr 122:397–402
Harambat J, Cochat P (2009) Growth after renal transplantation. Pediatr Nephrol 24:1297–1306
North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) 2008 annual report. Available at: https://doi.org/web.emmes. com/study/ped/annlrept/annlrept.html. Accessed 8 Oct 2009.
Alvarez-Garcia O, Garcia-Lopez E, Loredo V, Gil-Peña H, Rodriguez J, Ordoñez FA, Santos F (2010) Growth hormone (GH) administration improves longitudinal growth in rats with growth retardation induced by rapamycin. Pediatr Nephrol 25:31A
Warshaw BL, Hymes LC (2010) Linear growth in pediatric renal transplant recipients is similar with sirolimus vs. calcineurin inhibitors. Presented at the American Transplant Congress, San Diego (CA) 2010 (abstract).
Acknowledgements
The study was funded by the Instituto de Salud Carlos III from the Spanish Ministry of Science and Innovation and by the Fundación Nutrición y Crecimiento.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González, D., García, C.D., Azócar, M. et al. Growth of kidney-transplanted pediatric patients treated with sirolimus. Pediatr Nephrol 26, 961–966 (2011). https://doi.org/10.1007/s00467-011-1811-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1811-3